Introduction
Mistletoe parasitism constitutes a continuum of host specificity that ranges from specialized to generalized mistletoe-host interactions mediated by generalist and specialist seed vectors to mistletoe dispersal (Gibson and Watkinson, 1989; Reid, 1991; Norton and De Lange, 1999; Mathiasen et al., 2008; Watson, 2013; Okubamichael et al., 2017; Amico et al., 2019). The immediate environment of host trees plays a fundamental role in the life cycle of these parasitic plants. Mistletoes that parasitize multiple host tree species (host range) must confront heterogeneity in their environments (Norton and Carpenter, 1998). However, the consequences of this environmental spatial heterogeneity (hosts) have received little attention despite their ecological and evolutionary importance (Okubamichael et al., 2017). The establishment of hemiparasitic mistletoes is initially mediated by mistletoe-host compatibility (host resistance and mistletoe infectivity; Yan, 1993; Sargent, 1995), their ability to face hosts’ characteristics (Pennings and Callaway, 2002), extract their water and nutrients (Strong et al., 2000; Scalon and Wright, 2015), and the effectiveness of animal vectors to transmit their seeds successfully to one or several host tree species (host specificity; López de Buen and Ornelas, 1999, 2002; Aukema and Martínez del Rio, 2002; Fadini, 2011; Pérez-Crespo et al., 2016a). Phenotypic differences between host tree species entail different intrinsic conditions for the development and growth of mistletoes and eventually affect characteristics of mistletoes’ reproductive structures. Thus, traits that affect mistletoe dispersal (mediated by seed dispersers) and its successful establishment, such as fruit and seed size, would differ among mistletoes growing on different host tree species (Rodríguez-Mendieta et al., 2018; Lara et al., 2021).
The genus Psittacanthus Mart. (Santalales, Loranthaceae), distributed from Baja California, Sonora, and Tamaulipas in Mexico to northern Argentina, is the most species-rich genus of the family in the continent (ca. 110 species; Kuijt, 2009; Ornelas, 2019; Dettke and Caires, 2021). Psittacanthus species are distinguished by their large and bulky haustoria, and by the long colorful flowers presumably pollinated by hummingbirds, and few species by bumblebees and bats (Azpeitia and Lara, 2006; Ramírez and Ornelas, 2010; Guerra et al., 2014; Pérez-Crespo et al., 2016b; Fadini et al., 2018; Diniz et al., 2022), as well as by their large, lipid-rich, one-seeded fruits (Kuijt, 1967, 2018; López de Buen and Ornelas, 1999; Ramírez and Ornelas, 2012). Most fruits of Psittacanthus mistletoes frequently depend on frugivorous birds for seed dispersal (López de Buen and Ornelas, 1999; Lara et al., 2009; Ramírez and Ornelas, 2012; Díaz Infante et al., 2016), and their spatial, often aggregated, distribution patterns within and between hosts are strongly influenced by the foraging behavior of seed dispersers (Monteiro et al., 1992; López de Buen and Ornelas, 1999; Arce-Acosta et al., 2016; Pérez-Crespo et al., 2016a; Guerra et al., 2018). Birds swallow the whole fruit, or it is peeled before being swallowed, removing the epicarp and the sticky viscin (López de Buen and Ornelas, 1999; Lara et al., 2009; Ramírez and Ornelas, 2009). Once the bird has ingested the seed, it is either regurgitated and dropped or wiped on a branch, or gut-processed and defecated; if regurgitated or defecated the seed survives the digestive process with some of the viscin, which allows it to adhere to potential hosts (López de Buen and Ornelas, 1999; Lara et al., 2009; Ramírez and Ornelas, 2009, 2012). Although mistletoe seeds germinate readily in almost all situations, seeds must be freed of the epicarp and deposited by birds on the branches of susceptible host trees to have any chance of establishing (López de Buen and Ornelas, 2002; López de Buen et al., 2002; Lara et al., 2009; Ramírez and Ornelas, 2009; Fadini, 2011; Arruda et al., 2012; Díaz Infante et al., 2016).
