Introduction
El Reno is one of the most important sideoats grama [Bouteloua curtipendula (Michx.) Torr.] cultivars developed in USA (Alderson and Sharp, 1995); it is mainly used for grazing. Sideoats is considered an important forage species due to its drought tolerance. It can reach a dry matter production of 3.36 t ha-1 (Smith et al., 2004), with 67.3 % of in vitro organic matter digestibility (IVDOM) and 10.3 % crude protein (CP) (White, 1986). Sideoats is a key species native to North America, with good digestibility throughout the growing season (Craig et al., 2001).
Because of sideoats value, it is important to develop efficient tissue culture protocols, which are required to apply plant transformation technologies for genetic improvement of this species. Traditional breeding programs based on phenotypic evaluation, selection, and hybridization have been used in many species to increase forage production and nutritional quality (Wang et al., 2010); however, phenotypic breeding has limited applications in sideoats due to the involvement of apomixis as reproductive system (Quero et al., 2010). Molecular biology techniques and plant tissue culture have been used as tools for plant improvement, especially for genetic transformation with the purpose of incorporating features of agronomic interest, even in apomictic plants (Mancini et al., 2014). Protocols of tissue culture and regeneration have been developed for B. gracilis (Willd. Ex Kunth) Lag. Ex Griffiths (Aguado-Santacruz et al., 2001), B. eriopoda (Torr.) Torr. (Osuna and Barrow, 2004), and recently for a landrace of sideoats B. curtipendula (Bernal-Flores et al., 2015).
Sideoats is a recalcitrant species for plantlet recovering from in vitro cultured calli (Bernal-Flores et al., 2015); therefore, the objective of this study was to evaluate pre-regenerations treatments using cytokinins to increase the frequency of regenerable calli and to establish an efficient plantlet regeneration protocol for this species.
Materials and methods
Plant material and its conditioning
The study was developed at the Forage Improvement Area Samuel Roberts Noble Foundation, Ardmore, Oklahoma, USA. Mature caryopses from commercial sideoats cv. El Reno were disinfested using 70 % ethanol for three min and placed into 3 % calcium hypochlorite plus 0.1 % Tween 80 for 3 h under stirring, and then rinsed into autoclaved miliQ water, and left at 4 oC overnight. The next day, the caryopses were placed into 80 mL of autoclaved water and 2 mL of PPM (Plant Preservation Mixture; Plant Cell Technology®) stirred for 1 h, and rinsed five times with sterile miliQ water.
