INTRODUCTION
The genus Agave has approximately 200 species, 150 of them are distributed in Mexico (García-Mendoza, 2004). This plant has been used since ancient times as a source of food, fodder, medicine, fiber and as construction material, among other uses. Its main use today is in the production of alcoholic beverages such as pulque, mezcal, bacanora and tequila; the latter of them is produced from the species Agave tequilana (Weber) var. Azul (Gentry, 1982). This species is particularly important in Mexico, being the raw material for tequila, which, due to the denomination of origin for tequila, is only produced in Mexico, being an important source of foreign exchange. In 2018, production of tequila in Mexico reached 309.1 million liters, for which 1,138,800 t of A. tequilana were required (CRT, 2019).
The development of intensive agricultural practices in the production of A. tequilana has led to an excessive use of chemical fertilizers, resulting in environmental deterioration and increasing risks to human health, microfauna and wild flora (Gomiero et al., 2011). Using organic resources as microbial biofertilizers could reduce the use of chemical products and in turn could reduce their environmental impact. A particularly promising alternative is the use of arbuscular mycorrhizal fungi (AMF) as biofertilizer (HarrisValle et al., 2019). AMF form one of the most important symbiotic associations with plants, the arbuscular mycorrhizae (Schüßler et al., 2007). This association promotes plant growth through different mechanisms of action, such as producing substances that regulate plant growth, improving water absorption, increasing nutrient uptake (especially P, Cu, Zn, N, etc.) (Hodge and Fitter, 2010; Schüßler et al., 2007; Smith and Read, 2008; Smith and Smith, 2012) and increasing tolerance to different abiotic stresses such as drought (Azcón-Aguilar and Barea, 2015) and salinity (Harris-Valle et al., 2012). AMF also help to increase the diversity and population levels of rhizospheric microorganisms by inducing a suppressive effect on soil phytopathogens (De La Noval et al., 2007; Jung et al., 2012; Trinidad-Cruz et al., 2017a) and improving the physical properties of the soil by forming stable aggregates (Rillig and Mummey, 2006).
Other studies have evaluated the effect of AMF on different species of agave plants. Quiñones-Aguilar et al. (2016) evaluated the effect of native AMF consortia on the growth and plant quality of A. inaequidens; they reported a growth-promoting effect and high quality in agave plants inoculated with native AMF consortia compared to a commercial strain; they also reported an increase larger than 500 % in the total dry weight of plants with mycorrhizal consortia compared to plants without AMF. TrinidadCruz et al. (2017a) reported a significant increase (P ≤ 0.05), between 148 % and 239 % in total dry biomass of A. cupreata inoculated with native AMF consortia compared to agave plants without inoculum. Pimienta-Barrios et al. (2009) evaluated the effect of inoculating A. tequilana seedlings with Glomus fasciculatum and G. intraradices on photosynthesis, foliar development and growth; they reported that inoculation with G. intraradices increased photosynthesis and induced an increase in the mesophyll of the plants.
Robles-Martínez et al. (2013) evaluated the functional compatibility of different native AMF inocula in A. angustifolia; they found that at least one native consortium and one commercial inoculant significantly promoted the accumulation of aerial biomass in the plants, and also increased the number and length of the roots compared to non-inoculated plants. Martínez-López et al. (2009) evaluated the dry biomass of A. americana inoculated with native strains of G. intraradices (R. intraradices) from Nuevo León, Mexico, and a commercial INIFAP® strain (different from G. intraradices), and found no differences in the biomass of agave plants between the two types of inoculum (commercial or native); thus, the aim of this study was to assess the effect of different native AMF consortia obtained from the rhizospheric soil of Agave cupreata plantations in the state of Michoacán, Mexico on the growth of A. tequilana seedlings grown under greenhouse conditions.
