Introduction
Bahia is one of the Brazilian states with the greatest diversity of orchids, with 118 genera and 522 species, numerically surpassing several American countries such as Argentina and Chile (Johnson, 2001; Lehnebach, 2003; FFB, 2023). Floristic-taxonomic surveys of Orchidaceae in the state are concentrated in the Chapada Diamantina region, and in the Caatinga phytogeographic domain (see Azevedo and van den Berg, 2007; Vieira et al., 2014), although recently they have also been carried out in areas of transition between the Atlantic Forest and the Caatinga phytogeographic domains (e.g., Marinho and Azevedo, 2013; Rêgo and Azevedo, 2017; Azevedo et al., 2021). These surveys revealed new occurrences of orchid species in northeastern Brazil (e.g., Rêgo and Azevedo, 2017; Santos and Azevedo, 2022; Lima and Azevedo, 2023) and have encouraged the continuity of field efforts. Nevertheless, the orchidological flora of the remainder of the Atlantic Forest in Bahia, which today is reduced to just 6% of the state's area (originally corresponding to 36%; MPEB, 2023), requires greater conservation attention. The presence of species, including ornamental ones, which are restricted to microhabitats or forming small populations, being subject to local suppression, was reported for a fragment of restinga in the municipality of Salvador, capital of the state (Barberena et al., 2019a, 2021).
Restingas constitute a vegetation type associated with coastal sandy deposits from Quaternary and coastal rocky environments, and range from herbaceous-shrub to forest formations (CONAMA, 2009), occurring in the Atlantic Forest phytogeographic domain in the state of Bahia. The municipality of Salvador is adopted as a geopolitical divide, delimiting the north coast of the state, which has approximately 200 km, and the south coast, with more than 800 km (Menezes, 2007). The ecosystems of the north coast have been exposed to more intense anthropic pressures due to the recent improvement of the road network and growing population density (INEMA, 2023). In return, these regional changes resulted in the establishment of Conservation Units, among them the Área de Proteção Ambiental Litoral Norte do Estado da Bahia (APA Litoral Norte), aiming at the ecological-economic planning of this coastal portion (INEMA, 2023), as well as the intensification of botanical research (Gomes and Guedes, 2014). Comprehensive inventories of vascular plants carried out in the restingas on the north coast of the state made it possible to highlight Orchidaceae as one of the families with the greatest local species richness (Queiroz et al., 2012; Gomes and Guedes, 2014). Nonetheless, the APA Litoral Norte suffers from environmental conflicts, notably the disordered occupation of land, lack of basic sanitation, environmental impacts caused by pine and eucalyptus plantation areas, indiscriminate intensification of livestock farming, predatory fishing, degradation of mangroves, and the predatory tourism in coastal districts (Gomes and Guedes, 2014; INEMA, 2023).
In order to disseminate consistent and updated data on the flora of Brazilian restingas and encourage the adoption of regional conservation strategies, we carried out a survey of orchid flora in the Parque Natural Municipal da Restinga de Praia do Forte (PNMR Praia do Forte), which is completely included in the APA Litoral Norte, comprising an identification key, photographs, and information on population size, substrate and phenology of the species in the study area.
Material and Methods
Study area
The Parque Natural Municipal da Restinga de Praia do Forte is an integral protection Conservation Unit, with ca. 253 hectares, and is situated at coordinates 12º50'-12º57'S and 37º98'-38º01'W in the municipality of Mata de São João on the northern coast of Bahia, northeastern Brazil (DOMMSJ, 2008; Fig. 1). The vegetation comprises marshy areas and well-conserved fragments of shrub and restinga forest formations, the latter phytophysiognomy being mainly composed by Elaeis guineensis Jacq. and Syagrus schizophylla (Mart.) Glassman (Fig. 2). The local climate is ‘Af’ (tropical, without a dry season) and the annual precipitation is 1600-1900 mm, with an average temperature equal to or greater than 18 ºC in the coldest month (Alvares et al., 2013).
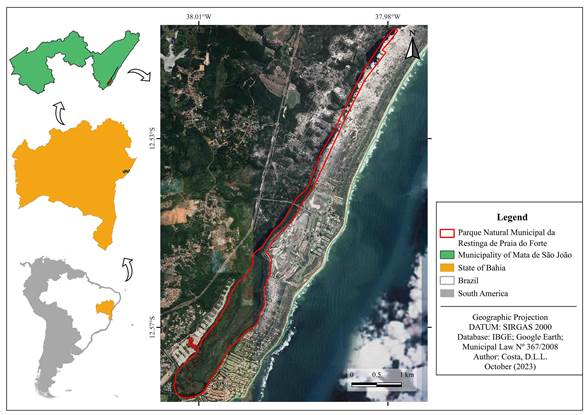
Figure 1: Map showing Parque Natural Municipal da Restinga de Praia do Forte (PNMR Praia do Forte) in the municipality of Mata de São João, Bahia, Brazil. Map produced by Deivid Lucas de Lima da Costa.

Figure 2: Parque Natural Municipal da Restinga de Praia do Forte (PNMR Praia do Forte) in the municipality of Mata de São João, Bahia, Brazil. A. marshy area and restinga forest formation (figure originally published in Barberena et al. (2018b)); B. details of a marshy area; C. shrub formation and lagoon; D. interior of a fragment of restinga forest formation. Photographs by Felipe Fajardo Villela Antolin Barberena.