Callus induction and pretreatments
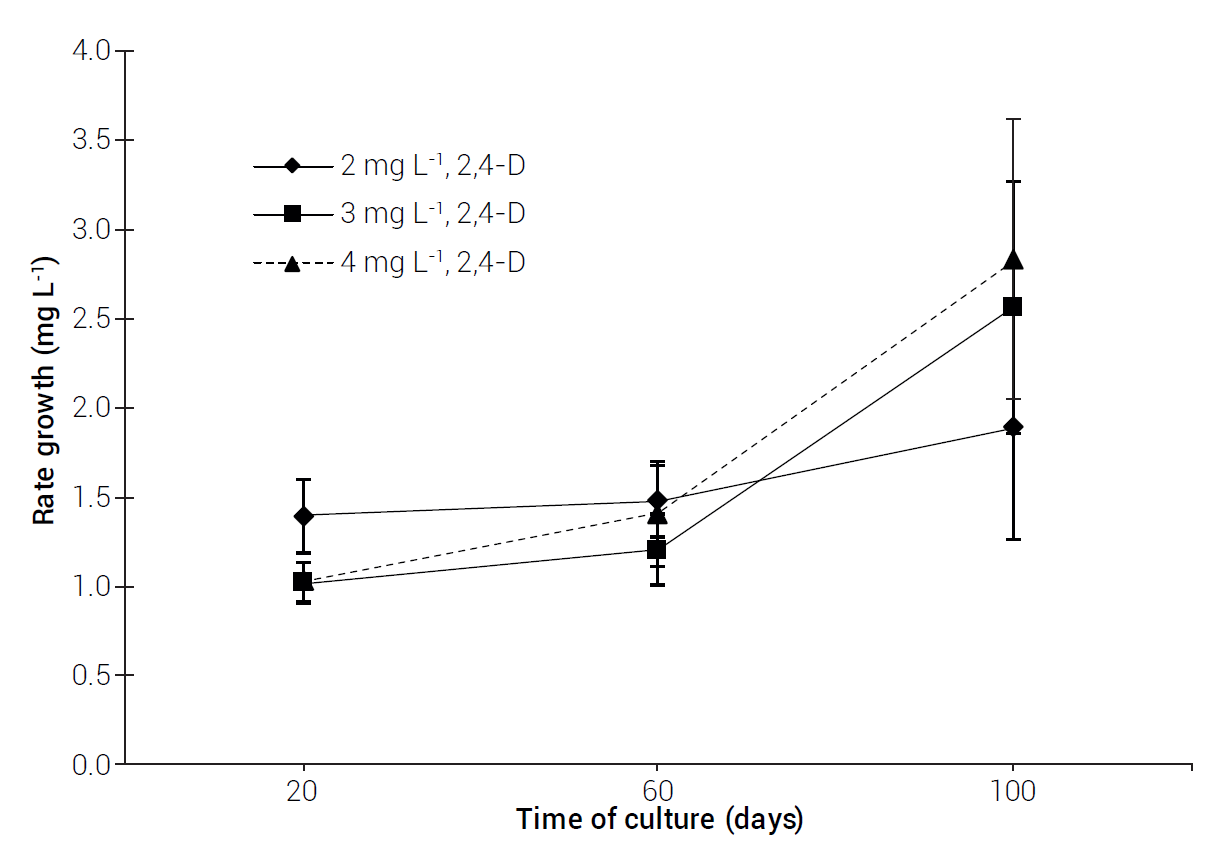
Preliminary studies were conducted for sideoats to develop the media to be used. Three 2,4-dichlorophenoxyacetic acid (2,4-D) concentrations (2, 3 and 4 mg L-1) and a unique 6-benzylaminopurine (BAP) concentration (0.15 mg L-1) were evaluated for callus induction. Callus induction media consisted of MS basal salts (4.3 g L-1) (Murashige and Skoog, 1962), supplemented with 30 g L-1 sucrose and two hormones (2,4-D and BAP). All media were solidified using 0.78 % agar (w/v), pH adjusted to 5.8 using 1N KOH, and autoclaved to 1.26 kg cm-2 at 120 oC. A total of 250 ± 10 caryopses were exposed to different 2,4-D levels and evaluated for a 100-day period in darkness at 24 ± 1 oC. After this process, two treatments were evaluated for increasing calli regeneration capacity: 1) MSB (BAP) and 2) MSBKT (BAP + kinetin + NN-phenyl-N-1,2,3,-thidiazol-5-yl-urea). Finally, a unique regeneration medium (MSBK) was used for promoting plant regeneration from calli. Regeneration calli were placed into a growth chamber (Percival Sci. Inc., Mod. CU1014, Perry, Iowa, USA) at 25 oC for a 12-h photoperiod and light intensity of 100 µE m-2 s-1. Regenerated plantlets were subsequently placed into rooting medium until plants reached complete development (Figure 1). Five friable type calli per 2,4-D level were randomly selected for growth rate determination (Hunt, 1982). Calli were weighed every 20 days using an analytical balance and subsequently changed to new culture media. Calli induction was estimated as calli formed/total seed cultured × 100.
Plant regeneration
The MSBK medium employed consisted of MS basal salts, 30 g L-1 sucrose, 2.0 mg L-1 6-BAP and 1.0 mg L-1 kinetin. A total of 25 calli pieces was placed on regeneration medium and subcultured every 20 days on the same media. Around 30 d after obtaining clearly differentiated plantlets, the material was transferred into rooting medium containing half-strength MS basal media supplemented with 8 g L-1 sucrose until achieving suitable root development.