MATERIALS AND METHODS
Arbuscular mycorrhizal fungi (AMF) and blue agave plants
In order to obtain native AMF consortia, samples of rhizospheric soil were taken from A. cupreata plantations at the mezcal denomination of origin region in Michoacán, México. The fungal consortia were obtained from different municipalities and labelled according to the name of the locality in which they were extracted. Consortia were identified as Dominant genera: Glomus, Scutellospora: El Huizachal (EH); Dominant genera: Acaulospora: Las Campesinas (LC,), Rancho Carlos Rojas (CR), El Limón (EL), Agua Dulce (AD); Dominant genera: Acaulospora, Glomus: Paso Ancho (PA), Barranca de las Nueces (BN) and Cerro del Metate (CM). The mycorrhizal consortia were previously propagated in trap pots for eight months using a variety of plants: sorghum (Sorghum spp.), alfalfa (Medicago sativa), bermuda grass (Cynodon dactylon) and agave (A. cupreata). The consortia were taxonomically identified and a total of 40 species were found, belonging to 13 genera, nine families and three orders (Trinidad-Cruz et al., 2017b).
The A. tequilana seedlings were propagated from bulbils collected from adult plants; the average weight of the seedlings was 2.8 ± 0.52 g at the beginning of the experiment. The bulbils came from blue agave plants from the Arenal region, in the state of Jalisco. The selected plants had produced an inflorescence.
Experimental conditions
A randomized complete blocks experimental design was used; the treatments consisted of the previously propagated native consortia EH, LC, CR, EL, AD, PA, BN and CM, along with the commercial inoculum MI (Mycorrhiza INIFAP® based on R. intraradices) used as positive control and a negative control without AMF. Five replicates were established per treatment. The experimental unit consisted of a pot with a blue agave plant, the roots were directly inoculated with the necessary amount of the corresponding inoculum containing 80 AMF spores. For the control without AMF, 10 g of sterile sand were used as inoculum. Black polyethylene bags (2 kg) were used as pots, each containing 1.5 kg of a sterile mixture of sand and soil (1:1 v/v) sterilized three times for 3 h at 120 ºC and 1.03 kg cm-2 of pressure. The pots were maintained at field capacity with deionized water. The experiment was established under greenhouse conditions for 300 days after transplantation (dat).
Evaluation of growth and microbiological variables
At 90, 180, 270 and 300 dat, the number of leaves and the plant height were recorded. At the end of the experiment, the fresh and dry biomass of the plants were quantified using an analytical balance (Mettler Toledo AT200, Columbus, Ohio, USA). Only half of the total fresh biomass was treated, the rest was used to determine mycorrhizal colonization, head diameter (digital vernier), leaf area (LICOR LI-3100 planimeter, Lincoln, Nebraska, USA), root length (tape measure), and root and foliage volume (water displacement method in a graduated glass test tube). In regard to the microbiological variables, the percentage of root colonization was determined using the root clearing and staining technique (Phillips and Hayman, 1970), and quantified using the method proposed by McGonigle et al. (1990). A sample of 100 g of substrate was taken from each pot to determine the number of spores using the wetsieving and decanting technique (Gerdemann and Nicolson, 1963) in combination with sucrose gradient centrifugation (Walker et al., 1982).
Data analysis
The Dickson index (DI), which assesses plant quality based on morphological characteristics, was calculated using the following formula (Dickson et al., 1960):
Where Total DB is the total plant dry biomass (g), PH is the plant height (cm), HD is the head diameter (mm), LDB is the leaf dry biomass (g) and RDB is the root dry biomass (g).
The relative mycorrhizal dependency index (RMDI), which assesses the increase in dry biomass due to the effect of mycorrhization, was calculated with the following formula:
Where DB+AMF is the dry biomass of mycorrhizal plants (g), DB-AMF is the dry biomass of non-mycorrhizal plants (g).
The response variables (fresh and dry biomass of the plants, head diameter, leaf area, root length, root and foliage volume, percentage of root colonization, number of spores, Dickson index and relative mycorrhizal dependency index) were subjected to analysis of variance; when the variables were significant, a multiple comparison of means test was performed using the Tukey test (P ≤ 0.05). These analyses were performed using the statistical software Statgraphics Centurion XV (StatPoint Inc., 2005).
