Introduction
It is estimated that around 50% of wetland area has been lost globally, with an even greater loss in some regions: the United States of America (53%), Australia (>50%), China (60%), New Zealand (>90%), and Europe (>90%; Mitsch and Gosselink, 2015). In Mexico, 6,968,452 ha of wetlands have been lost in the last 40 years; equivalent to 62.1% of the original area of these ecosystems (Landgrave and Moreno-Casasola, 2012). The principal causes of the deterioration and loss of wetlands in Mexico are demographic growth, urban and industrial growth, tourism developments, pollution, and the expansion of the area used for agricultural and cattle ranching activities (Moreno-Casasola, 2008). Raising livestock is an important economic activity throughout Mexico, and has become one of the main causes of the deterioration and destruction of natural resources (Guevara and Lira-Noriega, 2004). As currently practiced, it has a significant, negative impact on ecosystems, which has been documented for two of the most biologically diverse states in the country: Chiapas and Veracruz (Toledo, 1988). Between 1980 and 2020, livestock production in the state of Veracruz increased by ca. 55% due to the demand demand for beef and its price, as well as the economic needs of the population (SIAP, 2021). At present, Veracruz is the state with the highest cattle production in Mexico (Rodríguez-Medina et al., 2017; SIAP, 2021).
On the coast of Veracruz, raising livestock is a very common activity carried out in different ecosystems, such as dunes, freshwater swamps and marshes (Moreno-Casasola et al., 2016). Several types of coastal wetlands are used as flooded pastures, including mangroves, freshwater swamps, and marshes locally known as popales and tulares (Moreno-Casasola and Infante-Mata, 2010). Fortunately, the mangroves are now legally protected, so it is no longer permitted to cut them down to turn them into pastures (Moreno-Casasola et al., 2018). However, other types of wetlands are still used for cattle production. The freshwater swamps on the floodplains of Veracruz are dominated mainly by tree species such as Pachira aquatica Aubl., several species of Ficus L. and Annona glabra L.(Moreno-Casasola and Infante-Mata, 2010).
Annona glabra is native from the America’s tropical and subtropical regions, is distributed from the southern United States of America (Florida) to South America and the Caribbean (Niembro-Rocas et al., 2010), and is considered invasive in Australia (Setter et al., 2004). Freshwater swamps dominated by this species are distributed mainly in the vicinity of coastal lagoons and on depressional floodplains where there is no marine influence, and the water currents are calmer and quite shallow (Niembro-Rocas et al., 2010; Moreno-Casasola et al., 2012). These kind of swamps are most affected by the increase in the livestock frontier, since they remain flooded for the shortest length of time (Moreno-Casasola et al., 2018; González-Nochebuena, 2021) and because, unlike mangrove areas, these swamps have little or no salt stress (López-Rosas and Moreno-Casasola, 2022). In addition, they have fertile, organic soils, so it is common for them to be cut down for their wood, and then used to establish pastures (Moreno-Casasola et al., 2012, 2018; Espejel et al., 2016). This is how, from the 1970s to the present, the area of freshwater swamps in Veracruz has drastically decreased, almost leading to their disappearance, and leaving flooded pastures in their place with some isolated trees that were spared when the original forest was cut (Infante-Mata, 2011).
After a freshwater swamp is cleared, it is common for ranchers to plant exotic grasses, generally of African origin, to feed the livestock (López-Rosas et al., 2019). This results in the transformation and alteration of plant communities that are reflected in changes in the floristic composition and the physicochemical characteristics of the soil and water (Travieso-Bello et al., 2005; Rodríguez-Medina and Moreno-Casasola, 2013). These changes hinder the natural regeneration of the wetland. For that reason, and in order to partially mitigate the effects of wetland loss, some ecological restoration projects have been implemented in marshes and swamps. For example, at the La Mancha Coastal Research Center (CICOLMA) on the coast of central Veracruz, the ecological restoration of a popal invaded by the C4 African grass Echinochloa pyramidalis (Lam.) Hitchc. & Chase, was carried out (López-Rosas et al., 2005, 2019). On the other hand, restoration activities are also underway in a Pachira aquatica freshwater swamp in the Ciénaga del Fuerte Protected Natural Area in Tecolutla, Veracruz, where an increase in cover by the grass Leersia hexandra Sw. has become problematic (Vázquez-Benavides et al., 2020; Sánchez-Luna et al., 2022). Thus, the ecological restoration of degraded wetlands makes it possible to recover their functionality and the ecosystem services that these ecosystems provide. Before carrying out restoration projects, it is important to run experiments to test different approaches, evaluate the results (Ponzio et al., 2019), and keep track of the economic investment to reduce costs. In this context, the objectives of this study were to: 1) evaluate the percent survival and growth (height and stem diameter) of Annona glabra seedlings subjected to different experimental restoration treatments in a freshwater swamp transformed into a pasture and invaded by the exotic grass Echinochloa pyramidalis; 2) describe changes in the accompanying vegetation in the treatments used; and 3) estimate the cost of ecological restoration per seedling, per restoration technique, and per hectare.