Conditions in which mistletoes grow can promote differences in display of reproductive traits associated with the attraction of their seed dispersers and the gut processing of the fruits (Ramírez and Ornelas, 2012; Rodríguez-Mendieta et al., 2018; Lara et al., 2021). For instance, regurgitated fruits of Psittacanthus schiedeanus (Cham. & Schltdl.) G. Don are larger and the main bird consumers take longer to regurgitate seeds as compared to those gut-passed and defecated, while regurgitated seeds do not germinate after 30 days of incubation in the growth chamber (Ramírez and Ornelas, 2009; 2012). Fruit size traits also influence gut-retention time and germination of defecated seeds, in which germination of mistletoe seeds increased with fruit size and gut retention time (Ramírez and Ornelas, 2009). In Psittacanthus calyculatus G. Don., fruit and seed size variation and probability of formation and ripening of the fruit are associated with host species growing in different geographical locations (Rodríguez-Mendieta et al., 2018; Lara et al., 2021). Based on these previous studies, both host species and study site, and its interaction, would significantly affect fitness measures, indicating that mistletoes’ fruit size traits would be affected differently depending on the host species and their site of occurrence. Thus, the aim of this study is to characterize size variation of Psittacanthus schiedeanus ripe fruits growing on different host species in two locations of cloud forest.
Material and Methods
Study species
Psittacanthus schiedeanus is a mistletoe species with orange-to-yellow, self-compatible bisexual flowers organized in triads and pollinated mainly by hummingbirds (Ramírez and Ornelas, 2010). Its ripe fruits are purplish-black, lipid-rich, one-seeded, attached to a cupular pedicel (see definition of cupular pedicel in Suaza-Gaviria et al., 2016) and dispersed by a variety of birds (Fig. 1), frequently by Ptilogonys cinereus Swainson, 1827 (Ptilogonatidae), Bombycylla cedrorum Vieillot, 1808 (Bombycillidae) and Myiozetetes similis Spix, 1825 (Tyrannidae) (López de Buen and Ornelas, 1999, 2001; Ramírez and Ornelas, 2009, 2012). These mistletoes are characteristic of the canopy in the cloud forest edges from northeastern Mexico to the Guatemalan highlands (Ornelas et al., 2016). The host species range of Psittacanthus schiedeanus includes ca. 20 tree taxa native to cloud forests and cultivated host species (López de Buen and Ornelas, 1999; 2002). In central Veracruz, the most severe infections occur on deciduous and evergreen host trees such as Liquidambar styraciflua L. (Altingiaceae), Quercus germana Schltdl. & Cham. (Fagaceae), Platanus mexicana Moric. (Platanaceae) and Acacia pennatula (Schltdl. & Cham.) Benth. (Fabaceae) (López de Buen and Ornelas, 1999, 2002; Cocoletzi et al., 2016, 2020).
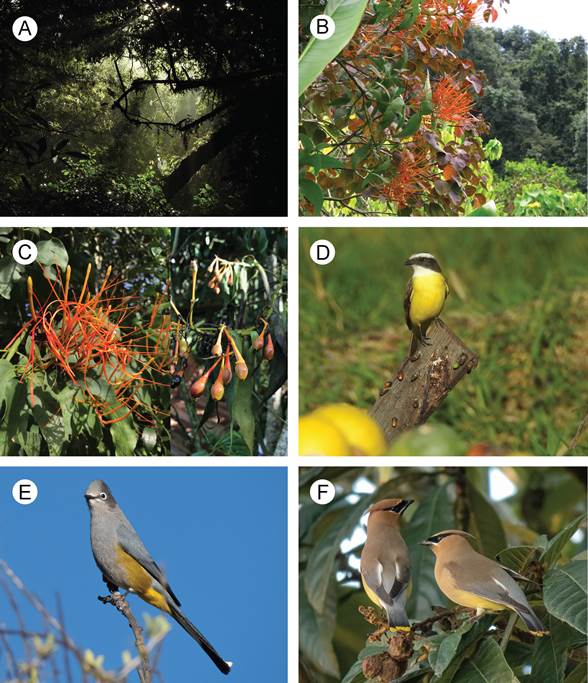
Figure 1: Habitat, morphology, and main seed dispersers of Psittacanthus schiedeanus (Cham. & Schltdl.) G. Don (Loranthaceae) mistletoes. A. habitat in central Veracruz; B. view of cloud forest canopy with some infected trees; C. inflorescence and infructescence of P. schiedeanus; D. Myiozetetes similis Spix, 1825 (Tyrannidae) perched on a tree trunk with germinating mistletoe seeds; E. Ptilogonys cinereus Swainson, 1827 (Ptilogonatidae); F. Bombycilla cedrorum Vieillot, 1808 (Bombycillidae). Photos by Ernesto A. López-Huicochea (A), Juan Francisco Ornelas (B-C), Fernando González-García (D), and Alberto Lobato (E-F).