RESULTS
The statistical analysis showed high variability in the different response variables between replicates, which can be attributed to the origin of the bulbils used in the experiment, since not all came from the same mother plant and not all were the same age. There were no significant differences in the number of leaves between the different treatments at the first three samplings; however, from 270 dat there were significant differences between the inocula, and those differences remained until the end of the experiment. The fungal consortium Barranca de las Nueces (BN) induced the largest number of leaves, surpassing by three leaves on average the treatments without mycorrhization, the fungal consortium Las Campesinas (LC) and the commercial inoculum (MI) produced the lowest number of leaves (Figure 1)
Blue agave plant growth was stimulated by AMF
The statistical analysis showed high variability in the different response variables between replicates, which can be attributed to the origin of the bulbils used in the experiment, since not all came from the same mother plant and not all were the same age. There were no significant differences in the number of leaves between the different treatments at the first three samplings; however, from 270 dat there were significant differences between the inocula, and those differences remained until the end of the experiment. The fungal consortium Barranca de las Nueces (BN) induced the largest number of leaves, surpassing by three leaves on average the treatments without mycorrhization, the fungal consortium Las Campesinas (LC) and the commercial inoculum (MI) produced the lowest number of leaves (Figure 1)
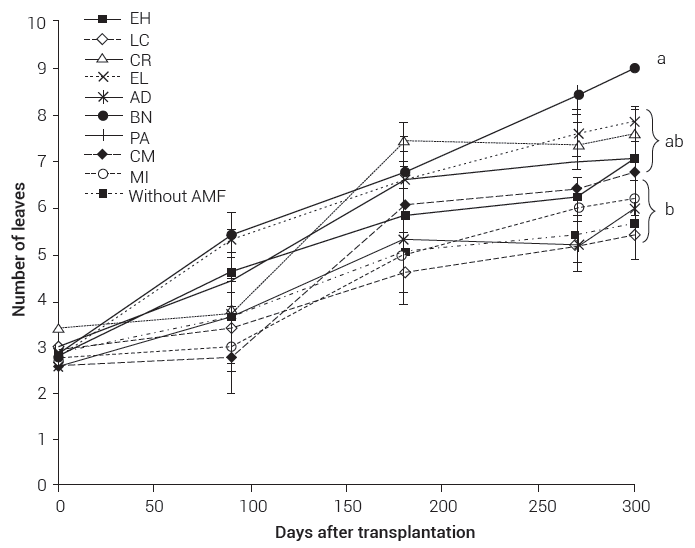
Figure 1. Effect of inoculation with arbuscular mycorrhizal fungi on the number of leaves of Agave tequilana plants grown under greenhouse conditions at 300 days after transplantation. Lines with the same letters are not statistically different (Tukey, P ≤ 0.05). The line on each point indicates the ± standard error.
Plant height did not show significant differences between treatments during the different samplings (Table 1). In general, the vegetative growth of agave plants inoculated with a native fungal consortium was significantly different (P ≤ 0.05) compared with non-inoculated agave plants (Figure 2), with significant increases (Tukey, P ≤ 0.05; Table 1) in fresh biomass, leaf area, root and foliage volume, number of leaves and head diameter.
Dry biomass, plant height and root length showed no significant differences between inoculated and noninoculated plants. The results of the positive control (MI) for all evaluated variables were statistically different from those obtained for the treatments with AMF consortia; however, the growth traits of A. tequilana plants inoculated with the native fungal consortium Barranca de las Nueces (BN) had the highest values (Table 1).
Table 1. Effect of inoculation with native arbuscular mycorrhizal fungi (AMF) on the growth of Agave tequilana plants grown under greenhouse conditions at 300 days after transplantation.
