Introduction
Humic substances (HS) are defined as a complex of organic substances that are the product of decomposition and resynthesis, in the soil, of plant and animal remains through the action of the microbiota. Its composition varies according to the source material, the biotic and abiotic components of the soil, and the associated mineralogy (García et al., 2019). Piccolo (2002) defined them as supramolecular associations of relatively small, self-assembling heterogeneous molecules, in which non-covalent forces predominate.
The main constituent elements of HS are C and O, with ranges from 50 to 60 % and from 30 to 35 %, respectively; to a lesser extent, they are H (4 to 6 %), N (2 to 6 %) and S (0.5 to 1.5 %) (Schnitzer, 1983). It is recognized that, when HA are applied to plants, several of their structural components are responsible for its bioactivity (García et al., 2016). Using chemometrics (PCR) to demonstrate the structure-activity relationship of HA, in addition to using chemical methods (elemental composition) and spectroscopic techniques (UV-Vis, infra-red FTIR, and nuclear magnetic resonance 13C CP-MAS NMR), aliphatic, aromatic, carboxyl and hydroxyl functional groups have been identified, as well as methoxyl, aryl, and O-aryl structures (of lignin), which explain their high biostimulant activity in plants (García et al., 2016). Humic substances or HA, due to their structural characteristics, have the ability to stimulate the antioxidant enzymatic system (peroxidases, catalases, superoxide dismutases) in charge of converting reactive oxygen species into harmless species for plants, increasing their tolerance to biotic and abiotic stresses (Cordeiro et al., 2011). Humic acids can change the functionality of cell membranes in the roots, causing reductions in hydraulic conductivity and, sequentially, promoting the growth of foliar organs, transpiration and resistance to water stress, the mechanism of action is known as colloidal stress (Asli and Neumann, 2010).
Among the abiotic stresses that affect hydraulic, edaphological and physiological conditions, seriously affecting the productivity of plants throughout the world, the most important is drought (Bodner et al., 2015; Rafique et al., 2020). Water stress affects plant growth, due to low availability of water in the soil, causing transpiration to exceed the absorbed water, and thereby causing a drastic decrease in water potential and cell turgor, which generates significant changes at the anatomical, physiological and biochemical level in most plants, seriously affecting the photosynthetic process and, as a consequence, the yield of many crops (Osakabe et al., 2014). Most plants face this water deficiency through different evolutionary adaptations at the physiological, anatomical, and cellular levels, which favor a greater entry of water and a more efficient use of it.
Maize is grown worldwide, and is also subject to the damage caused by water deficit. In Mexico, between 15 to 17 million hectares are cultivated under rainfed conditions annually, 50 % with maize. The yield of rainfed maize is considerably lower than irrigated maize (Inzunza-Ibarra et al., 2018). The grain yield in maize is affected by the intensity of water stress and the stage of development.
Recent literature reports that humic acids can be used to reduce the damage caused by water stress by stimulating the antioxidant enzyme system. Due this, the expression of genes that code for the synthesis of enzymes that destroy reactive oxygen species (ROS), peroxidase (POX), catalase (CAT), superoxide dismutase (SOD), is stimulated at the cytosolic level (García et al., 2016). The application of fulvic acids (FA) to maize plants stressed by drought stimulated the enzymes POX, CAT and SOD, exerting an effect of protection and preservation of growth (Anjum et al., 2011). In sugarcane subjected to water stress, it was also shown that the application of HA protects the plants by inducing the activity of antioxidant enzymes (Aguiar et al., 2016). Rodrigues et al. (2017), using commercial HS, obtained higher seedling vigor and a higher emergence rate, which directly affects the establishment in the initial stage and the viability of the seeds.
The aim of this study was to evaluate the effect of the application of humic acids (HA) extracted from vermicompost of domestic waste, at three different concentrations, on the germination and vegetative phases, with and without water stress induced by withholding irrigation, in three native populations of maize (Zea mays L.) from regions with drought in Oaxaca.
