Introduction
The genus Acianthera Scheidw. belongs to the family Orchidaceae and comprises little more than 300 species (Karremans, 2016; Karremans and Vieira-Uribe, 2020), which grow as epiphytes, lithophytes, or rarely as terrestrials in dry to wet forest, from Mexico to Uruguay and Northern Argentina, including the Antilles (Luer, 2004). Brazil is a center of diversity for the genus; about 135 species have been reported to be native to the country (Barros et al., 2015). As currently defined, Acianthera is divided into four monophyletic subgenera: A. subg. Acianthera, A. subg. Antilla (Luer) Karremans, A. subg. Brenesia (Schltr.) Karremans, and A. subg. Kraenzlinella (Kuntze) Karremans (Karremans et al., 2016).
Acianthera subg. Brenesia was originally proposed as genus Brenesia Schltr., based on a Costa Rican species described as Brenesia costaricensis Schltr. The plant is characterized by its sessile leaves, secondary stems or ramicaul covered by inflated sheaths, and particularly an inflorescence produced at the base of the secondary stem (Schlechter, 1923). The genus has afterwards mostly been treated under the synonymy of Pleurothallis R. Br. (Williams, 1956; Luer, 1986; Pupulin, 2002). DNA-based studies showed that Brenesia costaricensis is embedded within Acianthera instead of Pleurothallis, and the species was transferred accordingly (Karremans et al., 2016).
Luer (2004) resurrected Brenesia, broadening its circumscription to include other species that share the development of inflorescence from the base of the secondary stem. Among the species transferred were Pleurothallis herrerae Luer and P. johnsonii Ames, two species that are morphologically very similar to B. costaricensis. However, Luer also included Pleurothallis balaeniceps Luer & Dressler, P. lappiformis A.H. Heller & L.O. Williams, P. pan Luer, P. stonei Luer, P. tomentosa Luer, and P. uncinata Fawc., which, despite featuring a basal inflorescence, have been shown not to be closely related Brenesia costaricensis (Pridgeon et al., 2001). Thus, the trait in question, an inflorescence arising from the base of the secondary stem, turned out to be a homoplasic trait within Pleurothallidinae. The species related to Brenesia costaricensis constitute a monophyletic group, which is sister to Acianthera, Antilla Luer and Kraenzlinella Kuntze. Karremans et al. (2016) proposed broadening Acianthera to include all these species groups and recognized each one at a subgeneric level.
Species of Acianthera subg. Brenesia feature secondary stems with 3-5 internodes, covered by inflated, laterally compressed, and overlapping sheaths, fleshy and sessile leaves, a raceme emerging from the base or the apex of the secondary stem, gaping flowers, with fleshy, hirsute, or papillose sepals that do not fully extend. Members of this subgenus are distributed from Mexico to Panama (Bogarín et al., 2014; Solano, 2015; Karremans and Vieira-Uribe, 2020). So far, four species are recognized in the subgenus: Acianthera costaricensis (Schltr.) Pupulin & Karremans, the type species, distributed in Costa Rica and Panama; A. herrerae from Chiapas (Mexico) and Guatemala; A. johnsonii from Guatemala, Honduras, and El Salvador; and A. sotoana Solano from Oaxaca and Veracruz (Mexico). The latter three have been reported as occurring in Mexico. However, specimens previously believed to represent A. johnsonii in Solano and Soto (2003) have now been determined to correspond to a different, yet undescribed species. Meanwhile, specimens of the true A. johnsonii have been recently collected from the Sierra Madre of Chiapas. The genuine A. johnsonii as well as the misidentified Acianthera species are described and illustrated here.
The aim of the current study was to conduct a review of Acianthera subgenus Brenesia based on specialized literature, specimens deposited in scientific collections, in cultivation, or available in online databases. For each species in the group a morphological description, drawing or photo, information concerning nomenclature, distribution, habitat, phenology, conservation status assessment and comparison with similar taxa are provided.
Materials and Methods
For each taxon nomenclatural information was acquired from type specimens and original descriptions. Historical protologues were accessible from Biodiversity Heritage Library (BHL, 2023). The type specimens were available online from the herbaria AMES, AMO, CR, MO, and US (acronyms according to Thiers, 2023). An online search for additional specimens for the group was carried out through the Global Biodiversity Information Facility (GBIF, 2023), SEINet data portal (SEINet, 2023), and the citizen science network iNaturalista (Naturalista, 2021).
The descriptions for each species were based on cultivated specimens in the living orchid collections of the AMO Herbarium and Soconusco Botanical Garden (El Colegio de la Frontera Sur, Tapachula, Chiapas, Mexico), specialized literature (Luer, 1991, 2004, 2023; Sayers and Du Plooy, 2003; Solano and Soto, 2003; Nelson Sutherland and Ortiz-Kafati, 2007; Solano, 2010, 2015; Karremans et al., 2016), as well as on herborized specimens housed at the herbaria AMES, AMO, B, CR, ECO-TA-H, F, HEM, JBL, LAGU, MO, NY, OAX, SEL, RENZ, TEFH, US, W, and XAL (acronyms according to Thiers, 2023). Line drawings were prepared from fresh material with a drawing tube adapted to a stereomicroscope (Wild Heerbrugg Type 308700, Gais, Switzerland). Additionally, with the authors’ permission, the quality of the photographs presented here were edited with Adobe Photoshop® CS4.
For each species, information concerning nomenclature, distribution, habitat, phenology, conservation status, and representative specimens is provided here. A morphological comparison of each taxon with the other species of Acianthera subg. Brenesia was based on the specialized literature cited above. The known localities for each species were georeferenced and displayed on a map of Mexico and Central America (Fig. 1) using QGIS v. 3.32 software (QGIS, 2023). A key for the Mesoamerican subgenera of Acianthera, as well as for the species of Acianthera subg. Acianthera, was prepared. For each species the conservation status was assessed according to the IUCN Red List Criteria (IUCN, 2019).
Results
Taxonomy
Middle American species of Acianthera belong to three of the following four subgenera according to Karremans et al. (2016). No species of Acianthera subg. Antilla are known to occur in the region.
Key for the subgenera of Acianthera
1a. Stems with 3-5 internodes, covered by laterally compressed, overlapping, inflated sheaths ……………………………………….……………………….. Acianthera subg. Brenesia
1b. Stems with 2 internodes, covered by tubular, adpressed sheaths ………..……………….... 2
2a. Habit erect; flowers star-like; lateral sepals free or united up to 1/3 of their length; petals basally auriculate ………………………………………... Acianthera subg. Kraenzlinella
2b. Habit erect or pendulous; flowers bilabiate; lateral sepals totally united to each other; petals not basally auriculate ……………………………………………………………………….. 3
3a. Leaf margins entire, plants occurring on the mainland throughout the Neotropics ………………………………………………………………. Acianthera subg. Acianthera
3b. Leaf margins denticulate or erose, plants endemic to the Caribbean islands ………………………………………..………………………….. Acianthera subg. Antilla
Acianthera subg. Brenesia (Schltr.) Karremans, Harvard Pap. Bot. 21(2): 184. 2016.
≡ Brenesia Schltr., Repert. Spec. Nov. Regni Veg. Beih. 19: 200. 1923. TYPE: Brenesia costaricensis Schltr.