Data collection and analysis
Field work was conducted monthly from September 2016 to December 2017, and in July 2020 and August 2022, by employing the walking survey method (Filgueiras et al., 1994). Collected specimens were deposited in the herbarium ALCB, with duplicates in the herbaria MBM, MG, RB (including RB spirit) and UPCB. Collections of the herbaria ALCB, HRB, HUEFS and RB were examined in person (acronyms according to Thiers, 2023, continuously updated), as well as digital images of type specimens deposited in European herbaria (JSTOR, 2023). The Environmental Information Reference Center (database CRIA, 2023) was also consulted in the search for possible other orchid specimens from the park. The analyzed specimens were identified by comparison with specimens deposited in the ALCB herbarium and based on floristic-taxonomic articles on Orchidaceae. The morphological terminology followed Radford et al. (1974) and Dressler (1981).
Phenological and distribution data of the species in the phytophysiognomies of PNMR Praia do Forte were mostly obtained in the field and complemented by information available on specimen labels. Species with stems with five or more leaves were considered multifoliate, and inflorescences with 10 or more flowers were treated as multiflowered (Barberena et al., 2022). Populations with less than 50 individuals were considered small (Barberena et al., 2019a). Details of specimens and habitats were recorded using a Nikon Coolpix P600 camera (Nikon Corporation, Tokyo, Japan) and a LG Joy cell phone (LG Corporation, Seoul, South Korea). The accepted name and threat categories of the species are in line with Flora e Funga do Brasil (FFB, 2023). The geographic distribution of species was based primarily on the studies of authors who contributed to Flora and Funga do Brasil (FFB, 2023) and complemented with relevant literature (Barberena et al., 2019b; Karremans et al., 2020; Barberena et al., 2021), and with data obtained from POWO (2023) for species with extra-Brazilian distributions. We adopted the ecological categories proposed by Benzing (1990) for the epiphytic species, and elaborated a map of the area using QGIS software v. 3.30 (QGIS, 2023).
Results
Orchidaceae is represented by 14 genera and 16 species in the PNMR Praia do Forte (Fig. 3), most of which are native to the Neotropics (87%; 14 spp.), including eight restricted to Brazil, highlighting Catasetum gardneri Schltr. and Cyrtopodium flavum Link & Otto ex Rchb.f., which are also endemic to the Atlantic Forest. The two other species (Oeceoclades maculata (Lindl.) Lindl. and Polystachya concreta (Jacq.) Garay & Sweet.) are pantropical. Epidendrum L. and Vanilla Mill. are the only genera represented by two species each.

Figure 3: Species of Orchidaceae of Parque Natural Municipal da Restinga de Praia do Forte (PNMR Praia do Forte) in the municipality of Mata de São João, Bahia, Brazil. A. Brassavola tuberculata Hook.; B. Campylocentrum robustum Cogn.; C. Catasetum gardneri Schltr.; D. Cyrtopodium flavum Link & Otto ex Rchb.f.; E. Eltroplectris calcarata (Sw.) Garay & Sweet.; F. Encyclia oncidioides (Lindl.) Schltr.; G. Epidendrum cinnabarinum (Salzm.) Lindl.; H. Epidendrum orchidiflorum (Salzm.) Lindl.; I. Epistephium williamsii Hook.f.; J. Gomesa barbata (Lindl.) M.W. Chase & N.H. Williams.; K. Oeceoclades maculata (Lindl.) Lindl.; L. Pachygenium parvum (Cogn.) Szlach., R. González & Rutk.; M. Polystachya concreta (Jacq.) Garay & Sweet.; N. Prescottia leptostachya Lindl.; O. Vanilla palmarum (Salzm. ex Lindl.) Lindl.; P. Vanilla phaeantha Rchb.f. Photographs by Felipe Fajardo Villela Antolin Barberena (A, C, E-P) and Tainan da Silva Sousa (B, D). Figures originally published in Barberena et al. (2018b).
Campylocentrum robustum Cogn., Eltroplectris calcarata (Sw.) Garay & Sweet, Epistephium williamsii Hook.f., Gomesa barbata (Lindl.) M.W. Chase & N.H. Williams, O. maculata, P. concreta, Prescottia leptostachya Lindl., and Vanilla palmarum (Salzm. ex Lindl.) Lindl. occur only in restinga forest formations (50%), while the others are either exclusive to shrub formations (Cyrtopodium flavum Link & Otto ex Rchb.f., and Epidendrum orchidiflorum (Salzm.) Lindl.) or occur in both phytophysiognomies (Brassavola tuberculata Hook., C. gardneri, Encyclia oncidioides (Lindl.) Schltr., Epidendrum cinnabarinum (Salzm.) Lindl., Pachygenium parvum (Cogn.) Szlach., R. González & Rutk., and Vanilla phaeantha Rchb.f.). The species found are mainly terrestrial (44%; 6 spp.), although there are also characteristic holo-epiphytic species (25%), facultative holo-epiphytes (19%), accidental holo-epiphytes (6%) and secondary hemi-epiphytes (6%). We also highlight that Cyrtopodium holstii L.C. Menezes was collected in the district of Praia do Forte (A.M. Miranda et al. 5387 (UB0015899, digital image!)), but not found within the park.