Materials and methods
Obtaining and characterizing humic acids from vermicompost
Humic acids were obtained from vermicompost of urban solid waste, using the methodology proposed by the International Humic Substances Society (IHSS) and in accordance to Swift (1996).
Evaluation of the effects of the application of humic acids on seed germination
The germination experiments were developed under controlled conditions in a germination chamber (Biotronette, LabLine Instruments, Huntingburg, Iindiana, USA), with a relative humidity of 70 %, temperature 29/25 °C (day/night), and a 12 h photoperiod, with a light intensity equivalent to 200 μmol m-2 s-1. Maize seeds came from mass selections made by local farmers at three regions with recurrent drought in the state of Oaxaca, Mexico: Mixteca (Cajete-Caj), Istmo (Zapalote-Zap) and Valles Centrales (Cuilapam-Cuil). They belong to the races Bolita and Zapalote races, characterized by having short and early plants, with small ears and seeds, and medium tolerance to drought (Kato et al., 2009). Seeds were subjected to disinfestation with 2.5 % sodium hypochlorite for 30 min and then washed repeatedly to eliminate chloride residues and dried. They were soaked in HA solutions at four concentrations (0, 30, 35 and 40 mg L-1) for 12 hours (Canellas et al., 2019a; Pinos et al., 2019), with a total of 50 seeds per replicate. The experimental design was a complete combinatorial (4 × 3) bifactorial (factors were maize populations and HA concentrations) with four replicates. Germination lasted 10 days and was counted from the emergence of the radicle. The variables evaluated were germination percentage (% G) at five, eight, and 10 days; germination rate index (GRI) at five days; plant height (P-grw.h) in cm and root length (RL) in cm at five and 10 days (measured from the base of the stem to the tip of the upper leaf, and from the basal node of the stem to the tip of the longest root, respectively), number of roots (Nrs) at five and 10 days; shoot (Dry-mss.ap) and root (R.dry-mss) dry mass in g at 10 days.
Evaluation of the effects of the application of humic acids on plant height
The experiment was conducted in a greenhouse with relative humidity conditions between 80 and 90 %, temperature of 27 ± 2 ºC and natural photoperiod at central Mexico. A completely randomized design was used with four HA concentration (0, 30, 35 and 40 mg L-1), applied independently to the three maize populations. Plants were grown in high-density polyethylene bags (50 cm in diameter and 20 cm in height) with 15 kg of soil, one plant per bag. The soil used was Luvisol, with a sandy loam texture, neutral pH, and low organic matter content.
Humic acids were applied by foliar and root routes with a 20 L volume spray backpack, starting 12 days after emergence, with a frequency of twice a week until the end of the experiment (35 days after emergence). Each plant received 28 mL of HA at each application; with this volume, the dropping point was reached on the leaf blade. In this period all treatments were irrigated homogeneously for 20 min at a frequency of twice a week. The criterion to decide the frequency of irrigation was the range of water available in this sandy-loam soil, fluctuating from 80 to 30 %, equivalent to 10.4 and 14.4 centibars (cb). The available water was monitored using tensiometers and expressed in cb. Tensiometers were calibrated considering the specific critical value, which depends on the type of soil and plants involved. The recovery irrigation, carried out only to restore the root zone to field capacity, was applied when the soil water tension reached 14.4 cb, since at this point the average reading of the tensiometer exceeded the critical value (Hensley and Deputy, 1999).
Plant height measurements were performed, taken from the base of the stem to the tip of the longest leaf, using a Global Plus tape measure with a margin of error of 1 mm. Data were recorded at vegetative stage V7 (the sheath of leaf number 7 was visible).
Evaluation of the effects of humic acids on maize plants subjected to water stress
A (2 × 4) complete combinatorial bifactorial arrangement was used. Factor 1 was hydric condition with two levels, with (R) and without (Wd) irrigation for induced water stress. Factor 2 was HA concentrations with four levels (0, 30, 35 and 40 mg L-1). The eight resulting treatments were replicated four times and applied independently to seeds of the three maize populations. The experimental unit was as described for the first part of this study.