Rupicolous, terrestrial, or epiphytic, caespitose or short repent herb; rhizome or primary stem short, globose, with 3 internodes, roots cylindrical, flexuous, whitish; secondary stem or ramicaul erect, formed by 3-5 internodes; covered by laterally compressed, overlapping sheaths, inflated towards the apex, scarious, red spotted; leaf elliptic to oblong-elliptic, fleshy, sessile or attenuate at the base into a short conduplicate petiole; inflorescence from the apex or on the basal nodes of the secondary stem, racemose, multi-flowered; floral bracts obliquely funnel-shaped, obtuse, white or green translucent with reddish dots, overlapping; flowers tubular or subglobose, trigonous in trans-section; sepals fleshy, axially carinate, 5-7-veined, cellular-papillose or hirsute-papillose, lateral sepals fused into a concave, bifid synsepal; petals fleshy, subulate to linear-rhombic, 3-veined; lip fleshy, pandurate, unguiculate, 3-veined, lamina with a pair of submarginal calli extended along the middle part of the lip; column slender, cylindrical or semicylindrical, with a well-developed foot; stigmatic cavity and rostellum ventral; anther cap ventral, pollinia 2, ovoid, laterally compressed, yellow, with granulose caudicles; ovary trigonous, subpyramidal, articled to a cylindrical pedicel.
The five species now recognized in this subgenus can be identified with the following key.
Key to the species of Acianthera subg. Brenesia
1a. Leaf sessile; sepals scurfy or glandular-papillose ………………………………………..… 2
1b. Leaf attenuate at the base into a short conduplicate petiole; sepals long hairy or glandular-papillose ………………………………………………………………………….………… 3
2a. Flowers sub-globose in form, whitish in background and densely dark red dotted; sepals scurfy ………………………………………. A. johnsonii (Ames) Pridgeon & M.W. Chase
2b. Flowers tubular in form, yellow in background with red dots; sepals glandular-papillose …………………………….………………. A. rzedowskiarum Solano & Karremans sp. nov.
3a. Sepals mucronate; petals linear-rhombic, acuminate; lip ovate to oblanceolate, without uncinate lobes ………………………………………………………………………………. 4
3b. Sepals conspicuously apiculate; petals subulate-rhombic, long acuminate, with obtuse angles at the middle; lip pandurate, with uncinate lateral lobes …………………………………………….……. A. herrerae (Luer) Solano & Soto Arenas
4a. Raceme <3 cm long; leaves elliptic; lip oblanceolate; plants from Veracruz and Oaxaca (Mexico) …………………………..……………………………………. A. sotoana Solano
4b. Raceme >5 cm long; leaves elliptic to elliptic-ovate; lip ovate; plants from Costa Rica and Panama ………….…………………………. A. costaricensis (Schltr.) Pupulin & Karremans
Acianthera costaricensis (Schltr.) Pupulin & Karremans, Harvard Pap. Bot. 21(2): 184. 2016.
≡ Brenesia costaricensis Schltr., Repert. Spec. Nov. Regni Veg. Beih. 19: 200. 1923.
≡ Pleurothallis lateralis L.O. Williams, Ceiba 5(2): 84. 1956 (replacement name, not P. costaricensis Rolfe, Bull. Misc. Inform. Kew 1917(2): 80. 1917).
TYPE: COSTA RICA. (Alajuela, San Ramón), bois humides à San Pedro de San Ramon, 1200 m, IX.1921, A. M. Brenes 117 (holotype: B (destroyed), lectotype designated in Barringer (1986): AMES-28732!, isolectotype: CR-18505! (photo at AMES!)).
Epiphytic or terrestrial, shortly repent herb, up to 35 cm tall; secondary stem 3-13 cm long, cylindrical, formed by 3-4 internodes; leaf 8-23 × 2-7 cm, elliptic to elliptic-ovate, acute to obtuse, attenuate and conduplicate at the base; inflorescence up to 12 cm long, only from a basal node on the secondary stem, creeping, with up to 15 flowers, arranged perpendicularly to the rachis, most open at the same time, peduncle cylindrical, 2 cm long; floral bracts 5 mm long; flowers 25 mm long, tubular, gaping; sepals and petals yellow or orange with purple dots, lip yellow with purple spots in the basal middle, column white with purple stripes, anther purple; sepals long hairy on both surfaces, the hairs dark purple, apically mucronate, ciliate, 5-veined, abaxially muriculate along the veins, dorsal sepal 16-26 × 5-10 mm, oblong-elliptic, acute, apically recurved, lateral sepals 16-27 × 12-20 mm when spread out, fused along 2/3 of their length into a concave, obovate, deeply bifid synsepal, each one obliquely ovate-elliptic, obtuse; petals 9-11 × 2-2.4 mm, lanceolate-rhombic, long acuminate, ciliate along the upper 2/3; lip 4.5-5 × 2.3-2.5 mm, arching, 3-lobed, ovate, obtuse, lateral lobes erect, rounded, lamina with erect basal margins, channeled between the submarginal calli, and with a pyramidal reddish callus near the base; column 5-7 mm long, 1.5 mm wide, almost straight, clinandrium denticulate at the margin, with an incurved foot column, 3 mm long; stigmatic cavity obovate; rostellum laminar, convex; anther 0.8 × 0.6 mm, ovoid-spheric, pollinia 0.5 mm long; ovary 5 mm long, pyramidal, incurved, glandular pubescent, articled to a cylindrical pedicel, 1 mm long; capsule not seen.
Phenology: this species flowers from June to September.
Distribution and habitat: Acianthera costaricensis seems be restricted to Costa Rica and Panama, making it the southernmost member of Acianthera subg. Brenesia (Fig. 1). The plant is often found growing terrestrially on well-drained road-cuts among mosses or as an epiphyte in cloud forest, at 1000-1900 m elevation.
Taxonomic notes: this species described by Schlechter (1923) was long considered conspecific with Pleurothallis johnsonii Ames, described a few months later (Ames, 1923), and the two taxa have traditionally been treated as nomenclatural synonyms (Ames and Correll, 1952; Garay, 1956; Williams, 1956; Hamer, 1974; Luer, 1977; Pupulin, 2002; Bogarín et al., 2014). However, they were considered different species by Karremans et al. (2016). Acianthera costaricensis is recognized by its large habit, elliptic to oblong-elliptic leaf, attenuate and conduplicate at its base, with the raceme exclusively originating from a basal node of the secondary stem, bearing yellow or orange with purple blotched sepals, covered with long dark- purple hairs on both surfaces, lanceolate-rhombic, long acuminate petals, and ovate lip, with a pyramidal callus near the base (Figs. 2, 3). The most similar species are Acianthera sotoana and A. herrerae, especially in their habit and coloration of the flowers. Table 1 shows the characteristics that allow distinguishing these species.

Figure 2: Photo of the type of Brenesia costaricensis Schltr. in AMES. Reproduced with permission of The Orchid Herbarium of Oak Ames of Harvard University.

Figure 3: Acianthera costaricensis (Schltr.) Pupulin & Karremans. A. habit; B. flower; C. perianth; D. ovary, column and lip in lateral view; E. lip in ventral and lateral views; F. anther cap and pollinaria. Lankester Composite Digital Plate by Franco Pupulin, based on D. Bogarín 11373 (JBL-sprit) from Costa Rica.