Plant material
Fruits from Psittacanthus schiedeanus were collected from plants growing on several host tree species located in Tlalnelhuayocan (19º34'47"N, 96º57'38"W; at 1624 m a.s.l.) and Coapexpan (19º31'22"N, 96º58'02"W; at 1392 m a.s.l.) in the state of Veracruz, Mexico (Fig. 2). During the 2013 fruiting season (between January 9 and February 7), we collected 10-20 ripe fruits (Fig. 3A) from 86 individual mistletoes growing on different tree host species at the two localities (n=1619 ripe fruits, 627 from Tlalnelhuayocan and 992 from Coapexpan).
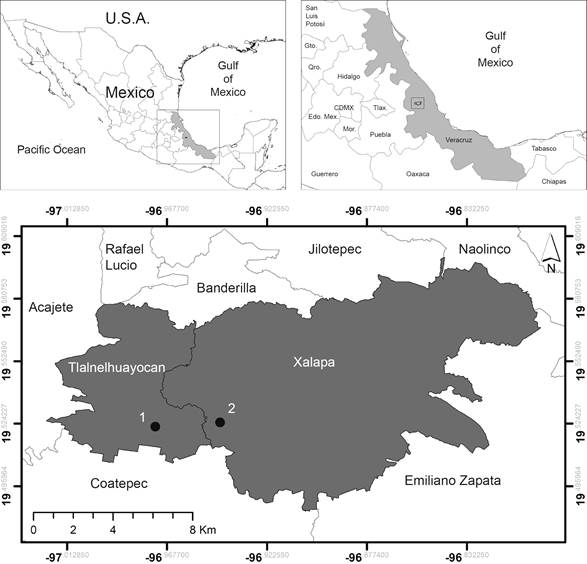
Figure 2: Location of sampling sites (1) Tlalnelhuayocan and (2) Coapexpan in central Veracruz, Mexico.

Figure 3: Fruits of Psittacanthus schiedeanus (Cham. & Schltdl.) G. Don (Loranthaceae). A. triads of fruits at various maturation stages. Note fruit color changes from green when immature but fully formed to purplish-black when ripen and the enlarged, brightly colored cupular pedicel that might serve the same function of attracting birds. Black arrows indicate developing unripe fruits of different sizes and white arrows ripe black fruits used for the analysis; B. detail of developing unripe and ripe fruits. Scale bar=1 cm. Photos by Juan Francisco Ornelas.
Fruit measurements
The fruits were taken to the molecular laboratory (LAMOLE) at Instituto de Ecología, A.C. to measure the length of the cupular pedicel and the lengths and widths of ripe fruits with a digital calliper (Mitutoyo, 500-173-30, Kawasaki, Japan) with a precision of 0.1 mm and individually weighed with an analytical balance (OHAUS PioneerTM, error 0.01 g, PA214, New Jersey, USA).
Data analysis
Fruit size differences among mistletoes growing on different host species were tested with one-way ANOVAs to determine the effects of host species on fruit size variation and Tukey’s pairwise tests to evaluate species differences in PAST v. 4.07 (Hammer et al., 2001). The models included host species and fruit size traits (length of the cupular pedicel, fruit length, fruit width, fruit weight) as response variables.
Results
The Psittacanthus schiedeanus fruit is ovoid (14.7 ( 10.2 mm), with a prominent cupular pedicel (22.4 mm in length; Table 1). All immature fruits are green that pass through a red or purple stage before becoming blackish or purplish-black at ripening (Fig. 3A). The enlarged, brightly colored cupular pedicel contrasts the purplish-black fruit (Fig. 3B).