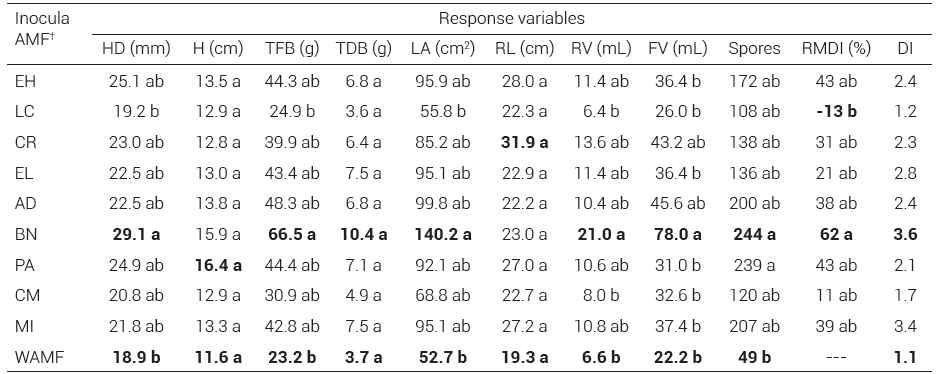
HD:head diameter, H: plant height, TFB: total fresh biomass, TDB: total dry biomass, LA: leaf area, RL: root length, RV: root volume, FV: foliage volume, Spores: spores in 100 g of substrate, RMDI: relative mycorrhizal dependency index, DI: dickson index. † EH: El Huizachal, LC: Las Campesinas, CR: Rancho Carlos Rojas, EL: El Limón, AD: Agua Dulce, PA: Paso Ancho, BN: Barranca de las Nueces, CM: Cerro del Metate, MI: Mycorrhiza INIFAP® based on R. intraradices, WAMF: without arbuscular mycorrhizal fungi. Means with the same letters are not statistically different (Tukey, P ≤ 0.05). The highest and lowest values of each response variable are shown in bold.
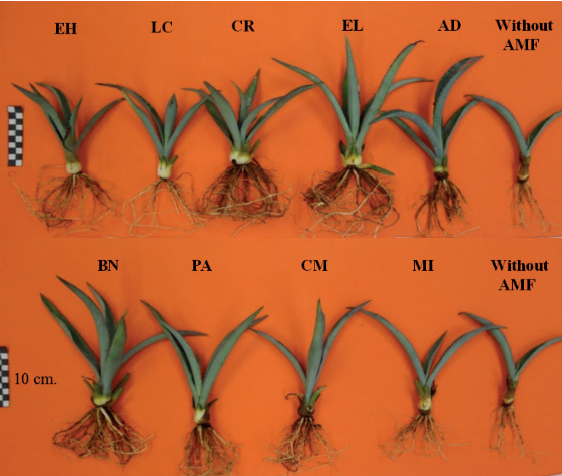
Figure 2. Aspect of Agave tequilana plants inoculated with different arbuscular mycorrhizal fungi (AMF) under greenhouse conditions 300 days after transplantation.
Regarding the total volume of the plants, an increase was observed when they were treated with any of the AMF inocula, compared to non-inoculated plants, but this increase was only statistically significant in the agave plants inoculated with the fungal consortium Barranca de las Nueces (BN) (Figure 3), which induced by 243 % the volume increase. Even though the statistical analysis showed no significant differences between the fungal consortia CR, EL and AD, they did induce an increase in the total volume of agave plants by more than 100 %, compared to the non-AMF treatment. The commercial inoculum MI increased by itself the total volume of the plants by 47 % (Figure 3)
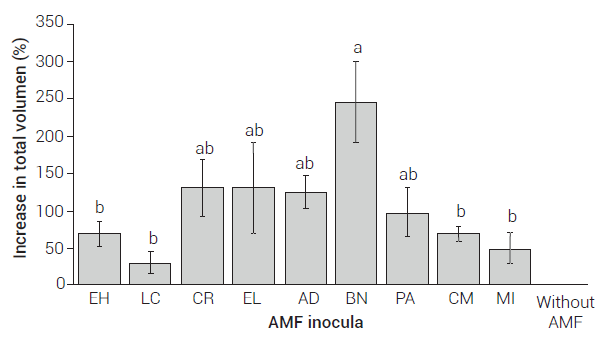
Figure 3. Increase in the total volume of Agave tequilana plants inoculated with different arbuscular mycorrhizal fungi (AMF), compared to plants without AMF 300 days after inoculation. Bars with the same letters are not statistically different (Tukey, P ≤ 0.05); the line at the upper part of the bar indicates the ± standard error.