Table 1: Summary of the main differences between species of Acianthera subgenus Brenesia (Schltr.) Karremans.
| Character | A. costaricensis (Schltr.) Pupulin & Karremans | A. herrerae (Luer) Solano & Soto Arenas | A. johnsonii (Ames) Pridgeon & M.W. Chase | A. rzedowskiarum Solano & Karremans sp. nov. | A. sotoana Solano |
|---|---|---|---|---|---|
| Leaf form | elliptic to oblong-elliptic | elliptic | elliptic, ovate, or ovate-oblong | elliptic to oblong-elliptic | elliptic |
| Leaf size (length × width) | 8-23 × 2-7 cm | 10-17 × 3.5-6.5 cm | 8-14 × 2.5-5.5 cm | 5-7 × 2-2.5 cm | 10-19 × 4-6 cm |
| Leaf base | attenuate and conduplicate | attenuate and conduplicate | sessile | sessile | sessile |
| Inflorescence origin | basal node on the secondary stem | basal node on the secondary stem | apex of the secondary stem, sometimes from the basal node of secondary stem | apex of the secondary stem, sometimes from the basal node of it | apex of the secondary stem, sometimes from the basal node of it |
| Flores per raceme | up to 15 | up to 7 | up to 9 | up to 7 | up to 10 |
| Flower arrangement | secund, perpendicular along the rachis | secund, subparallel along to the rachis | distichous, perpendicular along the rachis | secund, subparallel along to the rachis | secund (basal raceme) or distichous (apical raceme), perpendicular along the rachis |
| Flower color | yellow or orange, purple dotted | yellow, purple dotted | white, densely red dark dotted | yellow, dark red dotted | yellow-brown to yellow- orange, densely purple dotted |
| Flower form | tubular, gaping | rounded, semi-closed | sub-globose, semi-closed | tubular-ellipsoid, bilabiate | tubular, semi-close |
| Flower size (length) | 25 mm | 20 mm | 12-17 mm | 15-17 mm | 17-21 mm |
| Sepal apex | acute to obtuse, mucronate | acuminate, apiculate | obtuse (dorsal) or acute (lateral), shortly apiculate | acute, apiculate, recurved | subacute, mucronate |
| Sepal ornamentation | purple hairs | glandular-pubescent | glandular-papillose | cellular-papillose | glandular-pubescent |
| Sepal, veins | 5 | 3-5 | 5-7 | 7 | 5-7 |
| Petal form | lanceolate-rhombic, long acuminate, not angled at the middle | subulate-rhombic, long acuminate, prominently angled at the middle | obliquely oblanceolate to oblanceolate-rhombic, acute, not angled at the middle | linear-narrowly rhombic, acuminate, falcate, not angled at the middle | lanceolate-subrhombic, oblique, acuminate, not angled at the middle |
| Lip form | ovate, obtuse | pandurate, apiculate | ovate-pandurate, obtuse to rounded | ovate-pandurate, rounded | cordate-sagittate, acute |
| Lip, lateral lobes | erect, rounded | uncinate | absent | erect, rounded | erect, rounded |
| Lip, basal callus | pyramidal, erect | transverse lamella | V-like, low | ovate, erect | prominent, erect, incurved |
| Distribution | Costa Rica, Panama | Guatemala, Mexico (Chiapas) | Belize, Guatemala, El Salvador, Honduras, Mexico (Chiapas) | Guatemala, Mexico (Chiapas) | Mexico (Oaxaca, Veracruz) |
Conservation status: Data Deficient according to the IUCN (2019) categories and criteria. For a proper assessment of its risk category it is necessary to know if the species persists in the localities reported below, estimate the size and number of populations, identify the risk factors threatening its populations and habitat, and model its distribution to estimate the extent of occurrence and area of occupancy.
Additional specimens examined: COSTA RICA. Province Alajuela, district San Ramón, Las Nubes en San Ramón, 20.XI.1932, A. M. Brenes 156 (AMES, CR); La Paz de San Ramón, 30.VII.1932, A. M. Brenes 35 (NY). Province Guanacaste, district La Cruz, P.N. Guanacaste, Estación Biológica Maritza, cima del volcán Orosí, 1300 m, 21.VIII.2007, J. F. Morales 15522 (CR-INB); loc. cit., M. Grayum and A. Rojas (B, CR-INB). District Liberia, Fila del volcán Cacao, 1400-1520 m, 22.IX.1986, I. A. Chacón 2301 and A. M. Chacón (MO, SEL); SW slope of Cerro Cacao, cordillera de Guanacaste, 1450 m, 10.VIII.2007, M. H. Grayum 12590 and D. García (CR-INB, MO). Canton Bagaces, district Mogote, Parque Nacional Volcán Rincón de La Vieja, 1850-1900 m, recolectadas por I. Chinchilla, 9.II.2015, D. Bogarín 11373 (JBL). Province Heredia, canton Saripiquí, Tierras altas de Sarapiquí, Río Ángel, 29.VIII.1956, L. H. Lankester s.n. (CR). Province San José, canton Tarrazú, district San Lorenzo, ca. 5 km al sureste de Santa Marta, camino a Bajo Reyes, 1475 m, 20.XI.2008, D. Bogarín et al. 5670 (JBL(×2)). Canton Vázquez de Coronado, P.N. Braulio Carrillo, Estación Zurquí, cerca del túnel, 1500 m, 9.VI.1986, I. Chacón 1930 (CR-INB); Zurquí Station, along Sendero Natural, 1680 m, 22.IX.1990, S. Ingram 576 and K. Ferrell (CR). Without locality, 1868, A. R. Endres 96 (W (× 5)); without locality, A. R. Endres s.n. (W); without locality, 24.VIII.1954, L. H. Lankester s.n. (K). PANAMA. Province Coclé, corregimiento Antón, hills north of El Valle de Anton, 1000 m, 29.VI.1946, P. H. Allen 3575 (AMES, MO); in the hills above El Valle, 1000 m, collected 6.III.1976, pressed 8.VIII.1976, C. Luer 1053 et al. (SEL). Province Panamá Oeste, district Chorrera, corregimiento Santa Rita, Santa Rita Ridge, 1200 ft, VI.1951, H. P. Butcher s.n. (AMES).
Acianthera herrerae (Luer) Solano & Soto Arenas, Icon. Orchid. 5-6: 10. 2003.
≡ Pleurothallis herreraeLuer, Lindleyana 6: 100. 1991.
≡ Brenesia herrerae(Luer) Luer, Monogr. Syst. Bot. Missouri Bot. Gard. 95: 255. 2004.
TYPE: GUATEMALA. Province of Quetzaltenango, Zunil, terrestrial in wet, pine-oak forest, 2100 m, collected by J. Herrera in VIII.1987, flowered in cultivation, M. Dix & M. Dix 6522 (holotype: MO-5259587!, isotype: UVAL).