Hardening of the plants in the greenhouse
Plantlets were transplanted into pots containing a commercial soil mixture (growing medium 830®, Sungro, USA) and placed during eight days into a manually conditioned mist space (90-95% of relative humidity, 25 ±1 oC, 16 h light at 390 uE m-2 s-1); then, the regenerated plants were placed into a conditioning chamber for eight days under 16/8-h (light/dark) greenhouse conditions at 25 ± 1 oC. Development of regenerated plants was observed for up to 60 days.
Statistical analysis
Calli induction frequency and plant regeneration
A logistic regression model was used to analyze this variable (Hosmer and Lemeshow, 2000; Zhao et al., 2011). The treatment effect was included into the model using indicator variables and the reference cell method with treatment 2 as reference. Negative binomial regression was used to analyze plant regeneration values for the three 2,4-D levels and considering the effect of treatments prior to plant regeneration assessment, under a similar scheme to that of logistic regression. Goodness of fit of the proposed model was tested by the deviance information criterion (DIC). Data analysis was performed using the GENMOD routine and differences among treatments were tested by likelihood ratio tests using the SAS program Ver. 9.1 (SAS Institute, 2004).
Growth rate
A mixed model was used to analyze calli growth rate at three different ages. The model included treatment, time, individual effects and interaction time × treatment effects. Least square means were computed using the Tukey adjustment treatment comparisons. Data analysis was performed using the MIXED routine through SAS Ver. 9.1 for Windows (SAS Institute, 2004).
The proposed model was as follows:
Where yijk: response variable measured at time k for subject j and treatment i , μ: general mean, αi: effect of treatment i, γk: time effect, (αγ)ik: treatment × time interaction. bij ~NIID(0, σu2): random effect for subject j, treatment i, eijk ~NIID(0, σe2) : random error. bij and eijk are assumed to be independent.
Results and discussion
Calli induction and growth rate
Preliminary studies on sideoats showed that using defined concentrations of 2,4-D and BAP in a basal medium would be sufficient for callus induction initiation. Statistical differences (P ≤ 0.05) were observed between treatments. The likelihood ratio test using 2,4-D at 2 mg L-1 (M2) as reference for treatments comparison proved the efficiency of 2,4-D at 4 mg L-1 (M4) for calli induction (Table 1).
Table 1 Comparison among treatments in relation to the M2 treatment for maximum likelihood estimates, using logistic regression for the calli induction variable.
| Parameter | DF | Estimate | SE | Wald | P value |
| Intercept | 1 | 0.5500 | 0.1450 | 14.38 | 0.0001 |
| M3 | 1 | -0.4983 | 0.1956 | 6.48 | 0.0109 |
| M4 | 1 | -0.7121 | 0.1953 | 13.28 | 0.0003 |
Sideoats grama calli induction from caryopses showed to be critically dependent on 2,4-D levels. Four different calli types were observed among culture media regarding the different 2,4-D concentrations (Figure 2). Friable light-yellow colored calli were predominant for media containing 3 mg L-1 2,4-D (35.6 %), while semicompact calli were mainly observed (34.6 %) at the medium containing 4 mg L-1 2,4-D (Table 2). Calli immersed into a mucilaginous matrix were less frequent in all the evaluated media. Other calli, defined by a combined mass of calli (soft-watery and soft-dry calli) were observed in higher frequency at lower 2,4-D levels. Calli frequency induction in response to the 2,4-D concentration of 2 mg L-1 were significantly higher (P ≤ 0.05) than those induced with both 3 and 4 mg L-1 of 2,4-D.

Figure 2 Characteristic calli aspects of sideoats grama developed during the induction process. I) friable calli, II) semicompact calli showing friable callus at the top, III) callus immersed within a mucilaginous matrix, IV) soft-watery callus. Bar represents 100 µm.