Orchid species from PNMR Praia do Forte can be primarily distinguished by growth type (monopodial or sympodial); thickening of the stem (thickened or not in pseudobulbs); leaves characteristics, notably number (1-4 or multifoliate) and position (apical or distributed along the stem); form (cylindrical, flat or concave) and consistency of leaf blades (membranaceous, papyraceous, subcoriaceous or coriaceous); position (lateral, terminal or axillary) and type of inflorescence (raceme or panicle); in addition to the resupination of the flower (resupinate or non-resupinate) and division of the lip (entire or trilobed), as can be seen in the following key.
Taxonomic treatment
Key to the species of Orchidaceae of the PNMR Praia do Forte, Bahia, Brazil
1a. Herb with monopodial growth; inflorescence axillary .............................................. 2
1b. Herb with sympodial growth; inflorescence lateral or terminal ................................ 4
2a. Leaf blades conduplicate, with apex emarginate, noticeably asymmetrical; flowers calcarate; sepals and petals white ..................................... Campylocentrum robustum Cogn.
2b. Leaf blades convolute, with apex acute, acuminate or obtuse, symmetrical; flowers non-calcarate; sepals and petals yellow or green ......................................................................... 3
3a. Characteristic holo-epiphyte; leaves petiolate, blades ovate to oval-lanceolate; sepals and petals yellow .................................... Vanilla palmarum (Salzm. ex Lindl.) Lindl.
3b. Secondary hemi-epiphyte; leaves sessile, blades oblong; sepals and petals green ....................................................................................................... Vanilla phaeantha Rchb.f.
4a. Stem thickened in pseudobulb ................................................................................... 5
4b. Stem not thickened in pseudobulb ........................................................................... 10
5a. Leaves distributed along the stem ............................................................................. 6
5b. Leaves apical ............................................................................................................. 8
6a. Leaf blades conduplicate; inflorescence terminal .......................................................................... Polystachya concreta (Jacq.) Garay & Sweet
6b. Leaf blades plicate; inflorescence lateral .................................................................. 7
7a. Leaf blades narrow-elliptical; inflorescence in raceme; flowers non-resupinate; lip entire, margin noticeably fimbriate ............................................. Catasetum gardneri Schltr.
7b. Leaf blades linear; inflorescence in panicle (rarely raceme); flowers resupinate; lip trilobed, central lobe with entire margin .......... Cyrtopodium flavum Link & Otto ex Rchb.f.
8a. Leaf blades maculate; inflorescence in raceme; flowers calcarate ..................................................................................... Oeceoclades maculata (Lindl.) Lindl.
8b. Leaf blades non-maculate; inflorescence in panicle; flowers non-calcarate ............. 9
9a. Apical leaf 1; inflorescence lateral ............................................................................... Gomesa barbata (Lindl.) M.W. Chase & N.H. Williams
9b. Apical leaves 2-3; inflorescence terminal ......... Encyclia oncidioides (Lindl.) Schltr.
10a. Leaf blades cylindrical ............................................... Brassavola tuberculata Hook.
10b. Leaf blades flat to concave ...................................................................................... 11
11a. Stem with 1-4 leaves; leaves petiolate, blade membranaceous to papyraceous ...... 12
11b. Stem multifoliate; leaves sessile, blade subcoriaceous to coriaceous ..................... 14
12a. Leaf blades silvery-green; inflorescence glabrous; flowers non-resupinate, non-calcarate .................................................................................. Prescottia leptostachya Lindl.
12b. Leaf blades dark green to green; inflorescence pilose; flowers resupinate, calcarate .............................................................................................................................................. 13
13a. Leaf blades oblong to linear-lanceolate; flowers pilose; lip entire, margin entire to slightly undulate ..................... Pachygenium parvum (Cogn.) Szlach., R. González & Rutk.
13b. Leaf blades narrow elliptical to ovate; flowers glabrous; lip trilobed, central lobe with noticeably fimbriate margin ................... Eltroplectris calcarata (Sw.) Garay & Sweet
14a. Leaf blades reticulate-veined; sepals and petals lilac to lilac-white ............................................................................................... Epistephium williamsii Hook.f.
14b. Leaf blades obscurely parallel-veined; sepals and petals green or orange to red-orange .................................................................................................................................. 15
15a. Leaf blades oblong; flowers non-resupinate; lip trilobed, central lobe with noticeably fimbriate margin ................................ Epidendrum cinnabarinum (Salzm.) Lindl.
15b. Leaf blades ovate to lanceolate; flowers resupinate; lip entire, margin sinuate ............................................................................. Epidendrum orchidiflorum (Salzm.) Lindl.
1. Brassavola tuberculata Hook., Bot. Mag. 56: t. 2878. 1829. Fig. 3A.
≡ Bletia tuberculata (Hook.) Rchb.f., in W.G. Walpers, Ann. Bot. Syst. 6: 434. 1862.
TYPE: BRAZIL. Rio Janeiro, Rio de Janeiro, at the entrance of Botafogo Bay, s.d., H. Harrison s.n. (holotype: K000583999? (digital image!)).
Facultative holo-epiphytic herb with sympodial growth; stems not thickened in pseudobulbs; leaf 1, sessile, apical; blade dark green to green, non-maculate, rare maculate, fleshy, cylindrical, conduplicate, linear, apex acute; inflorescence in raceme, terminal, 1-3-flowered, glabrous; flowers resupinate, non-calcarate; sepals and petals yellowish to brownish-yellow, non-maculate; lip white, greenish-yellow at the base, entire, margin entire.