Terrestrial, short repent herb, up to 35 cm tall; secondary stem 3-12 cm long, formed by 3-4 internodes; leaf 10-17 × 3.5-6.5 cm, elliptic, obtuse, attenuate and conduplicate at the base; inflorescence 2.5 cm long, a raceme produced only from the basal node of the secondary stem, creeping, up to 5 simultaneous flowers, peduncle 1 cm long; floral bracts 5 mm long; flowers 20 mm long, rounded semi-closed, arranged subparallel along the rachis; sepals and petals yellow with purple dots, lip yellow; sepals glandular-pubescent on the abaxial surface, ciliate, apiculate, 3-5-veined, dorsal sepal 17-19 × 5-6.5 mm, lanceolate, acuminate, lateral sepals 19-21 × 12 mm when spread out, fused along 2/3 of their length into a concave, obovate, deeply bifid synsepal, each one ovate-lanceolate, acuminate, slightly oblique; petals 14 × 4 mm, subulate-rhombic, long acuminate, 3-veined, ciliate at the middle, with obtuse angles at the middle; lip 7.5 × 4 mm, arching, 3-lobed, pandurate, obtuse, apiculate, verrucose on the adaxial surface, lateral lobes uncinate, antrorse, erect, lamina with low submarginal calli at the middle, and a transverse lamella near the base; with a pair of diminutive, auricled lobes at the base; column 6 mm long, 1 mm wide, arching, clinandrium denticulate at the margin, with a straight foot column, 3 mm long; stigmatic cavity obovate; rostellum laminar, convex; anther 0.8 × 0.6 mm, ovoid-spheric, pollinia 0.5 mm long; ovary 3-4 mm long, pyramidal, glandular-pubescent, articled to a cylindrical pedicel, 3 mm long; capsule not seen.
Phenology: this species flowers from August to December.
Distribution and habitat: so far, Acianthera herrerae is only known from Chiapas in Mexico, and Guatemala (Fig. 1), where it grows as a terrestrial in wet Pinus-Quercus forests, at 2000-2100 m elevation (Luer, 1991).
Taxonomic notes: Acianthera herrerae is recognized by its large habit, the base of the leaf attenuate and conduplicate, creeping raceme produced only from the basal node of the secondary stem, yellow with purple blotched flowers, acuminate, apiculate, and glandular-pubescent sepals, and a conspicuously pandurate lip with uncinate erect lateral lobes (Figs. 4, 5). Acianthera costaricensis and A. sotoana are similar, especially in the size of the plant and the flower coloration. Table 1 shows the characteristics that allow distinguishing these species.
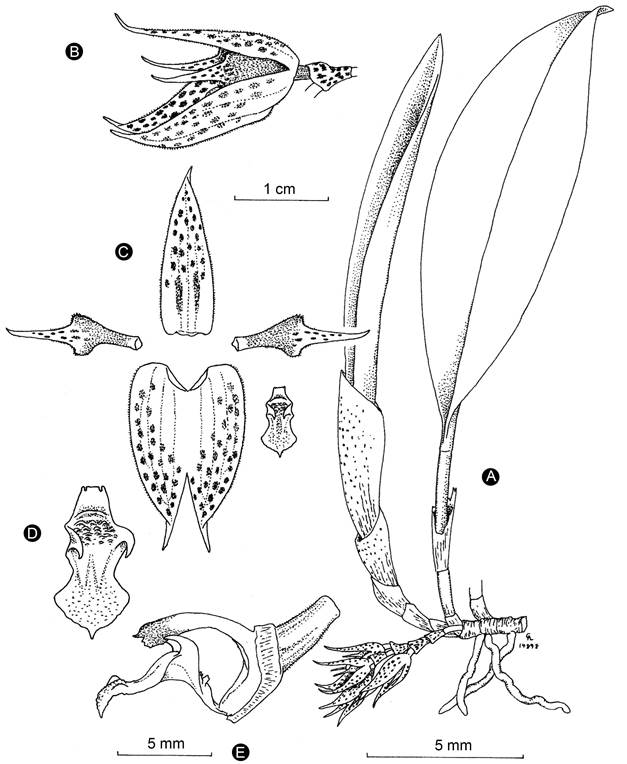
Figure 4: Acianthera herrerae (Luer) Solano & Soto Arenas. A. habit; B. flower; C. perianth; D. Lip; E. Lip, column, and ovary in lateral view. Drawing by Carlyle Luer based on the holotype specimen. Reproduced with permission of the Missouri Botanical Garden.

Figure 5: Acianthera herrerae (Luer) Solano & Soto Arenas. Photograph by Ron Parsons based on a cultivated specimen collected by Moises Behar s.n. in Guatemala.
Conservation status: Data Deficient according to the IUCN (2019) categories and criteria. For a proper assessment it is necessary to know if the species persists in the localities reported below, estimate the size and number of populations, identify the risk factors threatening its populations and habitat, and model its distribution to estimate the extent of occurrence and area of occupancy. Acianthera herrerae is poorly known and represented in scientific collections.
Additional specimens examined: GUATEMALA. Department Quetzaltenango, Zunil, collected by J. Herrera, M. Behar 80 (MO). Department San Marcos, barrancos 6 miles SW of town of Tajomulco, NW slopes of volcan Tajomulco, 2300-2800 m, 26.II.1940, J. A. Steyermark 36687 (F). Department Zacapa, Sierra Las Minas; Zacapa, upper slopes along rio Repollal to summit of mountain, 2100-2400 m, 12-13.I.1942, J. A. Steyermark 42505 (AMES, F).
Other specimen: MEXICO. Chiapas, región del volcán Tacaná, O.Suárez s.n. (photo from a plant cultivated at La Encantada, Oaxaca, Mexico (Suárez, 2004).
Acianthera johnsonii (Ames) Pridgeon & M.W. Chase, Lindleyana 16(4): 244. 2001.
≡ Pleurothallis johnsoniiAmes, Sched. Orch. 2: 21-22. 1923.
≡ Brenesia johnsonii (Ames) Luer, Monogr. Syst. Bot. Missouri Bot. Gard. 95: 255. 2004.
TYPE: GUATEMALA. Alta Verapaz, Chamá to Cobán, 3000 ft, 15.VIII.1920, H. Johnson 901 (holotype: AMES-74369!, isotypes: F-867639!, US-1081162!).
Terrestrial, rupicolous, or epiphytic, caespitose herb, up to 29 cm tall; roots 1.5-2 mm diameter; secondary stem 3-18 cm long, formed by 3-4 internodes; leaf 8-14 × 2.5-5.5 cm, elliptic, ovate, or ovate-oblong, acute, sessile, shortly conduplicate at the base; inflorescence 2.5-5 cm long, generally from the apex of the secondary stem, sometimes from the basal node of the secondary stem, apical raceme reclined on the adaxial surface of the leaf, with distichous flowers perpendicular along the rachis, basal raceme creeping, with secund flowers, rachis with up to 9 flowers, opening successively and eventually all open at the same time, peduncle 1-2 cm long, cylindrical, covered by a spathaceous, conduplicate sheath; floral bracts 3-6 mm long; flowers 12-17 mm tall, sub-globose, semi-closed, sepals whitish in background, but densely red dark dotted, petals, lip, column, and anther reddish; sepals glandular-papillose on both surfaces, concave, shortly apiculate, ciliate, 5-7-veined, dorsal sepal 11.3-14 × 6-8 mm, ovate-lanceolate to elliptic, obtuse, lateral sepals 12-13.3 × 4-6.5 mm, obliquely elliptic to ovate, acute, fused along almost 1/3 of their length into a deeply bifid synsepal; petals 7-8 × 2-3.3 mm, obliquely oblanceolate to oblanceolate-rhombic, acute, minute-ciliate or erose above the middle, glandular-pubescent, 3-veined, with a small auricled projection on the labellar base; lip 5.5-6 × 2.5-4 mm, apparently entire, clawed, arching; claw cuneate, 1 mm long, ovate-pandurate, obtuse to rounded, glandular-verrucose on the adaxial surface, the margins near the base somewhat erect, 3-veined, lamina channeled between the submarginal calli, with a V-like callus near the base; column 4-5 mm long, 1.3 mm wide, slightly arching, clinandrium entire, foot column almost 2-2.5 mm long; stigmatic cavity subquadrate, viscous at the interior, rostellum quadrate, membranous; anther sub-globose, pollinia obovoid; ovary 3-4 mm long, trigonous, glandular-papillose, articled to a cylindrical pedicel, 2 mm long; capsule 1.5 cm long, ellipsoid.