The evaluations were carried out at stage R1 (silks visible). The duration of the experiment was four months, from August to December 2018. For the treatments with induced water stress, irrigation was applied twice a week from sowing to 35 days (same procedure as the irrigation treatments described above), at which time irrigation was suspended, resuming again from day 55 and continuing until the end of the experiment. In both cases, equal volumes of water were used in each application.
At the end of the experiment, the following response variables were evaluated: P-grw-h, number of leaves (Nsh) (fully extended leaves were counted), Dry-mss.ap, R.dry-mss and total soluble protein content in leaf (Ts-pro) (Bradford, 1976).
Statistical data processing
The data were verified for normality and variance homogeneity. A one-factor or multifactorial analysis of variance (ANOVA) was performed, according to the experimental design in each case, using SAS software (Statistical Analysis System, Version 9.0). When significant differences were detected, the Tukey test for separation of means was performed (P ≤ 0.05).
Results
Effects of the application of vermicompost HA on germination of maize seeds
The effects of HA on germination of maize seeds were more evident at the highest concentrations and at eight and 10 days (Figure 1A). At 10 days, the concentration of 35 mg L-1 significantly stimulated (P ≤ 0.01) the growth of the radicle, 16.7 % higher than the control. On the contrary, at five days all HA concentrations delayed germination. Interestingly, the concentration of 35 mg L-1, which delayed germination by 9.7 % at five days, had the most favorable evolution, reaching a stimulus of 51.7 % at 10 days. The effects of HA on the germination rate index, GRI, are shown in Figure 1B. Humic acids decreased GRI at the three concentrations tested. The largest and significant (P ≤ 0.01) reduction was caused by the concentration of 35 mg L-1.

Figure 1 Effects of the application of three concentrations of humic acids (HA) extracted from vermicompost on germination of three native maize populations from Oaxaca, Mexico. Bars represent averages ± SE (standard error). Equal letters between HA doses imply the absence of significant differences (Tukey, P ≤ 0.05). GRI: germination rate index.
Once the seeds germinated, radicle growth is the most important issue. In this study, at five days, only the 30 mg L-1 treatment exerted a significant stimulus effect (P ≤ 0.01) on radicle growth; interestingly, this was the HA concentration that least affected the germination process; however, at 10 days, the 35 mg L-1 treatment showed more intense (4.4 % higher) and significant effects (P ≤ 0.01) than the control (Figure 1C).
The Nrs also constitutes an important phase of development and root expansion of plants in the soil. In this study, the application of HA to the seeds did not show intense effects on the subsequent emission of roots. At five days, only the concentration of 30 mg L-1 significantly (P ≤ 0.01) stimulated the emission of new roots (Figure 1D).
Production of shoot and root biomass also responded to the different concentrations of HA (Figure 1E). At 10 days, again the concentration of 30 mg L-1 showed a significant induction (P ≤ 0.01) in production of shoot biomass (AP) for this early growth phase.
Similarly, at 10 days, concentrations of 30 and 35 mg L-1 significantly (P ≤ 0.01) promoted root biomass production. Plant height is a character that represents the beginning of growth after germination. At five days, it was shown that concentrations of 30 and 40 mg L-1 exert a significant effect (P ≤ 0.01) in stimulating the seedling height in relation to the control. This effect was not preserved at 10 days, at which time only the concentration of 40 mg L-1 promoted growth, without statistical differences with respect to the control. The concentrations of 30 and 35 mg L-1 caused a significant delay in the seedling height in relation the control (P ≤ 0.01) (Figure 1F).
The effects of HA application of HA on the factor populations were also evaluated in the germination phase. The Cuil population showed the best response to the HA applications, superior to the Zap and Caj popuations (P ≤ 0.001) in the variables of seedling height, number of roots, and shoot and root dry mass.