Distribution and notes: northeastern Argentina, Bolivia, northern, northeastern, southern, southeastern and west-central Brazil, Paraguay (POWO, 2023; van den Berg, 2023). In PNMR Praia do Forte, Brassavola tuberculata occurs in shrub and restinga forest formations, and has a population of more than 100 individuals. The species has been observed with flowers and fruits from September to January.
Specimen examined: BRAZIL. Bahia, PNMR Praia do Forte, 16.IX.2016, fl, fr, F.F.V.A. Barberena et al. 374 (ALCB, UPCB).
2. Campylocentrum robustum Cogn., Fl. Bras. 3(6): 509. 1906. Fig. 3B.
TYPE: BRAZIL. Rio de Janeiro, Copacabana, 1871, A. Glaziou 5490 (holotype not indicated, lectotype designated by Pessoa and Alves (2019): C, isolectotypes: P00361631 (digital image!), W).
Characteristic holo-epiphytic herb with monopodial growth; stem not thickened in pseudobulb, multifoliate; leaves sessile, distributed along the stem; blade green, non-maculate, coriaceous, flat, conduplicate, oblong to narrow elliptical, apex emarginate, noticeably asymmetrical; inflorescence in raceme, axillary, 8-14-flowered, glabrous; flowers resupinate, calcarate; sepals and petals white, non-maculate; lip white, entire, margin entire.
Distribution and notes: northeastern and southeastern Brazil (Pessoa, 2023a). In PNMR Praia do Forte, Campylocentrum robustum occurs exclusively in restinga forest formations, its population being made up of more than 100 individuals. The species has been observed with flowers and fruits in September.
Specimen examined: BRAZIL. Bahia, PNMR Praia do Forte, s.d., fl, T. S. Sousa 69 (ALCB).
3. Catasetum gardneri Schltr., Orchis 8: 84. 1914. Fig. 3C.
TYPE: BRAZIL. Pernambuco, s.d., G. Gardner 1160 (holotype: K000294030 (digital image!)).
≡ Monachanthus fimbriatus Gardner, Bot. Mag. 65: t. 3708. 1839.
≡ Catasetum fimbriatum (Gardner) Rchb.f., in W. W. Saunders, Refug. Bot. 2: t. 83. 1872, nom. illeg.
Facultative holo-epiphytic herb with sympodial growth; stems thickened in pseudobulbs; leaves 2-4, sessile, distributed along the stem; blade green, non-maculate, chartaceous, flat, plicate, narrow-elliptical, margin entire, apex acute to acuminate; inflorescence in raceme, lateral, multiflowered, glabrous; flowers non-resupinate, non-calcarate; sepals and petals yellowish-green, non-maculate; lip yellowish-green, yellow at the center, entire, margin noticeably fimbriate.
Distribution and notes: northeastern and southeastern Brazil (Petini-Benelli, 2023). In PNMR de Praia do Forte, Catasetum gardneri occurs in shrub and restinga forest formations, and has a population of more than 100 individuals. It has been observed with flowers and fruits in December and January.
Specimen examined: BRAZIL. Bahia, PNMR Praia do Forte, 16.XII.2016, fl, F. F. V. A. Barberena and T. S. Sousa 393 (ALCB).
4. Cyrtopodium flavum Link & Otto ex Rchb.f., Iconogr. Bot. Exot. 3: 7, t. 214. 1830. Fig. 3D.
TYPE: BRAZIL. Without any other locality, “Hort. Berol.”, s.d., s.leg. (holotype: not found).
Terricolous herb with sympodial growth; stems thickened in pseudobulbs, multifoliate; leaves sessile, distributed along the stem; blade pale green to green, non-maculate, chartaceous, flat, plicate, linear, apex acute to acuminate; inflorescence in panicle, rare raceme, lateral, multiflowered, glabrous; flowers resupinate, non-calcarate; sepals and petals yellow to greenish-yellow, non-maculate; lip yellow, trilobed, central lobe with entire margin.
Distribution and notes: northeastern, southern and southeastern Brazil (Batista and Bianchetti, 2023). In PNMR Praia do Forte, Cyrtopodium flavum occurs in shrub formations, and has a population of more than 100 individuals. It has been observed with flowers from November to January and fruits from September to January.
Specimen examined: BRAZIL. Bahia, PNMR Praia do Forte, 8.XI.2016, fl, T. S. Sousa 23 (ALCB).
5. Eltroplectris calcarata (Sw.) Garay & Sweet, J. Arnold Arbor. 53(3): 390. 1972. Fig. 3E.
≡ Neottia calcarata Sw., Fl. Ind. Occid. 3: 1413. 1806.
≡ Stenorrhynchos calcaratum (Sw.) Rich., De Orchid. Eur.: 37. 1817.
≡ Collea calcarata (Sw.) Lindl., Bot. Reg. 9: t. 760. 1823.
≡ Pelexia calcarata (Sw.) Cogn., I. Urban, Symb. Antill. 6: 328. 1909.
≡ Centrogenium calcaratum (Sw.) Schltr., Beih. Bot. Centralbl. 37(2): 452. 1920.
≡ Spiranthes calcarata (Sw.) Jiménez, Phytologia 8: 326. 1962.