Table 1: Fruit size differences among Psittacanthus schiedeanus (Cham. & Schltdl.) G. Don plants growing on different hosts in the municipalities of Coapexpan and Tlalnelhuayocan, Veracruz, Mexico.
| Host species | Family | n | Cupular pedicel length (mm) | Fruit length (mm) | Fruit width (mm) | Fruit weight (g) |
|---|---|---|---|---|---|---|
| Coapexpan | ||||||
| Liquidambar styraciflua L. | Altingiaceae | 290 | 20.947±±2.957e | 14.678±0.985ad | 10.425±0.690c | 0.932±0.159cd |
| Quercus germana Schltdl. & Cham. | Fagaceae | 32 | 23.243±3.248acd | 15.115±1.044ad | 10.578±0.648ac | 0.961±0.177acd |
| Acacia pennatula (Schltdl. & Cham.) Benth. | Fabaceae | 431 | 23.596±3.674a | 14.416±1.115a | 10.241±0.855a | 0.883±0.188a |
| Leucaena leucocephala (Lam.) de Wit | Fabaceae | 179 | 22.317±3.683c | 15.078±1.700c | 10.584 ±0.769c | 0.997±0.188e |
| Crataegus mexicana Moc. & Sessé ex DC. | Rosaceae | 60 | 18.655±2.581b | 13.190±1.423b | 9.189±0.577b | 0.737±0.156b |
| Tlalnelhuayocan | ||||||
| Liquidambar styraciflua L. | Altingiaceae | 418 | 22.702±2.997c | 15.055±1.334d | 10.293±0.810a | 0.944±0.173d |
| Ostrya virginiana (Mill.) K. Koch. | Betulaceae | 80 | 26.700±3.117d | 15.025±1.221d | 10.147±0.706a | 0.915±0.018ad |
| Psidium guajava L. | Myrtaceae | 70 | 20.839±3.386e | 14.388±1.356a | 9.971±0.632b | 0.886±0.896ad |
| Quercus laurina Bonpl. | Fagaceae | 59 | 19.562±2.084b | 13.540±0.705bc | 9.572±0.360b | 0.736±0.072be |
| One-way ANOVA | F 8,1618=50.30 | F 8,1618=28.06 | F 8,1616=28.73 | F 7,1613=25.13 | ||
| P<0.0001 | P<0.0001 | P<0.0001 | P<0.0001 | |||
| Coapexpan | 992 | 22.280±3.697 | 14.560±1.297 | 10.304±0.833 | 0.911±0.187 | |
| Tlalnelhuayocan | 627 | 22.708±3.496 | 14.834±1.356 | 10.170±0.777 | 0.915±0.174 | |
| Total | 1619 | 22.446±3.625 | 14.666±1.326 | 10.252±0.814 | 0.913±0.182 |
A total of 1619 mistletoe fruits were measured. These mistletoe fruits were collected from plants growing on Acacia pennatula (n=431), Liquidambar styraciflua (n=290), Leucaena leucocephala (Lam.) de Wit (n=179), Crataegus mexicana Moc. & Sessé ex DC. (n=60), and Quercus germana (n=32) in Coapexpan, and on Liquidambar styraciflua (n=418), Ostrya virginiana (Mill.) K. Koch. (n=80 fruits), Psidium guajava L. (n=70) and Quercus laurina Bonpl. (n=59) in Tlalnelhuayocan (Table 1). Liquidambar styraciflua was the only host tree species found in both localities.
On average, mistletoes on Liquidambar styraciflua and Ostrya virginiana produced heaviest, longest and widest fruits at Tlalnelhuayocan (Fig. 4A-C), whereas in Coapexpan the heaviest, longest and widest fruits were from mistletoes on Quercus germana and Leucaena leucocephala, as compared to those on the other host species (Table 1). Fruits with longest cupular pedicels were found on plants growing on Acacia pennatula and Quercus germana in Coapexpan and Ostrya virginiana and Liquidambar styraciflua in Tlalnelhuayocan (Fig. 4D), and the smaller fruits were found generally on cultivated host trees (e.g., Crataegus mexicana, Psidium guajava; Table 1).