Effects of the application of HA at stage V7 of three maize populations
Results obtained at V7 allowed to observe the effect of the application of HA on plant height (Figure 2). Cuil population was the only one in which the concentrations of 30 and 35 mg L-1 significantly delayed (P ≤ 0.001) plant growth in realtion to the control. In the Caj and Zap populations, all three concentrations significantly increased plant growth compared to the control (P ≤ 0.001). The highest applied concentration, 40 mg L-1, exerted the most intense effects on plant growth, with increases of 110, 105 and 146 % for the Caj, Cuil and Zap populations, respectively.

Figure 2 Effects of the application of three concentrations of humic acids (HA) on plant height of three native maize populations from Oaxaca, Mexico in the vegetative phase, 35 days after emergence. Bars represent averages ± SE (standard error). Equal letters between treatments imply the absence of significant differences (Tukey, P ≤ 0.05).
Effects of HA applied to plants under conditions of induced water stress
For the Caj population, the plant height and shoot biomass in the plants subjected to water stress, did not differ significantly from the plants under normal irrigation (Figure 3). The number of roots and the dry mass of the root was significantly reduced (P ≤ 0.01) due to the limitation of irrigation, even with the application of HA. Interestingly, the application of HA to the Caj population significantly increased (P ≤ 0.01) protein synthesis in plants with irrigation limitations.

Figure 3 Effect of the application of humic acids (HA) on growth parameters and protein content (PW - shoot dry mass) of maize plants of Cajete population. A) HA concentration factor (0, 30, 35, 40 mg L-1), B) hydric condition factor (R - normal irrigation, Wd - induced water deficit). Bars represent averages ± SE (standard error). Equal letters between treatments imply the absence of significant differences (Tukey, P ≤ 0.05).
The effect of HA concentrations applied to plants was also differentiated. The 30 mg L-1 concentration promoted the largest significant increases in plant height, number of leaves, dry mass of shoots and roots by 29, 23, 30 and 32 %, respectively.
The total protein content in the plants was significantly stimulated with the application of the concentration of 40 mg L-1. In terms of crop science, these results are important because they indicate that lower concentrations induce favorable effects on plant growth, while higher concentrations stimulate protein synthesis (García et al., 2012) (Figure 3).
In the Cuil population, the variables plant height and biomass produced, in plants with induced drought and with HA application, did not differ significantly from those that grew under normal irrigation regime. The application of HA to plants with induced drought promoted a total amount of protein greater than 100 % compared to plants with regular irrigation. This result reaffirms that in Cuil population, HA could induce protein synthesis (García et al., 2012).
For this population, the application of HA in a concentration of 30 mg L-1 significantly stimulated plant height, number of leaves, shoot and root dry mass, and total protein content by 6, 30, 20, and 45 %, respectively. Contrary to what was observed in the Caj population, in the Cuil population the concentration of 30 mg L-1 showed a more uniform effect on both growth parameters and protein content. This could be an advantage in terms of crop science, since the same concentration exerts the most promoting effects on all variables (Figure 4).

Figure 4 Effect of the application of humic acids (HA) on growth parameters and protein content (PW - shoot dry mass) of maize plants of Cuilapam population. A) HA concentration factor (0, 30, 35, 40 mg L-1), B) hydric condition factor (R - normal irrigation, Wd - induced water deficit). Bars represent averages ± SE (standard error). Equal letters between treatments imply the absence of significant differences (Tukey, P ≤ 0.05).
In the Zap population, the effects of HA application on drought-induced plants were less evident than for Cuil population. There were no significant differences between plants with induced drought and plants with regular irrigation, although the values of plant height and number of leaves were low.