TYPE: DOMINICAN REPUBLIC. Without any other locality, s.d., O. P. Swartz s.n. (holotype: S-R-3752 (digital image!)).
Terricolous herb with sympodial growth; stems inconspicuous, not thickened in pseudobulbs; leaves 1-2(-3), petiolate, basal; blade dark green, non-maculate, membranaceous, flat, convolute, narrow-elliptical to ovate, apex acute to slightly acuminate; inflorescence in raceme, terminal, multiflowered, pilose; flowers resupinate, calcarate; sepals and petals white to greenish-white, non-maculate; lip white, trilobed, central lobe with noticeably fimbriate margin.
Distribution and notes: Bahamas, Bolivia, northeastern, southern and southeastern Brazil, Cayman Island, Colombia, Cuba, Dominican Republic, Ecuador, Haiti, Jamaica, Paraguay, Peru, Puerto Rico, Suriname, Trinidad and Tobago, United States of America (Florida), Venezuela, Windward Islands (Guimarães, 2023; POWO, 2023). In PNMR Praia do Forte, Eltroplectris calcarata occurs exclusively in restinga forest formations, and has a population of more than 100 individuals. It has been observed with flowers and fruits in September and October.
Specimen examined: BRAZIL. Bahia, PNMR Praia do Forte, 16.IX.2016, fl, fr, F. F. V. A. Barberena et al. 376 (ALCB, MBM, MG, RB spirit).
6. Encyclia oncidioides (Lindl.) Schltr., Orchideen 210. 1914. Fig. 3F.
≡ Epidendrum oncidioides Lindl., Edwards's Bot. Reg. 19: t. 1623. 1833.
TYPE: BRAZIL. Rio de Janeiro, s.d., A. Harrison s.n. (holotype: K000583895 (digital image!)).
Facultative holo-epiphytic herb with sympodial growth; stems thickened in pseudobulbs; leaves 2-3, sessile, apical; blade green, non-maculate, coriaceous, flat, conduplicate, oblong to linear, apex acute to rounded; inflorescence in panicle, terminal, multiflowered, glabrous; flowers resupinate, non-calcarate; sepals and petals brownish-yellow to brownish, maculate; lip white to yellow, trilobed, central lobe with entire to undulate margin.
Distribution and notes: northern, northeastern, southern and southeastern Brazil (Bastos et al., 2023). In PNMR Praia do Forte, Encyclia oncidioides occurs in shrub and restinga forest formations, and has a population of more than 100 individuals. It has been observed with flowers from September to December and with fruits from October to January.
Specimen examined: BRAZIL. Bahia, PNMR Praia do Forte, 16.IX.2016, fl, F. F. V. A. Barberena and T. S. Sousa 377 (ALCB, MBM, MG, RB).
7. Epidendrum cinnabarinum (Salzm.) Lindl., Gen. Sp. Orchid. Pl. 106. 1830-1840 (1831). Fig. 3G.
TYPE: BRAZIL. Bahia, “in fruticetis sabulosis”, s.d., P. Salzmann s.n. (holotype: K000293879 (digital image!), isotypes: FI011800, G00168900 (digital image!), K000293528 (digital image!), K00293553 (digital image!), LE00001439 (digital image!), LE00006535 (digital image!), MPU017820 (digital image!), MPU017821 (digital image!), MPU017822 (digital image!), MPU017823 (digital image!), P00467527 (digital image!), TUB009605).
Accidental holo-epiphytic herb with sympodial growth; stems not thickened in pseudobulbs, multifoliate; leaves sessile, distributed along the stem; blade green to yellowish-green, non-maculate, coriaceous, flat, conduplicate, oblong, obscurely parallel-veined, apex obtuse to acute; inflorescence in raceme, terminal, multiflowered, glabrous; flowers non-resupinate, non-calcarate; sepals and petals orange to red-orange, non-maculate; lip orange-yellow to orange, trilobed, central lobe with noticeably fimbriate margin.
Distribution and notes: northeastern Brazil (Pessoa, 2023b). In PNMR Praia do Forte, Epidendrum cinnabarinum occurs mainly in shrub and infrequently in restinga forest formations and has a population of more than 100 individuals. It has been observed with flowers from October to January and with fruits in December and January.
Specimen examined: BRAZIL. Bahia, PNMR Praia do Forte, 19.I.2017, fl, F. F. V. A. Barberena and T. S. Sousa 396 (ALCB).
8. Epidendrum orchidiflorum (Salzm.) Lindl., Gen. Sp. Orchid. Pl. 103. 1830-1840 (1831). Fig. 3H.
TYPE: BRAZIL. Bahia, “in fruticetis sabulosis”, s.d., P. Salzmann s.n. (holotype: K000293877 (digital image!), isotypes: FI011826, G168860 (digital image!), K00293555 (digital image!), K00293556 (digital image!), LE00006551 (digital image!), MPU 013726 (digital image!), MPU 013727 (digital image!), MPU 013728 (digital image!), P00484658 (digital image!), W0027678 (digital image!).
Terricolous herb with sympodial growth; stems not thickened in pseudobulbs, multifoliate; leaves sessile, distributed along the stem; blade green to purple, non-maculate, coriaceous, flat, conduplicate, ovate to lanceolate, obscurely parallel-veined, apex acute to rounded; inflorescence in raceme, terminal, 9-10-flowered, glabrous; flowers resupinate, non-calcarate; sepals and petals green, non-maculate to maculate; lip green, entire, margin sinuate.