Table 2 Calli induction frequency from sideoats grama cv. El Reno caryopses under three 2,4-D levels.
| 2,4-D (mg L-1) | Types of calli (%) | Calli induction frequency (%) | |||
| I | II | III | IV | ||
| 2 | 31.0 ± 2 ab | 26.4 ± 3 ab | 3.7 ± 2 c | 38.9 ± 4 a | 62.2a |
| 3 | 35.6 ± 5 a | 27.2 ± 3b | 9.2 ± 2 c | 28.0 ± 3 b | 51.4ab |
| 4 | 32.1 ± 3 ab | 34.6 ± 4 a | 5.6 ± 3 c | 27.7 ± 5 bc | 46.0c |
I: friable calli, II: semicompact calli, III: calli showing mucilaginous matrix, IV: other calli (compact, soft-watery callus). Means followed by different letters in the columns are statistically different (Tukey, P < 0.05).
Significant differences (P ≤ 0.05) were observed through time in calli induced by different media (Table 3). Two growth phases were observed at the three tested 2,4-D levels: a slow growth phase (first 20 days), followed by a rapid growth phase until the end of the evaluation period (Figure 3). At the beginning of the evaluation, calli grown at the lowest 2,4-D level showed higher rate of growth in comparison to those grown at 3 and 4 mg L-1 2,4-D. Friable calli showed the highest growth rates, while brownish-colored calli displayed lower growth rates and finally died (data not shown).
Table 3 Statistical significance of variation sources of sideoats grama cv. El Reno growth rates.
| variation source | DF | F value | P value |
| Treatment | 2 | 4.34 | 0.0142 |
| Time | 2 | 81.13 | <0.0001** |
| Treatment × time | 4 | 3.31 | 0.0117 |
** Highly significant at P ≤ 0.05.
Pretreatment and plant regeneration
Regeneration response was strongly influenced by cytokinins, but at a greater extent with BAP alone (P ≤ 0.05), while the combination of BAP, kinetin and TDZ showed little effect on plant regeneration (Table 4). Calli cultured on MSB media developed bud-like structures (Figure 4b), mainly within friable calli. A similar response was observed for calli grown on MSBKT media (Figure 4c), while callus placed directly on regeneration media developed numerous root-like structures (Figure 4a) but were unable to regenerate plantlets.
Table 4 Plant regeneration from calli pretreated using three 2,4-D levels and different levels of cytokinins for Bouteloua curtipendula cv. El Reno (n=5).
| Callus induction media (2,4-D level) | Cytokinins in pretreatments (mg L-1) | Regeneration media | Total plantlets regenerated | Plantlets regenerated per 25 calli | ||
| BAP | KIN | TDZ | ||||
| ck | 0.0 | 0.0 | 0.0 | MSBK | 0 ± 0.0 | 0.0 |
| 2 mg L-1 | 3.0 | 0.0 | 0.0 | 2 ± 0.4c | 0.1 | |
| 0.1 | 1.5 | 0.5 | 4 ± 0.5bc | 0.2 | ||
| ck | 0.0 | 0.0 | 0.0 | MSBK | 0 ± 0.0 | 0.0 |
| 3 mg L-1 | 3.0 | 0.0 | 0.0 | 8 ± 0.5ab | 0.3 | |
| 0.1 | 1.5 | 0.5 | 2 ± 0.4c | 0.1 | ||
| ck | 0.0 | 0.0 | 0.0 | MSBK | 0 ± 0.0 | 0.0 |
| 4 mg L-1 | 3.0 | 0.0 | 0.0 | 11 ± 0.7a | 0.4 | |
| 0.1 | 1.5 | 0.5 | 3 ± 0.4c | 0.1 | ||
ck: control media (2, 3 and 4 mg L-1), MSB: 2,4-D (2, 3 and 4 mg L-1) + BAP (3 mg L-1), MSBKT: 2,4-D (2, 3 and 4 mg L-1)+ BAP (0.1 mg L-1) + kinetin (1.5 mg L-1) + TDZ (0.5 mg L-1), MSBK: BAP (2.0 mg L-1) + kinetin (1.0 mg L-1). Data represent total regenerated plantlets per treatment ± standard error. Means followed by different letters within columns indicate significant differences (Tukey, P ≤ 0.05).