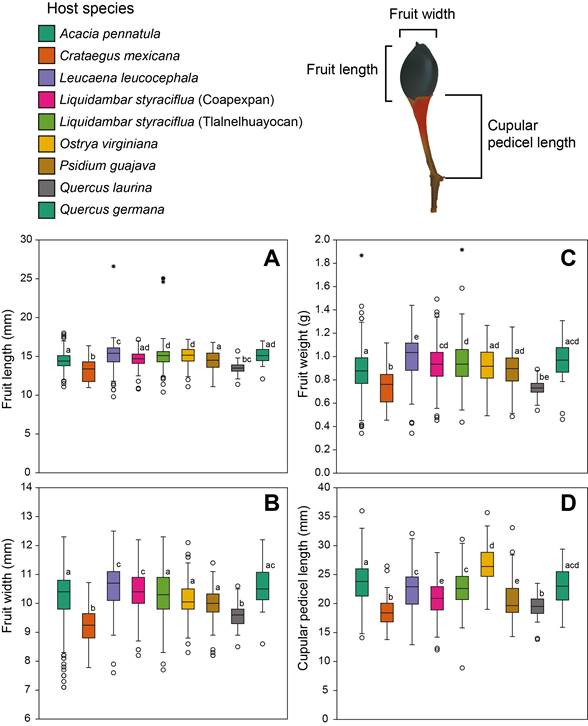
Figure 4: Effects of host species on Psittacanthus schiedeanus (Cham. & Schltdl.) G. Don fruit size measurements taken from fresh ripe fruits. A. fruit length (mm); B. fruit width (mm); C. fruit weight (g); D. cupular pedicel length (mm). Letters indicate significant differences (P<0.05) after post hoc mean comparisons (Tukey’s test). Illustration by Julieta Ornelas Peresbarbosa.
Discussion
Effects of host species on fruit size
In this study, we characterized size variation of Psittacanthus schiedeanus fruits growing on different host species in two populations. We found mistletoes growing on naturally distributed hosts (Liquidambar styraciflua, Quercus germana, Ostrya virginiana, Acacia pennatula) produced larger fruits than those on cultivated host species (Crataegus mexicana, Psidium guajava). These results suggest that reproductive responses of mistletoes (fruit size traits) are affected by host species, and these host-associated differences can promote differences in display associated with the attraction of their mutualistic seed dispersers and the consumption and gut processing of the fruits (Ramírez and Ornelas, 2009, 2012; Rodríguez-Mendieta et al., 2018; Lara et al., 2021).
Variation in fruit size of Psittacanthus mistletoes has been reported among geographically isolated populations (e.g., Díaz Infante et al., 2016) and among mistletoes growing on different host tree species (Lara et al., 2009; Ramírez and Ornelas, 2009; Rodríguez-Mendieta et al., 2018; Lara et al., 2021). Fruits (and cupular pedicels) of P. schiedeanus are larger than those of P. calyculatus (Lara et al., 2009; Ramírez and Ornelas, 2009; Rodríguez-Mendieta et al., 2018; Lara et al., 2021) and P. auriculatus Eichler (Díaz Infante et al., 2016; Pérez-Crespo et al., 2016b). However, host-mediated effects on mistletoes’ fruit external morphology need to be further evaluated in other Psittacanthus species. For instance, fruit size variation could relate to selection and handling by fruit consumers to mediate seed dispersal. That is, larger fruits and seeds would have a greater potential for dispersal and successful establishment, assuming that these have more resources for haustorium development and subsequent interaction with the host (e.g., Gonzáles et al., 2007; Ramírez and Ornelas, 2009, 2012). Although fruits collected from two localities and from different host species showed significant differences, the differences within sites were numerically small. However, larger fruits may be preferentially taken by frugivores with longer gut passage rates, achieving a greater likelihood of seed attachment, seed germination and seedling survival on most preferred host species and for long-distance seed dispersal (Ramírez and Ornelas, 2009; 2012).
Host quality and generalist mistletoes
Psittacanthus schiedeanus is a generalist mistletoe species (host species range) that can parasitize multiple tree host taxa considered seasonally deciduous and evergreen species (López de Buen and Ornelas, 1999; 2002). The leaf phenology differences among the sampled host tree species (deciduous vs. evergreen), and thus their physiology and availability of resources to mistletoes, might explain in part the observed fruit size variation in P. schiedeanus mistletoes (Table 1), which are evergreen, regardless of the foliar habit of their hosts. The phenology of deciduous and evergreen host tree species implies that they have different strategies to regulate water balance and resource allocation in the plant, potentially affecting the attached fruiting mistletoes (Escher et al., 2004; Takashima et al., 2004).