Root biomass production recorded a slight non-significant increase in drought-treated and HA-treated plants, compared to those grown under normal irrigation conditions. In plants under stress and with HA application, the total protein content was significantly higher than in plants grown with normal irrigation. With the application of HA at a concentration of 40 mg L-1, a more intense and significantly different effect was registered than with the other concentrations applied and than the control. At this concentration, HA promoted plant height, number of leaves, root biomass, shoot biomass and total protein content by 19, 17, 15, 61 and 100 %, respectively. These results indicate a promising development of efficient crop science practice, using HA in this population (Figure 5).
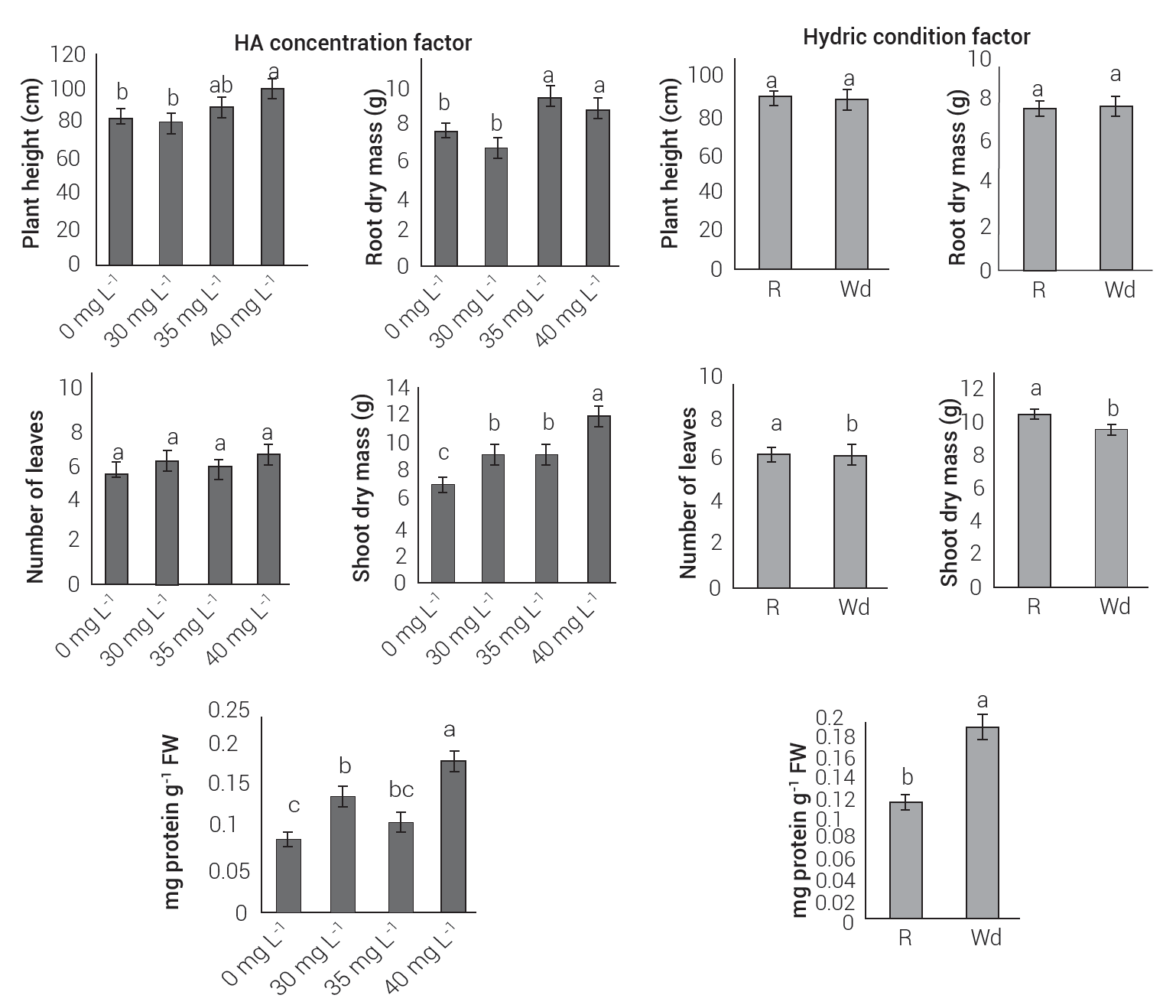
Figure 5 Effect of the application of humic acids (HA) on growth parameters and protein content (PW - shoot dry mass) of maize plants of Zapalote population. A) HA concentration factor (0, 30, 35, 40 mg L-1), B) hydric condition factor (R - normal irrigation, Wd - induced water deficit). Bars represent averages ± SE (standard error). Equal letters between treatments imply the absence of significant differences (Tukey, P ≤ 0.05).