Phenology: this species flowers from May to November.
Distribution and habitat: Acianthera johnsonii is known to occur in Mexico (Chiapas), Belize (Sayers and Du Plooy, 2003), Guatemala, El Salvador, and Honduras (Nelson Sutherland and Ortiz-Kafati, 2007). In Mexico the species is only known from the Sierra Madre of Chiapas, on the Cerro Boquerón and in the El Triunfo Biosphere Reserve, from (914)-1200 to 2400 m elevation. The species grows as a terrestrial, rupicolous, or epiphyte, on humus carpets in Pinus forest, Pinus-Quercus forest, and secondary vegetation derived from cloud forest, often on calcareous substrate.
Taxonomic notes: specimens from the Altos of Chiapas previously determined as A. johnsonii (Solano and Soto, 2003) are described here as a new species. However, its presence in Mexico is confirmed with specimens collected in southeastern Chiapas (Sierra Madre of Chiapas), close to the border with Guatemala. According to Karremans and Vieira-Uribe (2020), A. johnsonii is recognized by its raceme emerging from the apex or base of the secondary stem, reddish, almost globose flowers, and glandular-papillose, non-hairy sepals (Figs. 6, 7). This species is similar to A. costaricensis and in the past they have been considered conspecific (Ames and Correll, 1952; Hamer, 1974; Luer, 1977; Solano and Soto, 2003). However, A. costaricensis differs notably in several traits; A. rzedowskiarum sp. nov. is similar in its habit. Table 1 shows the characteristics that allow distinguishing these species.

Figure 6: Acianthera johnsonii (Ames) Pridgeon & M.W. Chase. Photograph by Anne Damon, based on A. Damon s.n. (ECO-TA-H), from Chiapas, Mexico.

Figure 7: Acianthera johnsonii (Ames) Pridgeon & M.W. Chase. Photograph by Ron Parson from a cultivated specimen.
Garay (1956) considered Brenesia costaricensis as morphologically the same as Pleurothallis johnsonii, and indicated that “Recent collections submitted by Mrs. R(uth) Oberg extend the range of the species to southern Mexico…”, note attached to the holotype sheet of Pleurothallis johnsonii at AMES (Fig. 8). Those specimens have not been located at AMES. However, Mrs. Oberg and Robert Dressler visited San Cristobal de las Casas and from there they went to the Ocotal lagoons (northeast Chiapas), where they collected orchids (Oberg, 1959). Oberg’s specimens probably came from Los Altos of Chiapas and corresponded to A. rzedowskiarum sp. nov., which grows in the periphery of San Cristobal de las Casas. A photo of A. johnsonii was published, as Pleurothallis johnsonii, by Behar and Tinschert (1998: p. 112).

Figure 8: Holotype of Pleurothallis johnsonii Ames. Reproduced with permission of The Orchid Herbarium of Oak Ames of Harvard University.
Conservation status: Data Deficient according to the IUCN (2019) categories and criteria. For a proper assessment of its risk category it is necessary to know if the species persists in the localities reported below, estimate the size and number of populations, and model its distribution to estimate the extent of occurrence and area of occupancy. In Mexico Acianthera johnsonii is known from few localities and is scarcely represented in scientific collections, even though the region where it grows has been well explored floristically in recent years. The known Mexican localities are within a region where some anthropogenic factors put its biodiversity at risk: habitat loss and transformation for shifting and permanent agriculture, cattle ranching, timber extraction, transformation of the traditional coffee plantations into full-sun ones, increase in human settlements, and extraction for illicit wildlife traffic (Solano et al., 2016). A proposal to extend the Tacana Volcano Biosphere Reserve (where ecosystems above 1500 m are well-conserved in comparison with those from lower elevations), connecting it with the Cerro Boquerón (CONANP, 2011), would increase the area protected, the variety of environments, the species that inhabit them, and the biological connectivity of their ecosystems (Solano et al., 2016).
Additional specimens examined: COSTA RICA. Cultivation, sent to the MSBG in X.1975 from C.R. by John Hall s.n. (SEL). EL SALVADOR. Department La Libertad, municipality Quezaltepeque, Boquerón, interior of San Salvador volcano, 1800 m, 3.VIII.1969, F. Hamer 73 (AMES, LAGU, SEL). GUATEMALA. Department Quetzaltenango, locality unknown, 1600 m, 20.VIII.1948, J. Renz 4826 (RENZ). HONDURAS. Department Comayagua, municipality Meambar, Agua Caliente, vaguada de los ríos Chamo y Humuya, 35 km E lago Yojoa, 220 m, 22.XI-31.XII.1980, C. Nelson 6392 et al. (TEFH); Meambar, 610 m, 30.VII.1933, J. B. Edwards 468 (AMES); El Achote, hills above the plains of Siguatepeque, 1350 m, 8.IX.1936, T. G. Yuncker 6398 et al. (AMES, NY); Siguatepeque, km 132 on Tegucigalpa highway, 1372 m, 17.IX.1932, J. B. Edwards 247 (AMES), 248 (AMES, NY). Department Francisco Morazán, municipality Districto Central, 45 km NO de Tegucigalpa, unión de los ríos S. Isidro y Las Moras, 1300 m, 23.VIII.1991, C. Nelson 12025 and R. Andino (TEFH); 45 km NO de Tegucigalpa, quebrada de San Isidro, 5 km N y 2 km O de Zambrano, 1300 m, 10-15.VIII.1991, C. Nelson 11797 and R. Andino (TEFH); 45 km NO de Tegucigalpa, orilla quebrada de La Protección, 3 km S y 5 km O de Zambrano, 1200 m, 12.IX.1991, C. Nelson and R. Andino 12355 (TEFH); 45 km NO de Tegucigalpa, quebrada de Las Moras, 6 km N y 2 km O de Zambrano, 1300 m, 10-15.VIII.1991, C. Nelson 11890 and R. Andino (TEFH). Department Intibucá, municipality Yamaranguila, montaña Camaco en los alrededores de Yamaranguila, 2.VII.1973, C. Bejarano 135 (TEFH). Without locality, cultivated by H. H. Morgan, X.1975, J. Nepple s.n. (SEL); without locality, donated to SEL by George Kennedy, flowered in cultivation, 5.VIII.1976, C. Luer 1042 (SEL). MEXICO. Chiapas, municipality Motozintla, Boquerón - Buenavista, camino Boquerón Buenavista a Finca Hamburgo, A. Damon s.n. (ECO-TA-H). Municipality Siltepec, La Lucha, Reserva de la Biosfera El Triunfo, 21.VII. 2005, H. Reyes-Escobar 46 (HEM). BELIZE. Cayo District, Mount Margaret, 18.IX.1999, B. Sayers 99/775 (MO).