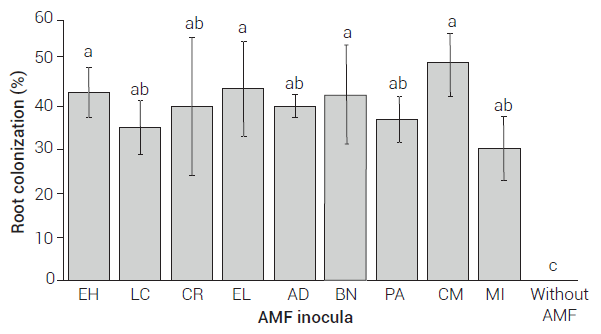
AMF colonization and propagation in agave as a host plant
Regarding the percentage of mycorrhizal colonization and the production of spores in the growth medium, no significant differences were found between AMF inocula. The colonization percentages ranged between 30 and 49 % (Figure 4), while spore production ranged between 107 and 243 per 100 g of substrate (Table 1).
Mycorrhizal dependency and blue agave plant quality index
The mycorrhizal dependency of the blue agave plants showed high variability among the different inocula; there were significant differences only between the fungal consortia BN and LC (Table 1). Agave plants inoculated with the BN consortium showed a 62 % increase in dry biomass compared to agave plants without AMF, whereas the agave plants inoculated with the LC consortium showed a 13 % decrease, accumulating less dry biomass than agave plants without mycorrhization, despite having a mycorrhizal colonization percentage of 35 %.
Regarding the Dickson index, which indicates the quality of a plant as a function of its growth parameters (the higher the value of the index, the higher the quality of the plant), the different inocula produced plants of different quality (Table 1). Most of the plants inoculated with an AMF had higher quality than the agave plants without mycorrhization (Figure 2), except for the plants inoculated with the fungal consortia CM and LC, which obtained a quality value similar to that of the plants without AMF. Plants inoculated with the BN consortium reached the highest plant quality level, corresponding to the highest values in the different growth variables evaluated.
DISCUSSION
It has been demonstrated in many plant species [papaya (Carica papaya), maize, pepper (Capsicum annuum), etc.] that AMF promote vegetative growth through various mechanisms, perhaps the most important is the improvement of plant nutrition (Quiñones-Aguilar et al., 2014) by increasing the acquisition of nutrients through the activity of the hyphae of mycorrhizal fungi (Smith and Read, 2008). In the present study all the AMF consortia evaluated promoted vegetative growth of A. tequilana, and in some cases, the increase was statistically significant compared to non-mycorrhized agave plants. Similar results have been reported for A. tequilana (Ruiz et al. , 2011), A. inaequidens (Quiñones-Aguilar et al., 2016), A. cupreata (Trinidad-Cruz et al., 2017a) and other agave species such as A. deserti (Cui and Nobel, 1992), A. angustifolia (Robles-Martínez et al., 2013) and other species with CAM metabolism such as Ferocactus acanthodes, Opuntia ficus-indica (Cui and Nobel, 1992) and O. albicarpa (Estrada-Luna and Davies, 2008), among others. This increase in growth could be attributed in part to an increase in the photosynthetic rate of agave plants or to an improvement in the acquisition of water and nutrients due to the activity of mycorrhizae, which act as an extension of the roots (Fernández-Lizarazo and Moreno-Fonseca, 2016; Pimienta-Barrios et al., 2009; Smith and Read, 2008).
Cui and Nobel (1992) studied the nutritional status, water acquisition capacity and gas exchange capacity of A. deserti plants inoculated with AMF and their results showed an increase in the phosphorus content in roots and leaves, higher hydraulic conductivity in roots and better CO2 absorption, which was reflected in the larger size of mycorrhized plants compared to non-mycorrhized. The fact that at least one native fungal consortium induced a higher increase in some of the evaluated growth traits compared to the commercial inoculum (MI) could be related to the diversity of AMF species present in the native consortia, as indicated by Trinidad-Cruz et al. (2017b).
Similar results were reported by Quiñones-Aguilar et al. (2016) with A. inaequidens and by Trinidad-Cruz et al. (2017a) with A. cupreata inoculated with native consortia. In this regard, Gavito and Varela (1995) and Bashan et al. (2000) reported that inocula consisting of more than one AMF species provide higher benefits to their hosts. It is also known that native mycorrhizal consortia tend to be more effective than consortia constituted by exotic species or by a single species (Gavito and Varela, 1995; Requena et al., 2001); the reason for this is that fungi adapt to certain specific environmental conditions and introducing them into different environments can cause some maladjustments (Hodge, 2000).