Distribution and notes: northern, northeastern, southeastern and west-central Brazil, Colombia, Guyana, Peru, Venezuela (Pessoa, 2023b; POWO, 2023). In PNMR Praia do Forte, Epidendrum orchidiflorum occurs exclusively in shrub formations, and has a population of more than 100 individuals. It has been observed with flowers and fruits from October to January.
Specimen examined: BRAZIL. Bahia, PNMR Praia do Forte, 16.XII.2016, fl, F. F. V. A. Barberena and T. S. Sousa 392 (ALCB).
9. Epistephium williamsii Hook.f., Bot. Mag. 90: t. 5485. 1865. Fig. 3I.
TYPE: BRAZIL. Bahia, William s.n. (not found). Lectotype (designated by Carvalho et al., 2016): original drawing reproduced in t. 5485 in Curtis’s Botanical Magazine.
Terricolous herb with sympodial growth; stems not thickened in pseudobulbs, multifoliate; leaves sessile, distributed along the stem; blade green, non-maculate, subcoriaceous to coriaceous, flat to concave, conduplicate, ovate to elliptical, reticulate-veined, apex acute; inflorescence in raceme, terminal, multiflowered, glabrous; flowers resupinate, non-calcarate; sepals and petals lilac to lilac-white, non-maculate; lip white with lilac veins, trilobed, central lobe with undulate margin.
Distribution and notes: northern, northeastern, southeastern and west-central Brazil, Guyana, Paraguay, Venezuela (Meneguzzo, 2023a; POWO, 2023). In PNMR Praia do Forte, Epistephium williamsii occurs exclusively in a marshy area near the borders of the park associated with restinga forest formations, and has a population of less than 50 individuals. It has been observed with flowers and fruits in January.
Specimen examined: BRAZIL. Bahia, PNMR Praia do Forte, 19.I.2017, fl, F. F. V. A. Barberena and T. S. Sousa 398 (ALCB).
10. Gomesa barbata (Lindl.) M.W. Chase & N.H. Williams, Ann. Bot. (Oxford) 104(3): 395. 2009. Fig. 3J.
≡ Oncidium barbatum Lindl., Coll. Bot.: t. 27. 1821.
≡ Alatiglossum barbatum (Lindl.) Baptista, Colet. Orquídeas Brasil. 3: 87. 2006.
TYPE: BRAZIL. s.d., Gardner s.n. (holotype: K000501739 (digital image!)).
Characteristic holo-epiphytic herb with sympodial growth; stems thickened in pseudobulbs; leaf 1, sessile, apical; blade green, non-maculate, chartaceous, flat, conduplicate, oblong, apex acute to slightly retuse; inflorescence in panicle, lateral, multiflowered, glabrous; flowers resupinate, non-calcarate; sepals and petals yellow, maculate; lip yellow, trilobed, central lobe with noticeably ciliate margin.
Distribution and notes: northeastern and southern Brazil (Meneguzzo, 2023b). In PNMR Praia do Forte, Gomesa barbata occurs exclusively in restinga forest formations, and has a population of less than 50 individuals. It has not been observed with flowers or fruits. However, there are regional records of the species flowering in October (L. P. Queiroz 883).
Specimen examined: BRAZIL. Bahia, PNMR Praia do Forte, 6.VIII.2022, st, T.S. Sousa 51 (ALCB).
Additional specimen examined: BRAZIL. Bahia, Mata de São João, arredores da cidade, fl, 24.X.1984, L. P. Queiroz 883 (HUEFS).
11. Oeceoclades maculata (Lindl.) Lindl., Gen. Sp. Orchid. Pl. 237-238. 1830-1840 (1833). Fig. 3K.
≡ Angraecum maculatum Lindl., Coll. Bot. 3: t. 15. May 1821.
≡ Limodorum maculatum (Lindl.) G. Lodd., Bot. Cab. 5: t. 496. Jun 1821.
≡ Aerobion maculatum (Lindl.) Spreng., Syst. Veg., ed. 16. 3: 718. 1826.
≡ Eulophia maculata (Lindl.) Rchb.f., in W.G. Walpers, Ann. Bot. Syst. 6: 647. 1863.
≡ Eulophidium maculatum (Lindl.) Pfitzer, Entwurf. Anordn. Orch.: 87. 1887.
≡ Graphorkis maculata (Lindl.) Kuntze, Revis. Gen. Pl. 2: 662. 1891.
TYPE: BRAZIL. Cult. in England, without data (lectotype designated by Turner (2016): Lindley, Coll. Bot. 3: t. 15. 1821).
Terricolous herb with sympodial growth; stems thickened in pseudobulbs; leaf 1, sessile, apical; blade green, maculate, coriaceous, flat to slightly concave, conduplicate, oblong to slightly elliptic, apex acute; inflorescence in raceme, lateral, 4-9-flowered, glabrous; flowers resupinate, calcarate; sepals and petals brownish, non-maculate; lip white, pink at the lateral margins, trilobed, central lobe with slightly undulate margin.