Discussion
The concentration of 35 mg L-1 induced the highest germination percentage, with increases of up to 51.7 % from five to 10 days compared to the control (Figure 1A). Similar results were reported by Bento et al. (2020) in maize seed germination using humic substances extracted from hydrochar, who reported a significant effect on germination and associated it with a high content of phenols present in the structure of humic substances. On the other hand, of the three evaluated populations, Cuil showed the best responses to HA applications, when evaluating the different growth variables. Similar results were reported by Rodrigues et al. (2017) using maize and applying commercial humic substances, obtaining better results in the length of the shoot and shoot and in root dry mass.
The next experiment was developed to extend the results achieved in the germination stage to the early vegetative stage (V7), to confirm the permanence of the effects of HA at a stage closer to the real field conditions. The application of humic substances has been shown to stimulate the growth of maize plants in different ways and with different concentrations. Similar results have been reported by Eyheraguibel et al. (2008), using maize and applying humic-like-substance on the development of the whole plant from seed to harvest. The application of HA from vermicompost to maize plants showed effects on the increase of radical exudates, which implies a greater interaction with soil nutrients and with the rhizospheric microbiota (Canellas et al., 2019b).
The effects of humic acids on crops under water stress conditions have been widely addressed in literature (Bulgari et al., 2019). Regarding the study in plants that grow under conditions of water stress, interestingly, the application of HA to the Caj population significantly increased (P ≤ 0.01) protein synthesis in plants with irrigation limitation. This result may indicate a protective effect induced by HA through a possible synthesis of osmoprotectants, enzymes and aquaporins, effects that have already been reported by García et al. (2012) and Olaetxea et al. (2016). The application of different concentrations of HA to plant species subjected to water stress results in protective effects; specifically, the 30 mg L-1 concentration had the greatest effect in all the populations evaluated under water deficit, obtaining the best results with the Caj population. In addition to genetic diversity (Kato et al., 2009), physiologically this may be possible due to the effect of the optimal concentration; furthermore, the effects of (HS) on most aspects of the plant depend on the source of origin, the molecular weight of the fraction and the concentration used (Nardi et al., 2002). Aguiar et al. (2016), in sugarcane, attributed the effect to the increase in the activity of the enzymes POX, CAT and SOD. In sorghum plants, Shen et al. (2020) reported a reduction in ROS as an effect of increased activity of antioxidant enzymes. Hernández et al. (2018) reported protective effects of HA against water stress in two rice cultivars, the concentrations that exert these effects are different for each cultivar.
Conclusions
In the germination phase, the application of HA in concentrations of 35 and 40 mg L-1 promotes a higher percentage of germination and radicle and shoot growth. The Cuilapam population registers the best response to the application of HA, with the highest germination and growth values. In the vegetative phase V7, the three maize populations register greater growth in plant height in response to the application of HA in a concentration of 40 mg L-1. In plants subjected to water stress, tolerance to this condition, reflected in the growth variables, depends on the maize population that receives the HA application and on the concentration used. The common response in all three populations is the increase in total protein content when plants are subjected to water stress and receive HA applications.











 nueva página del texto (beta)
nueva página del texto (beta)