Robles-Martínez et al. (2013) evaluated the effect of different native AMF consortia on A. angustifolia and found that at least one native consortium promoted the increase of aerial biomass compared to non-inoculated plants. Quiñones-Aguilar et al. (2016) and Trinidad-Cruz et al. (2017a) studied native AMF consortia isolated from the rhizosphere of A. cupreata inoculated into A. inaequidens and A. cupreata. Mycorrhized agave plants with native consortia showed higher growth, plant quality and resistance to Fusarium oxysporum while plants of A. angustifolia inoculated with AMF showed an increase in the number and length of roots; however, MartínezLópez et al. (2009) found no differences in the increase of biomass induced by the inoculation of R. intraradices strains of different origin (commercial and native) into A. americana plants.
The discrepancy between the results of this study and those reported by Martínez-López et al. (2009) may be explained by the fact that in the present study, the native inocula used were consortia, rather than singlespecies inocula, and the interaction between one or more species present in the consortia could yield better results, compared to commercial strains (Robles-Martínez et al., 2013). Even though there is no plant-fungus specificity, the effect of each fungus on its host can vary; it can promote growth, increase nutrient and water uptake capacity, or induce resistance to biotic or abiotic stresses, among other benefits (Smith and Gianinazzi-Pearson, 1988); thus, fungi have differential effectiveness (Hodge, 2000; QuiñonesAguilar et al., 2016; Robles-Martínez et al., 2013).
Another possible explanation of the increased growth of agave plants inoculated with native consortia is that, due to the way in which the evaluated consortia were propagated (trap pots), other beneficial microorganisms, such as plant growth-promoting rhizobacteria, likely multiplied and contributed to promote the growth of the agave plants. De la De la Torre-Ruiz et al. (2016), studying A. americana plants inoculated with Rhizobium daejeonense, Acinetobacter calcoaceticus and Pseudomonas mosselii, observed a significant effect (P ≤ 0.05) on growth and sugar content. Ruiz et al. (2011), working with A. tequilana seedlings inoculated with G. intraradices and Gluconacetobacter diazotrophicus, reported a higher dry weight compared to treatments where more than one bacterial strain were inoculated.
In the present study, there might have been positive effect caused by the interaction between AMF and rhizobacteria in the treatments with native inocula, but this effect would have been unlikely to occur in plants without AMF because the growth medium was previously sterilized, nor could it have occurred in the treatment with the monospecific inoculum (MI).
Regarding the microbiological variables, the results obtained here showed a mycorrhizal colonization percentage of 49 %. In another study on A. inaequidens inoculated with native AMF, Quiñones-Aguilar et al. (2016) reported mycorrhizal colonization percentages ranging from 20 to 45 %. Pimienta-Barrios et al. (2009) reported colonization percentages of more than 70 % in young A. tequilana plants inoculated with AMF (G. fasciculatum and G. intrarradices); Cui and Nobel (1992) found mycorrhizal colonization percentages of up to 64 % in A. desertii plants inoculated with a native AMF consortium (G. epigaeum, G. fascieulatum and three other species of Glomus spp.). In contrast, Robles-Martínez et al. (2013) found colonization percentages of 35 % in A. angustifolia plants inoculated with a native AMF consortium native to the Tlacolula district in the state of Oaxaca.
The observed differences in mycorrhizal colonization may be explained by the conditions and duration of the experiments, the AMF species used, the origin of the inocula, and the affinity of the host for the AMF species. Daniels Hetrick and Bloom (1986) evaluated the influence of different plant hosts as marigold (Calendula officinalis), red clover (Trifolium pratense), sorghum, tomato (Solanum lycopersicum) and asparagus (Asparagus officinalis) on the production of AMF spores (G. fasciculatum, G. macrocarpum and G. mosseae) and on mycorrhizal colonization; these authors mentioned that there may be different affinity levels between hosts and AMF, even at the lowest amount of inoculated spores. Likewise, OchoaMeza et al. (2009) reported colonization percentages of up to 24.9 % in A. angustifolia plants grown under field conditions and 32 AMF spore morphotypes associated with the rhizosphere of these plants, corresponding to the following genera: Glomus (17), Acaulospora (10), Archeospora (2), Diversispora (1) and Pacispora (2).