Distribution and notes: native to Central Africa and Gulf of Guinea Island, introduced into Southeastern Mexico, Florida, and several countries of Central and South America, including all regions of Brazil (Machnicki-Reis and Smidt, 2023; POWO, 2023). In PNMR Praia do Forte, Oeceoclades maculata occurs exclusively in restinga forest formations, and has a population of less than 50 individuals. It has been observed with flowers and fruits in July.
Specimen examined: BRAZIL. Bahia, PNMR Praia do Forte, 27.VII.2020, fr, F. F. V. A. Barberena et al. 416 (ALCB).
12. Pachygenium parvum (Cogn.) Szlach., R. González & Rutk., Polish Bot. J. 46(1): 5. 2001. Fig. 3L.
≡ Stenorrhynchos parvum Cogn., in C. F. P. von Martius & auct. suc. (eds.), Fl. Bras. 3(6): 537. 1906.
≡ Pelexia parva (Cogn.) Schltr., Beih. Bot. Centralbl. 37(2): 404. 1920.
TYPE: BRAZIL. Minas Gerais, Serra do Cipó, 22.IV.1892, C. Schwacke 8404 (holotype: BR0000006585457 (digital image!), isotype: RB00542665 (digital image!)).
Terricolous herb with sympodial growth; stems inconspicuous, not thickened in pseudobulbs; leaves 1-4, petiolate, distributed along the stem; blade green, non-maculate, papyraceous, flat, convolute, oblong to linear-lanceolate, apex acute to acuminate; inflorescence in raceme, terminal, multiflowered, pilose; flowers resupinate, calcarate; sepals green to white-greenish, non-maculate; petals white, non-maculate; lip white, with a green longitudinal stripe, entire, margin entire to slightly undulate.
Distribution: northeastern and southeastern Brazil (Barberena et al., 2021; Meneguzzo, 2023c). In PNMR Praia do Forte, Pachygenium parvum occurs in shrub and restinga forest formations, and has a population of less than 50 individuals. It has been observed with flowers and fruits in September.
Specimen examined: BRAZIL. Bahia, PNMR Praia do Forte, 18.IX.2016, fl, F. F. V. A. Barberena and T. S. Sousa 386 (ALCB, RB spirit).
13. Polystachya concreta (Jacq.) Garay & Sweet, Orquideología 9(3): 206. 1974. Fig. 3M.
≡ Epidendrum concretum Jacq., Enum. Syst. Pl.: 30. 1760.
TYPE: MARTINIQUE. Bois du Pl. Larcher, 2.VIII.1936, M. Privault 136 (neotype designated by Mytnik-Ejsmont & Baranow 2010: P00419738 (digital image!)).
Characteristic holo-epiphytic herb with sympodial growth; stems thickened in pseudobulbs; leaves 2-3, sessile, distributed along the stem; blade green, non-maculate, subcoriaceous, flat to slightly concave, conduplicate, oblong, apex acute; inflorescence in panicle, terminal, multiflowered, glabrous; flowers non-resupinate, non-calcarate; sepals and petals pale green, non-maculate; lip white to greenish-white, trilobed, central lobe with slightly undulate margin.
Distribution and notes: pantropical, including northern and west-central Brazil (Meneguzzo, 2023d; POWO, 2023). In PNMR Praia do Forte, Polystachya concreta occurs exclusively in restinga forest formations, and has a population of less than 50 individuals. It has been observed with flowers and fruits in February.
Specimen examined: BRAZIL. Bahia, PNMR Praia do Forte, 12.II.2017, fr, F. F. V. A. Barberena and T. S. Sousa 401 (ALCB).
14. Prescottia leptostachya Lindl., Edwards's Bot. Reg. 22: sub t. 1915 (err. typ. t. 1916). 1836. Fig. 3N.
TYPE: BRAZIL. Bahia, “in fruticetis sabulosis”, s.d., P. Salzmann s.n. (holotype K-L000293872 (digital image!), isotypes: K000293363 (digital image!), K000293364 (digital image!), P00366657 (digital image!), W646 (digital image!)).
Terricolous herb with sympodial growth; stems inconspicuous, not thickened in pseudobulbs; leaves 1-3, petiolate, basal; blade silvery-green, non-maculate, membranaceous, flat, convolute, elliptical to oblong, apex acute to acuminate; inflorescence in raceme, terminal, multiflowered, glabrous; flowers non-resupinate, non-calcarate; sepals and petals green, non-maculate; lip green, entire, margin entire.
Distribution and notes: northeastern Brazil, restricted to the state of Bahia (Meneguzzo, 2023e). In PNMR Praia do Forte, Prescottia leptostachya occurs exclusively in restinga forest formations, and has a population of less than 50 individuals. It has been observed with flowers and fruits in January and February.
Specimen examined: BRAZIL. Bahia, PNMR Praia do Forte, 12.II.2017, fr, F.F.V.A. Barberena and T.S. Sousa 400 (ALCB).
15. Vanilla palmarum (Salzm. ex Lindl.) Lindl., Gen. Sp. Orchid. Pl. 436. 1830-1840 (1840). Fig. 3O.
≡ Epidendrum palmarum Salzm. ex Lindl., Gen. Sp. Orchid. Pl.: 436. 1840.
TYPE: BRAZIL. Bahia, s.d., P. Salzmann s.n. (holotype: K000293881 (digital image!), isotypes: G00190804 (digital image!), G00190913 (digital image!), K000293255 (digital image!), LE00006463 (digital image!), MPU013457 (digital image!), MPU018359 (digital image!), MPU018360 (digital image!).