Cocoletzi et al. (2020) investigated the physiological performance during development of P. schiedeanus on deciduous (Liquidambar styraciflua) and evergreen (Quercus germana) host trees in a cloud forest in eastern Mexico. They found that P. schiedeanus mistletoes decreased the photosynthetic reactions of carbon metabolism in L. styraciflua, whereas photosynthetic light reactions and nutritional status decreased in Q. germana. That is, P. schiedeanus usurped photosynthetic resources from its host, being notably more pronounced in the deciduous than evergreen species (Cocoletzi et al., 2020). Further comparative studies on fruit size variation in Psittacanthus generalist species might need increasing the sampling of additional seasonally deciduous and evergreen host tree species, accompanied with environmental information (moisture, luminosity) as well as nutritional and leaf phenology information (soil type, deciduousness), particularly during months of fruit production of P. schiedeanus preceded by leaf senescence in deciduous L. styraciflua.
Fruit size traits and seed dispersers
Beyond the abundance and characteristics of the host, explaining fruit size trait variation (resources and genetic variation) in P. schiedeanus (host/mistletoe interactions), host-associated differences in fruit size traits might influence interactions with seed dispersers that determine the fate of the seeds (López de Buen and Ornelas, 1999; Rodríguez-Mendieta et al., 2018). For instance, host-associated differences in fruit size traits affect its attractiveness and consumption by seed vectors (Ramírez and Ornelas, 2009; 2012) and, eventually, might induce reproductive isolation of mistletoe populations infecting different host species (Rodríguez-Mendieta et al., 2018; Lara et al., 2021). The enlarged, brightly colored cupular pedicel in contrast to the purplish-black fruit might serve the same function of attracting birds.
Although numerous bird species consume mistletoe fruits of P. schiedeanus, Bombycilla cedrorum, Ptilogonys cinereus, and Myiozetetes similis are the main consumers and the most efficient seed dispersers in the study region, because they regurgitate or defecate complete viable seeds singly in safe sites, and those seeds germinate in about one week (López de Buen and Ornelas, 1999). From upper branches, B. cedrorum and P. cinereus defecate complete and viable P. schiedeanus seeds that fell on branches or to the ground. In contrast, M. similis regurgitates and drops the seeds, or wipes them on a branch, after ingestion. Because of this behavior, M. similis would be the disperser more likely to deposit the seeds on the branches (López de Buen and Ornelas, 1999, 2001). Liquidambar styraciflua is the most preferred host species by these three bird species; more birds visited L. styraciflua more often than would be expected by its local abundance, infested trees and mistletoe abundance (López de Buen and Ornelas, 1999). Residence time on the most abundant host species is three times higher for B. cedrorum (30 min) than that for P. cinereus (9 min) (López de Buen and Ornelas, 1999). Also, B. cedrorum birds defecate more mistletoe seeds and more seedlings survived onto branches of the most frequently infected host tree species, L. styraciflua, than those on other frequently infected host tree species (López de Buen and Ornelas, 1999, 2002). Moreover, gut retention times of P. schiedeanus mistletoe fruits collected from L. styraciflua were longer when consumed by P. cinereus (ca. 30 min), but germinated faster (11 days), as compared to those gut-processed and defecated by B. cedrorum (ca. 27 min, 13 days; Ramírez and Ornelas, 2009). Although gut passage probably assists in separation of the large seed from its epicarp and viscin layer, and assimilation of large amounts of lipids that inhibit germination in P. schiedeanus fruits, the effects of fruit size differences by host species, foraging behavior and gut retention rates of the different interacting seed dispersers on seed germination and seedling establishment need to be evaluated further using fruit choice experiments (see also Ramírez and Ornelas, 2009; 2012).
Conclusions
We describe variation in fruit size traits of Psittacanthus schiedeanus infecting different host tree species. Although these differences might vary across space (Díaz Infante et al., 2016; Rodríguez-Mendieta et al., 2018; Lara et al., 2021), consistent fruit size differences may be the result of host differences in architecture and canopy density, leaf phenology and host-resource differences (i.e., conditions under which the hemiparasites are growing; ‘host quality’ hypothesis; Watson, 2009; Cocoletzi et al., 2020). Further study on fruit selection and fruit handling by seed dispersers is needed to discuss whether the observed fruit size variation correlates with fruit selection and handling by main consumers, and with successful attachment and establishment to host branch after gut passage.











 nueva página del texto (beta)
nueva página del texto (beta)