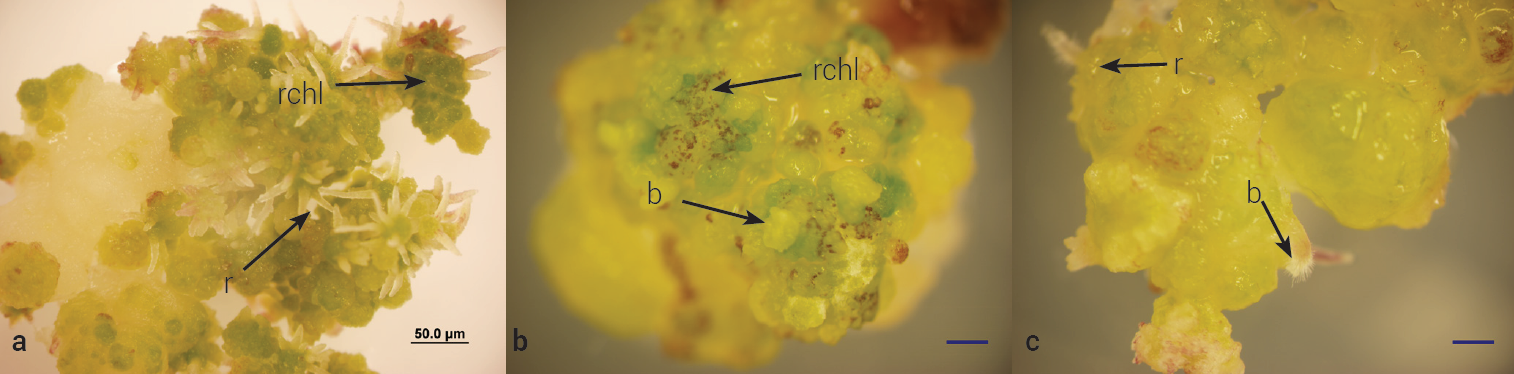
Figure 4 Bouteloua curtipendula cv. El Reno calli subcultured on three different media after initiation of calli. a) calli placed on MSBK medium (control), showing abundant root structures (r) and chlorophyll accumulation (chl), b) calli pretreated on MSB medium showing bud-like structures, c) calli pretreated on media MSBKT. Bar represents 50.0 μm.
Pretreated calli showed strong chlorophyll accumulation and developed more friable sections in relation to other calli types. Pigmented calli occurred within a 10 days period for pre-treated calli and continued until these were placed on regeneration medium.
MSBTK media
Pretreatment stimulated cells toward plant regeneration and this effect showed to be better with BAP than when this hormone was combined with kinetin and TDZ. The MSBK regeneration media supplemented with BAP and kinetin was responsible for completing the regeneration process and it was more evident in calli from 3 and 4 mg L-1 2,4-D levels.
Morphogenic calli derived from mature caryopses (Figure 5a), pretreated with cytokinins and subcultured on MSBK regeneration media, achieved complete plant regeneration (Figure 5b). More efficient plant regeneration responses were observed in friable and semicompact calli (Figure 5c). Soft, watery and brownish calli, as well as those immersed in mucilaginous matrix did not register plant regeneration. Plants showing extensive root development were transplanted into pots and placed in a greenhouse (Figure 5d). Tissue culture and plant regeneration represent an important step for genetic improvement through plant transformation for many forage species (Burris et al., 2009; Lenis-Manzano et al., 2010), especially for those that are apomictic (Giri and Praveena, 2015; Quero et al., 2010).