The inocula used in the present study also contained a great diversity of AMF species (10 on average) associated with the rhizosphere (Trinidad-Cruz et al., 2017a), which might indicate that the roots of agave plants are likely colonized by several species at the same time. The results of this study showed a spore density of up to 243 spores in 100 g of substrate, a relatively lower value than those reported by Ochoa-Meza et al. (2009), who found between 400 and 700 spores per 100 g of A. angustifolia rhizosphere soil in the mountainous area of Sonora, Mexico.
The relatively low density of spores found in this study could be due to the conditions of the growth medium, the environment, the age of the plants and the AMF species used. It has also been shown that sporulation is associated with the propagation substrate or growth medium (Douds and Schenck, 1990; Ferrera et al., 1993). Camacho-Camacho et al. (2013) evaluated the efficiency of different substrates for obtaining AMF spores from native fungal consortia under greenhouse conditions and reported that the production of spores varied according to the substrate mixture used. A substrate composed of a mixture of sand, peat moss and compost, with sorghum as trap plant, yielded the largest number of spores (866 in 100 g of substrate), while the lowest number was obtained with sand as the substrate (98 spores in 100 g of substrate). Based on these results, the authors indicated that organic matter probably stimulates the production of AMF spores. Since the growth medium used in this study was poor in both organic matter (0.98 %) and nutrients (5, 15 and 35 ppm N, P and K respectively), this could explain the low levels of spore production.
The relative mycorrhizal dependency index showed values from 11 to 62 %; this variation can be attributed to the diversity of AMF species in each consortium and their affinity for the corresponding plant species. Some plant species are heavily reliant on mycorrhizals. QuiñonesAguilar et al. (2014) report a RMDI of up to 99 % for papaya plants inoculated with Glomus sp. In agave plants, a wide range of mycorrhizal colonization values have been reported, depending on the growing conditions, the AMF species used and the variety of each agave plant, among other factors; however, if the colonization values found in this study are compared to those reported by PimientaBarrios et al. (2009) for the same species (less than 60 %) and by Quiñones-Aguilar et al. (2016) for A. inaequidens (more than 79 %), the mycorrhizal dependency observed in the present study could be classified from medium to low.
In this study agave plants inoculated with the LC consortium showed a negative RMDI, indicating that agave plants inoculated with this particular consortium yielded less dry biomass than non-inoculated agave plants. Smith and Smith (2012) and Johnson et al. (1997) pointed out that in some cases mycorrhizal can act as a parasite, taking carbon compounds from plants while providing them with few benefits, reflecting that their growth is equal to or less than that of non-mycorrhized plants. This could have occurred in this study at agave plants inoculated with the LC consortium.
CONCLUSIONS
Inoculation with native AMF consortia obtained from the rhizosphere of A. cupreata promoted the growth of A. tequilana plants compared to non-inoculated plants. The native consortium BN (Ambispora appendicula (Spain, Sieverding & Schenck) C. Walker, Acaulospora denticulata Sieverding & S. Toro, Acaulospora scrobiculata Trappe, Acaulospora spinosa C. Walker & Trappe, Acaulospora sp. 4, Claroideoglomus etunicatum (W. N. Becker & Gerd.) C. Walker & A. Schüßler, Diversispora aurantia (Błaszk., Blanke, Renker & Buscot) C. Walker & A. Schüßler, Glomus deserticola Trappe, Bloss & J. A. Menge, Glomus sp. 1, Rhizophagus diaphanum (C. Cano & Y. Dalpé) C. Walker & A. Schüßler) was associated with the highest vegetative growth and the highest quality of plants. Native LC consortium could be acting as a parasite, since agave plants inoculated with this consortium did not show an increase in growth. The use of mycorrhizal consortia on A. tequilana plants in a nursery could represent an alternative to the use of chemical fertilizers for the production of the raw material for tequila.











 nueva página del texto (beta)
nueva página del texto (beta)