Additional Naturalista observation. HONDURAS. Department Lempira, municipality San Manuel Colohete, 14.48136°N, -88.663696°W, 15.XI.2019, H. Vega s.n. (Naturalista, 2023a).
Acianthera rzedowskiarum Solano & Karremans, sp. nov. Figs. 9, 10, 11.
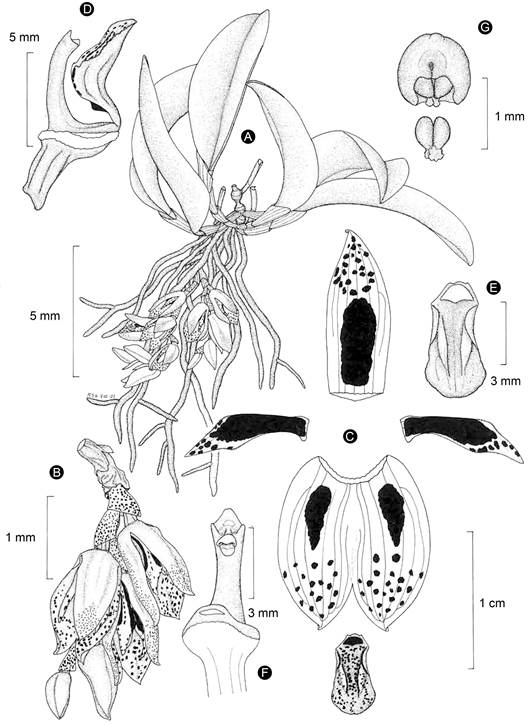
Figure 9: Acianthera rzedowskiarum Solano & Karremans. A. habit; B. inflorescence; C. perianth; D. lip, column, and ovary in lateral view; E. lip; F. column in ventral view; G. anther cap and pollinarium. Line drawing by Rodolfo Solano based on the holotype.
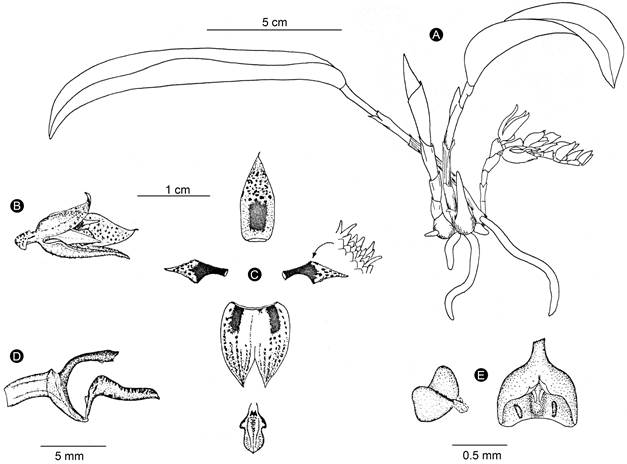
Figure 10: Acianthera rzedowskiarum Solano & Karremans. A. habit; B. flower; C. perianth; D. lip, column, and ovary in lateral view; E. anther cap and pollinarium. Line drawing by Eric Hágsater based on E. W. Greenwood and J. P. Brenan 572 (AMO), from Chiapas, Mexico.

Figure 11: Acianthera rzedowskiarum Solano & Karremans. A. photograph by Rodolfo Solano based on the holotype; B. photograph by Edward W. Greenwood, based on W. R. Thurston 1433 (AMO); C. photograph by Rodolfo Solano based on the holotype; D. photograph by Ron Parson from a cultivated specimen, apparently from Guatemala.
TYPE: MEXICO. Chiapas, Oxchuc, carretera Ocosingo - San Cristóbal de las Casas, entre Ocosingo y Oxchuc, taludes de carretera con vegetación húmeda, ca. 1800 m, 26.VI.1996, M. Soto 7984a (holotype: AMO!).
Species similar to Acianthera johnsonii from which it differs by its smaller plants, yellow blotched flowers with dark red dots, cellular-papillose sepals, linear-narrowly rhombic, acuminate, falcate petals, and shortly 3-lobed, oblong-ovate, rounded lip.
Rupicolous or terrestrial herb, up to 9 cm tall; roots 0.6-0.8 mm diameter; secondary stem 1.5-3 cm long, formed by 3-4 internodes, covered by scarious sheaths; leaf 5-7 × 2-2.5 cm, elliptic to oblong-elliptic, acute, arching, attenuate and sessile at the base; inflorescence generally from the base of the stem, sometimes from the apex, 3-4.5 cm long, basal raceme creeping, with secund flowers, apical raceme reclined on adaxial surface of the leaf, with distichous flowers, rachis with up to 7 flowers, 3-5 open simultaneously, peduncle 5-7 mm long, cylindrical, covered at the base by a spathaceous, conduplicate sheath, 5 mm long, with other tubular, membranous bract, 7 mm long; floral bracts 4.5-5.5 mm long; flowers 15-17 mm tall, tubular-ellipsoid, trigonous in trans-section, semi-closed at the start, progressively opening and becoming bilabiate, sepals yellow, dark red dotted, petals red dark in basal 2/3, yellow in apical 1/3, lip yellow with red dark spots, column dark red with a yellow foot, anther reddish; sepals recurved and apiculate at the apex, axially carinate, glandular-papillose, 5-7-veined, dorsal sepal 12 × 4.4 mm, oblong-lanceolate, acute, channeled, lateral sepals 12.6 × 10.5 mm, fused along 2/3 of their length into an ovate, concave, deeply bifid synsepal, each one elliptic and acute when spread out, forming a chain at their bases; petals 9 × 2.1-2.2 mm, fleshy, linear-narrowly rhombic, acuminate, falcate, widened above the middle, 3-veined, with a small and auricled projection on the labellar base, ciliate above the middle; lip 5.8 × 3 mm, fleshy, arching, 3-lobed, ovate-pandurate, rounded, 3-veined, lateral lobes erect, rounded, lamina channeled between the submarginal calli along the middle part part of the lip, with an ovate erect callus near the base; column 5.5 mm long, 1.5 mm wide, with an arching, cylindrical body, clinandrium trapezoidal, entire; stigmatic cavity subquadrate, viscous at the interior, rostellum quadrate, membranous, recurved at their margins; anther 0.9 mm long and wide, semi-globose, pollinia 0.5 mm long; ovary 3.8-4 mm long, trigonous, glabrous, pedicel terete; capsule 9 mm long, 3 mm wide, ellipsoid, subtrigonous, with ribs and dehiscence lines thickened.
Etymology: the specific name honors the contributions to the knowledge of the Mexican flora during almost six decades by the matrimony of Jerzy Rzedowski and Graciela Calderón de Rzedowski (both recently deceased), affectionately known as the Rzedowski.
Phenology: this species flowers from August to October.