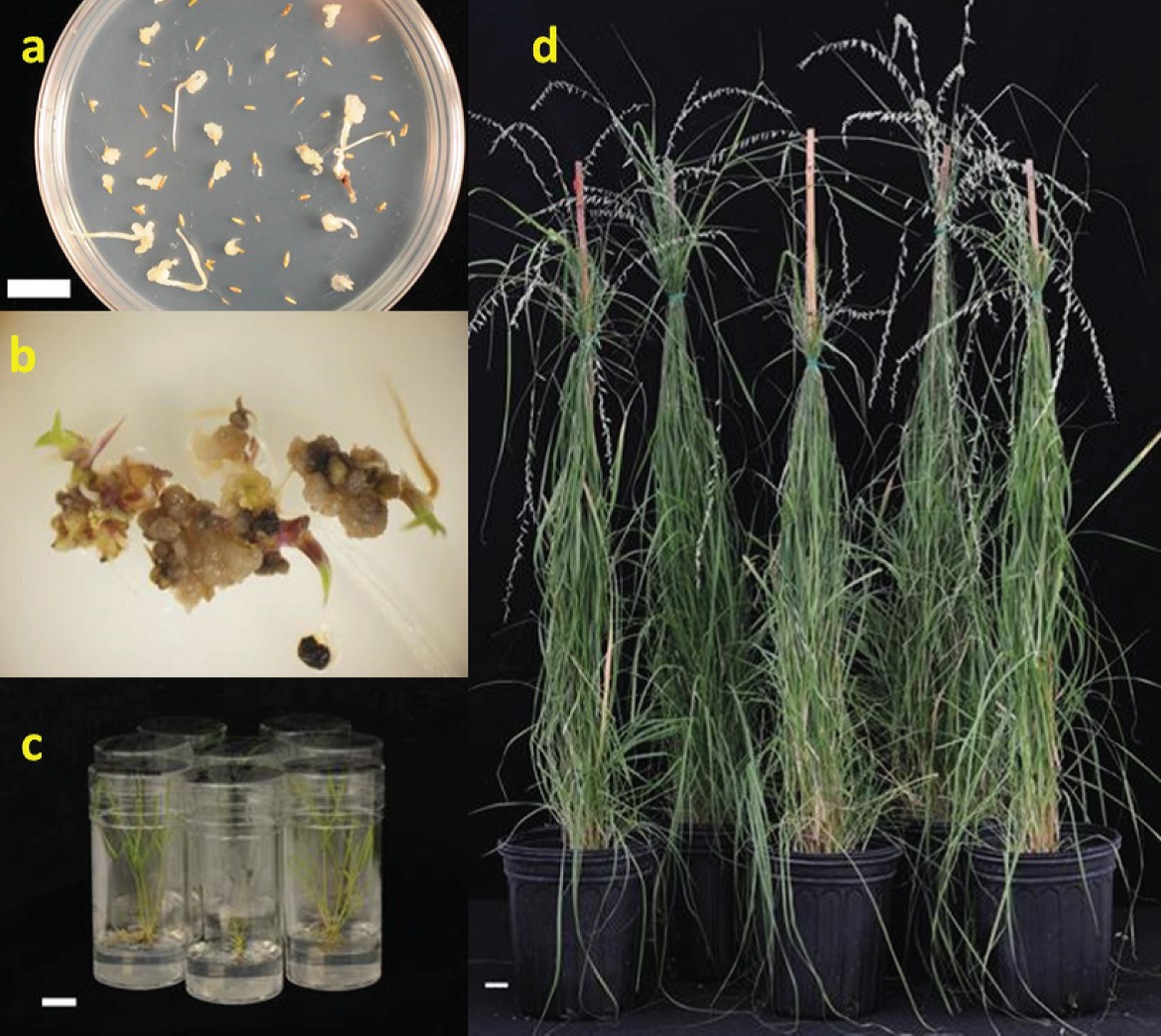
Figure 5 In vitro plant regeneration for B. curtipendula cv. El Reno. a) mature caryopses on medium supplemented with 2,4-D, b) regenerated plantlets, c) plants on maintenance media, d) plants grown in pots (60-d old). Bar represents 2 cm.