Characteristic holo-epiphytic with monopodial growth; stems not thickened in pseudobulbs, multifoliate; leaves petiolate, distributed along the stem; blade green to yellowish-green, non-maculate, coriaceous, flat, convolute, ovate to oval-lanceolate, apex acute to obtuse, symmetrical; inflorescence in raceme, axillary, multiflowered (flowers open one by one consecutively), glabrous; flowers resupinate, non-calcarate; sepals and petals yellow, non-maculate; lip yellow, entire, margin undulate.
Disribution and notes: northern, northeastern, southeastern and west-Central Brazil, Colombia, Ecuador, French Guiana, Guyana, Peru, Suriname, Venezuela (Barberena et al., 2019b; FFB, 2023; POWO, 2023). In PNMR Praia do Forte, Vanilla palmarum occurs exclusively in restinga forest formations, and has a population of more than 100 individuals. It has been observed with flowers and fruits from September to January.
Specimen examined: BRAZIL. Bahia, PNMR Praia do Forte, 22.X.2016, fl, F. F. V. A. Barberena and T. S. Sousa 390 (ALCB).
16. Vanilla phaeantha Rchb.f., Flora 48(18): 274. 1865. Fig. 3P.
TYPE: CUBA. s.d., C. Wright 3351 (lectotype designated by Karremans et al. (2020): K000463762 (digital image!), specimen on the left; isolectotypes: W43472 (W0253997 digital image!), illustration; AMES 00090738, specimen on the left; MO 2056007, segment in top left corner; G 00190805, specimen on the far right; GOET 008712, flower; MA 607142, top, center segment; BM 000062803).
Secondary hemi-epiphytic with monopodial growth; stems not thickened in pseudobulbs, multifoliate; leaves sessile, distributed along the stem; blade green, non-maculate, coriaceous, flat, convolute, oblong, apex acute to acuminate, symmetrical; inflorescence in raceme, axillary, multiflowered (flowers open one by one consecutively), glabrous; flowers resupinate, non-calcarate; sepals and petals green, non-maculate; lip white, with longitudinal yellow veins, yellow-green to yellow at the apex, entire, margin undulate.
Distribution: Bahamas, northern, northeastern, southeastern and west-central Brazil, Colombia, Costa Rica, Cuba, Dominican Republic, El Salvador, Jamaica, southeastern Mexico, Panama, Trinidad-Tobago, United States of America (Florida), Venezuela, Windward Islands (Karremans et al., 2020; FFB, 2023; POWO, 2023). In PNMR Praia do Forte, Vanilla phaeantha occurs in shrub and restinga forest formations, and has a population of more than 100 individuals. It has been observed with flowers in January.
Specimen examined: BRAZIL. Bahia, PNMR Praia do Forte, 12.II.2017, fl, F. F. V. A. Barberena and T. S. Sousa 399 (ALCB).
Discussion
A preliminary checklist of the Angiosperm flora of the PNMR Praia do Forte included nine species of Orchidaceae (DOMMSJ, 2013). However, the nomenclature of these taxa is outdated and there is no voucher associated with the list presented, making it impossible to confirm taxonomic identities. Thus, this is the first work to present a taxonomic study of Orchidaceae in the PNMR Praia do Forte, with photos, diagnostic descriptions and testimonial material for the species.
Five species of orchids from the PNMR Praia do Forte (Cyrtopodium flavum, Eltroplectris calcarata, Encyclia oncidioides, Epidendrum cinnabarinum and Gomesa barbata) had their status for conservation evaluated at the national level and were classified as Least Concern (FFB, 2023). The other species have not yet been evaluated, but, as they have wide geographic distributions, it is likely that they are also not at imminent risk of extinction. Nonetheless, many Orchidaceae species are subject to predatory collection due to their high ornamental value, often resulting in population reduction or local suppression, this scenario being more likely in areas where the plants are more easily accessed (Cruz et al., 2003; Barberena et al., 2018a), as is the case with restingas. In the PNMR Praia do Forte, we observed that some species have small populations (<50 individuals) and restricted local distribution, and, therefore, require the attention of managers, in order to implement possible management and conservation actions and prevent them from being suppressed locally. This is the case of Epistephium williamsii, Gomesa barbata, Oeceoclades maculata, Pachygenium parvum, Polystachya concreta and Prescottia leptostachya.
The other species occur in several localities or throughout the park and have populations of more than 100 individuals, which is why, for the moment, we consider them not to be locally threatened. Even so, we have records of predatory collections of flowering individuals of the ornamental species Cyrtopodium flavum and Encyclia oncidioides in the PNMR Praia do Forte, which also reinforces the need for greater surveillance and environmental education actions among local residents and tourists. The orchid flora of the PNMR Praia do Forte is very similar to that of the Área de Proteção Ambiental das Lagoas e Dunas do Abaeté - a Conservation Unit for Sustainable Use in Bahia and also a restinga fragment - with which it shares 12 species (Barberena et al., 2019a, 2021). This floristic similarity may be related to the relative proximity between the areas, which are approximately 60 km apart each other. In this way, joint conservation and ecosystem protection strategies can be planned regionally.











 nova página do texto(beta)
nova página do texto(beta)