Distribution and habitat: so far, this species is only known from Los Altos (The Highlands) of Chiapas, Mexico. It is likely to occur in Guatemala too. Acianthera rzedowskiarum grows as a rupicolous or terrestrial along road-cuts and cliffs within Pinus-Quercus forests, or in secondary vegetation derived from it, often on calcareous substrate, from 1700 to 2420 m elevation. The species is sympatric with other members of Pleurothallidinae recently described from Chiapas, including Stelis zootrophionoides Castañeda-Zárate & Ramos-Castro and Stelis mirandae Beutelsp. & Mor.-Mol. Interestingly, the currently known distribution of these three species seems to be restricted to Los Altos of Chiapas (Ramos-Castro et al., 2012; García-González et al., 2015; Beutelspacher-Baigts and Moreno-Molina, 2018).
Taxonomic notes: among Acianthera subg. Brenesia this species is recognized by its small habit, creeping inflorescence produced from the stem base, semi-closed yellow flowers with reddish dots, and linear-narrowly rhombic and falcate petals (Figs. 9, 10, 11). The most similar species is A. johnsonii (Figs. 5, 6). Table 1 shows the characteristics that allow distinguishing between both taxa.
Photos of A. rzedowskiarum were previously published. It appeared with the name A. johnsonii in Soto et al. (2007: fig. 0010) and Beutelspacher-Baigts and Moreno-Molina (2018: p. 201), and as an unnamed species in Karremans and Vieira-Uribe (2020: p. 45). Photographs published on iNaturalista (Naturalista, 2023b) show a specimen similar to A. rzedowskiarum recorded at Quiche, Guatemala, which, unlike the specimens from Chiapas, has sepals with larger red spots.
Conservation status: Data Deficient according to the IUCN (2019) categories and criteria. For a proper assessment of its risk category it is necessary to know if the species persists in their localities reported below, estimate the size and number of populations, and model its distribution to estimate the extent of occurrence and area of occupancy. Acianthera rzedowskiarum is known from few localities and is scarcely represented in scientific collections, it may have not been noticed by botanists working in Los Altos of Chiapas or Guatemala, even though these regions have been well-explored floristically. In Chiapas the species grows in a relatively accessible area where the forests persist as remnant fragments and are close to human settlements and the urbanized area of San Cristobal de las Casas. Natural vegetation in this region is facing anthropogenic effects due to the expansion of urban areas, extraction of wood and firewood, opening of lands for agriculture and sheep grazing, and extraction of plants for commercial and/or ritual purposes (García-González et al., 2015; Jiménez-López et al., 2019). Nonetheless, in this region remnant forests are within small ecological reserves where the species can grow. There is no knowledge of the populations of the species and its habitat in Guatemala.
Additional specimens examined. MEXICO. Chiapas, municipality Comitán de Domínguez, I. Moreno-Molina s.n. (photo in OAX). Municipality Huixtán, 30.5 km on road San Cristóbal de las Casas - Ocosingo, 27.X.1977, E. W. Greenwood and J. P. Brenan 572 sub E. Hágsater 5495 (AMO, MEXU, XAL). Municipality San Cristóbal de las Casas, W. R. Thurston 6241 (AMO, XAL); behind Hotel Molino de la Albarrada, 2420 m, 25.X.1975, E. W. Greenwood s.n. (SEL). Unknown locality, D. E. Breedlove 52457 (CAS); unknown locality, E. W. Greenwood 123 (AMO); unknown locality, W. R. Thurston 1433 (photo in AMO).
Additional Naturalista observations. MEXICO. Chiapas, municipality Chamula, San Juan Chamula, Yaalichin, 16.833602°N, -92.680288°W, 26.V.2022, N. Ramírez-Marcial s.n. (Naturalista, 2023c). GUATEMALA. Department Quiché, municipality San Antonio Ilotenango, 15.025308º N, -91.172828º W, 9.IX.2018, L. Diaz s.n. (Naturalista, 2023b).
Acianthera sotoanaSolano, Lankesteriana 9(3): 447-450. 2010.
TYPE. MEXICO. Oaxaca, municipio Totontepec Villa de Morelos, Cañón del Río Toro, sobre el banco del río, 1800 m, colectado IX.1977, prensado 23.VII.1983, O. Suárez sub E. Hágsater 5536 (holotype: AMO!, isotype: AMO!).
Rupicolous or occasionally epiphytic herb, up to 36.5 cm tall; roots 1.2-2 mm diameter; secondary formed by 4-5 internodes, 4-17.5 cm long, 1.6-3.5 mm diameter, covered by scarious sheaths; leaves 10-19 × 4-6 cm, elliptic, obtuse, recurved at the apex, sessile; inflorescence 5-6.5 cm long, up to 10 simultaneous flowers, generally from the basal node of the secondary stem and creeping, sometimes from the apex of the secondary stem and reclined on the adaxial surface of the leaf, peduncle 1-3 cm long, 2 mm diameter, with 2 (basal peduncle) or 5 (apical peduncle) obliquely infundibuliform bracts, obtuse, overlapping, 6-7.7 mm long, the apical peduncle covered at the base by a scarious spathaceous bract; floral bracts 7.9-8.7 mm long; flowers 17-21 mm long, tubular, semi-close, yellowish-brown to yellowish orange, densely purple dotted on both surfaces, petal yellowish-brown with purple dots, lip orange with purple dots, column and anther purple; sepals glandular-pubescent on both surface, hairs dark red, ciliate, apically mucronate, 5-7-veined, dorsal sepal 17-18.5 × 5 mm, lanceolate to oblong-lanceolate, subacute, lateral sepals 18.4-21.5 × 10-13.4 mm, fused along 2/3 of their length into an oblong-lanceolate, concave, deeply bifid synsepal; petals 11.2-12.3 × 2 mm, lanceolate-subrhombic, oblique, acuminate, 3-veined, ciliate along the upper 2/3; lip 5.5 × 2.1 mm when spread out, arching, 3-lobed, conduplicate, cordate-sagittate, acute, lateral lobes erect, rounded, lamina channeled between the submarginal calli, with a prominent, incurved, reddish callus near the base; column 5 mm long, 1 mm wide, slightly arching, with an incurved foot column, clinandrium 3-dentate; stigmatic cavity obovate, rostellum laminar, convex; anther 1 × 0.8 mm, ovoid-spheric, pollinia 0.6 mm long; ovary pyramidal, glandular pubescent, articled to a cylindrical pedicel; capsule not seen.
Phenology: this species flowers between June and December. The fruits begin to develop in October; between December and March they ripe and release the seeds.
Distribution and habitat: endemic to Mexico. The species is known from the southern Sierra Madre Oriental and the Sierra Los Tuxtlas, Veracruz, and Northern Mountains of Oaxaca (Fig. 1). The populations are located between 1125 and 1800 m elevation, in areas with cloud forest where permanent streams are commonly present. The specimens grow on rocks on top of a layer of moss or organic matter, but sometimes they become epiphytes.
Taxonomic notes: the species was previously reported from Chiapas by Solano (2015) based on a sterile specimen (Damon s.n., ECO-TA-H). In the meantime, it flowered and was confirmed to correspond to A. johnsonii instead. Therefore, at this time A. sotoana is exclusively known to occur in northern Oaxaca and central and southern Veracruz. A photo of A. sotoana was included by Flores-Palacios and Valencia-Díaz (2007), under the name Pleurothallis cf. herrerae, from a specimen traded in a market of the city of Xalapa, Veracruz. Acianthera sotoana (Figs. 12, 13) is very similar to A. costaricensis and it has been suggested that both could be conspecific (Karremans and Vieira-Uribe, 2020). Acianthera sotoana can be distinguished from A. costaricensis according to the traits that are compared between both taxa in Table 1.