Both callus induction and plant regeneration processes occur as a response to factors such as plant genotype, availability of nutrients and auxin and cytokinin type and amount, both exogenous and endogenous (Aina et al., 2012; Rakshit et al., 2010). Although optimal response is linked to genotype, endogenous auxin and cytokinin balance for callus induction and plant regeneration (Visser et al., 1992), the interaction between genotype and exogenous hormone supply defines calli type and amount (Aina et al., 2012; Rakshit et al., 2010). Efficient plant regeneration responses were obtained when applying higher 2,4-D levels and BAP. This auxin is related to growth-promotion and synthesis of both DNA and RNA (Mustafa and Khan, 2012), as well as promotion of changes in DNA methylation, resulting in reprogramming of differentiated cells (Chen et al., 2001).
Pretreatment using MSBKT had higher efficiency at lower 2,4-D levels, while the MSB pretreatment was more efficient for plant regeneration at any 2,4-D level. The highest plant regeneration responses were obtained using MSB pretreatment, while regeneration efficiency was significantly (P ≤ 0.05) enhanced to higher levels of 2,4-D. Regeneration efficiency has been described as a quantitative trait, which often varies among plant species (Aina et al., 2012); it is affected by culture media, cell density, plant growth regulators, conditioned media (Manninen, 1997), genotype, embryogenic callus morphology (Bregitzer, 1992), explant source and age (Aina et al., 2012). The effects of 2,4-D in combination with cytokinins and physical growth conditions have been widely and successfully tested for barley (Castillo et al., 1998) and B. gracilis plant regeneration (Aguado-Santacruz et al., 2001); however, better plant regeneration was obtained by using dicamba in B. eriopoda (Osuna and Barrow, 2004), osmotic stress in B. curtipendula (Bernal-Flores et al., 2015) and low concentrations of naphthalene-acetic acid for apomictic C4 grasses (Lenis-Manzano et al., 2010; Ma et al., 2018).
Calli directly placed on regeneration media (MSBK) without pretreatment (2,4-D+MSBK) did not regenerate plantlets, similar to reports on Cynodon dactylon L. (Zang et al., 2006). MSB and MSBKT treatments of calli promoted plant regeneration in Sideoats, but the level of response was dependent on 2,4-D concentrations. Here, MSB pre-regeneration treatment promoted higher regeneration rates for all treatments. Although calli developed chlorophyll in MSB and MSBKT media, this effect did not improve regeneration response, as it has been reported for some dicots (Visser et al., 1992). The use of TDZ improved the regenerative response for recalcitrant Urochloa spp. (Yaguinuma et al., 2018) and has also been effectively used for species in which purine-type cytokinins were ineffective (Lu, 1993).
MSB treatment for 10 days in calli stimulated a better regeneration response at 3 and 4 mg L-1 of 2,4-D than calli cultured directly on regeneration medium (MSBK). Pre-regeneration treatments based on BAP and other cytokinins, mainly kinetin and BAP, improved callus induction and plant regeneration response in other species (Castillo et al., 1998; Mustafa and Khan 2012) and their effects have been related to nitrogen accumulation, decreased calcium levels and boron uptake (De Oliveira et al., 2010). Cytokinins stimulated chlorophyll production in calli for all 2,4-D concentrations. Studies on hormonal balance have linked of endogenous levels in calli to plant regeneration response (Huang et al., 2012). For calli induced in C. dactylon, the use of 6-benzylaminopurine from 0.1 to 0.5 mg L-1 resulted in regenerable compact yellowish callus (Zang et al., 2006).
Conclusions
The development of an in vitro culture protocol for sideoats grama is an important step for genetic improvement of this species. The best response on calli induction was obtained at lower 2,4-D concentrations, while the most efficient pre-regeneration treatment for improving regenerative response of sideoats was based on BAP and higher 2,4-D levels. A method for increasing the regenerative response of calli consists of induction of morphogenic responses on media containing 2,4-D followed by cytokinins pretreatments. This study is expected to improve the biotechnological management of sideoats grama cv. El Reno.











 nueva página del texto (beta)
nueva página del texto (beta)