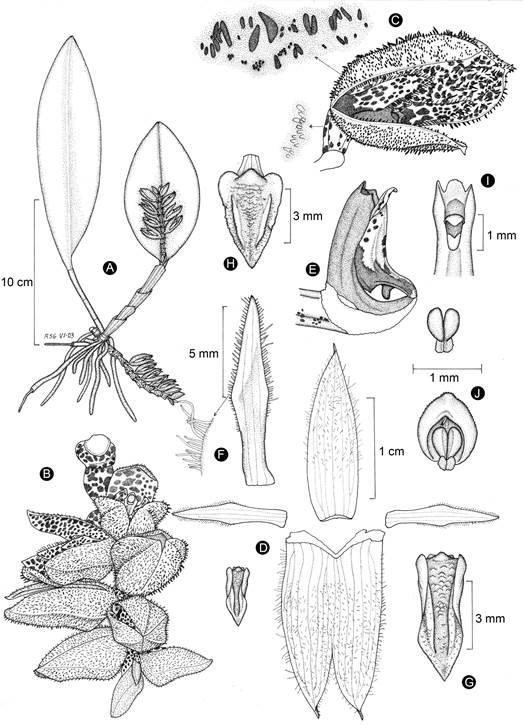
Figure 12: Acianthera sotoana Solano. A. habit; B. inflorescence; C. flower with detail of the dark red hairs on the sepals abaxial surface and glandules on the ovary; D. perianth; E. lip, column, and ovary in lateral view; F. petal with detail of the marginal hairs; G. lip in natural form; H. lip spread out; I. column upper part; J. anther cap and pollinarium. Line drawing by Rodolfo Solano, based on the holotype.

Figure 13: Acianthera sotoana Solano. A-C. photographs by Edward Greenwood based on the type E. W. Greenwood s.n. (AMO); D. photograph by Roberto Castro based on a specimen from Veracruz, Mexico.
Conservation status: Data Deficient according to the IUCN (2019) categories and criteria. For a proper assessment of its risk category it is necessary to know if the species persists in its localities reported below, estimate the size and number of populations, and model its distribution to estimate the extent of occurrence and area of occupancy. Localities of this species are in or close to protected natural areas: Pico de Orizaba-Cofre de Perote National Park (Veracruz), Los Tuxtlas Biosphere Reserve (Veracruz) and Santa María Huitepec Communal Reserve (Oaxaca), where there is still favorable habitat for the species. However, outside these protected areas (especially in Veracruz) the habitat has been severely transformed for shifting and permanent agriculture, cattle ranching, transformation of the traditional coffee plantations into full-sun ones and increase in human settlements. An additional risk factor could be the occasional extraction of specimens for illicit trade, as was reported by Flores-Palacios and Valencia-Díaz (2007).
Additional specimens examined: MEXICO. Oaxaca, municipality Totontepec Villa de Morelos, cañón del Río Toro, IX.1977, E. W. Greenwood s.n. (AMO); cañón del Río Toro, IX.1977, pressed 20.VI. 1981, O. Suárez 566 (AMO), 2500 (OAX); Santa María Huitepec, río Llano, 13.III 2009, R. Solano and A. Martínez s.n. (OAX). Veracruz, municipality Ixhuacán de los Reyes, desviación a Guadalupe Camujapan, 19.X.2008, M. Castañeda-Zárate and S. Ramos-Castro 154 (XAL!); loc. cit., 16.XII.2008, M. Castañeda-Zárate and S. Ramos-Castro 342 (XAL); Guadalupe Camujapan, 19.X.2008, J. Viccón and M. Castañeda-Zárate 199 (MEXU). Municipality Pajapan, cima del volcán San Martín Pajapan, 2.IX.2007, T. Krömer and E. Otto 2954 (MEXU). Municipality Tatahuicapan de Juárez, faldas del volcán Santa Martha, 28.VIII.2008, T. Krömer et al. 3696 (MEXU). Municipality Xalapa de Enríquez, mercado ambulante que se establece los días viernes en Av. Encanto, entre Av. Américas y calle Z, VI.2003, A. Flores-Palacios and S. Valencia-Díaz s.n. (XAL).
Discussion
In this study we adhere to Karremans (2016) in recognizing Brenesia as part of Acianthera, rather than as a distinct genus, as treated by Luer (2023) in volume VII of Flora Mesoamericana. Luer’s treatment of Brenesia encompassed three species: B. costaricensis, B. herrerae, and B. johnsonii. In Flora Mesoamericana, A. costaricensis and A. herrerae were regarded as endemic to Costa Rica and Guatemala, respectively. Meanwhile, A. johnsonii was considered as a variable species distributed from Oaxaca and Chiapas (Mexico) to Panama; this delimitation included what is now recognized as A. sotoana and A. rzedowskiarum. Furthermore, in Flora Mesoamericana the reports of A. johnsonii in Costa Rica and Panama were based on specimens determined under that name at AMES (Brenes 156, Allen 3575, respectively), which we now reassigned to A. costaricensis. Luer passed away in 2019 and the editors of this Flora did not update his treatment for Brenesia (as well as other Pleurothallidiane groups prepared by the author).
The five species recognized here in Acianthera subg. Brenesia extend from central Veracruz and northern Oaxaca (Mexico) to Panama. Acianthera costaricensis represents the southernmost species, A. sotoana the northernmost one and restricted to Mexico, and A. johnsonii as the most widely distributed taxon. The former Mexican specimens initially labeled as A. johnsonii, from the Altos of Chiapas, were inaccurately determined; they, in fact, correspond to the species described here as A. rzedowskiarum, which had been classified under A. johnsonii in previous checklists of Mexican Orchidaceae (Soto et al., 2007; Beutelspacher-Baigts and Moreno-Molina, 2018). Nonetheless, we confirm the presence of A. johnsonii in southeastern Chiapas.
Acianthera subg. Brenesia exhibits a close relationship with Acianthera subgenera Antilla and Kraenzlinella, which form a well-supported monophyletic group occupying the basal branch within Acianthera (Karremans et al., 2016). This highlights the importance of Mesoamerican and Antillean regions in the evolutionary history and diversification of a genus that currently presents a higher richness in Brazil, where slightly under half of the 300 Acianthera species have been documented (Barros et al., 2015). This context makes it interesting for future studies that facilitate estimations of the origin time and the geological events related to the diversification of the genus’ basal lineage.
All species within Acianthera subg. Brenesia were categorized here as Data Deficient, as our knowledge is primarily limited to the number of localities for each species; However, many of them were recorded from specimens collected over 50 years ago in El Salvador, Guatemala, and Honduras, regions we have not visited. We believe that for a thorough assessment and assignment of risk categories, the following steps are imperative: i) validation of each species’ continued presence where recorded; ii) estimation of population number, size, and long-term viability for each taxon; iii) identification of risk factors threatening each species and its habitat; and iv) modelling the distribution of each species to estimate its extent of occurrence and area of occupancy. So, assigning Data Deficient category to each taxon underscores the need for more information and acknowledges the possibility that forthcoming research might be required to determine the extinction risk of these species.











 nueva página del texto (beta)
nueva página del texto (beta)