Materials and Methods
Study area
The study was carried out in a coastal area in central Veracruz, Mexico, in the municipality of Actopan (between 19°33'19'' and 19°33'13''N; and between 96°22'41'' and 96°22'45''W; Fig. 1). This area is located in a Private Conservation Area belonging to the “Residencial Ecológico Diada La Mancha”, on Federal Highway 180 Veracruz - Poza Rica between kilometers 193 and 195, south of the La Mancha Lagoon. The study area is located between mangroves and coastal dune communities, separated by a dirt road. The dunes are covered by grassland, medium forest, low deciduous forest, and fragments of swamp dominated by Annona glabra in the lower areas. The study area underwent a land-use change approximately 50 years ago when cattle and the forage grass E. pyramidalis were introduced (Sánchez-García, 2020). In 2018, before setting up the experiment, cattle was excluded from the property, as a condition for the decree to create the Private Conservation Area (SEDEMA, 2018).

Figure 1: Location of the study area and assignment of treatments in the flooded pasture in the municipality of Actopan, in the state of Veracruz, Mexico. A. distribution of the experimental blocks; B. distribution of the 16 quadrats subjected to the 15 treatments + one control within each block.
The study area is currently a flooded grassland dominated by the exotic African grass E. pyramidalis (Fig. 1). To a lesser extent there are also native hydrophytic species in the area, such as Pontederia sagittata C. Presl, Typha domingensis Pers., Thalia geniculata L., Echinodorus paniculatus Micheli, and different species of sedges, as well as isolated trees of A. glabra from the original swamp (Sánchez-García, 2020).
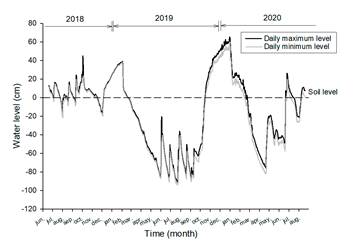
The hydroperiod, one of the main environmental factors defining type of wetlands, was monitored with a HOBO® model U20L sensor (Level Loggers; ONSET, Cape Cod, Massachusetts, USA) to measure the water level (Fig. 2). The level of wetland flooding increases at the beginning of the rainy season in June and reaches its highest in the “nortes” season (November-February), when the strong northerly cold fronts cause the mouth of the lagoon to close, and with the arrival of surface runoff and subsurface freshwater from the Sierra de Manuel Díaz and the coastal dunes (Yetter, 2004).
Experimental design
The restoration treatments were carried out in nine 10 × 10 m blocks evenly distributed throughout the study area (Fig. 1A). Within each block, 16 quadrats measuring 1.5 × 1.5 m were delimited, with a separation of 1.33 m between adjacent quadrats (Fig. 1B). A randomized block experimental design was applied with a random assignment of 15 treatments and one control in each block (nine replications).
Before the start of the experiment, vegetation was cut with a machete to ground level in all quadrats of each block. The 15 treatments were the result of the combination of two factors: restoration technique (five levels) and the pretreatment of A. glabra seedlings (three levels).
The levels of the restoration technique were:
1. No modification: the vegetation that regrew was maintained.
2. Plastic cover: the vegetation was covered at ground level with a thick, black plastic sheet.
3. Pontederia L.: ten individuals of P. sagittata approximately 50 cm in height were sown, systematically distributed in the quadrat to compete with the grass. The plants were obtained from a population that is next to the study area. After sowing, the plants were allowed to acclimate for one month. Dead individuals were replaced until ten individuals had established. Pontederia sagittata was selected because it is common and dominant in marshes, and it has been reported as accompanying herbaceous vegetation within swamps dominated by A. glabra in the state of Veracruz (Infante-Mata et al., 2011; Moreno-Casasola et al., 2017).
4. Soil removal: the soil was removed to a depth of 37 cm, cutting the roots and rhizomes found at that depth until a uniform and porous soil consistency was achieved.
5. Raised soil level: the ground level was raised by forming a 20-cm-high mound of soil to lower the water level and reduce the flooding time in these experimental quadrats.
Once the restoration techniques had been applied, in September 2019, four A. glabra seedlings were transplanted into each quadrat. The origin or pretreatment of these seedlings was the second experimental factor, called seedling pretreatment, and had three levels:
1. Nursery seedlings without fertilizer (NNF): propagules (seeds and small seedlings) of A. glabra were collected from a freshwater swamp located near the study area and transferred to a rustic nursery nearby. The propagules were grown under nursery conditions, and from them, after approximately eight months, seedlings were obtained. These seedlings were watered weekly without any type of fertilizer. The experiment began with seedlings of average height of 43.3±1.0 cm and diameter of 5.1±0.1 mm.
2. Nursery seedlings with fertilizer (NWF): with these seedlings the same procedure was carried out as in the previous treatment. However, starting at sixth months of age these seedlings were sprayed weekly with foliar fertilizer (Gro-green©, GRO GREEN fertilizantes, S.A. de C.V., Gómez Palacio, Mexico). The experiment began with seedlings of average height of 42.1±1.0 cm and diameter of 5.2±0.1 mm.
3. Wetland seedlings: A. glabra seedlings were collected from two sites (to obtain the desired number of seedlings): i) a freshwater swamp dominated by A. glabra located in CICOLMA, and ii) a freshwater swamp located near the study area. The seedlings were transferred haphazardly to the same nursery. They were allowed to acclimate for three months. The experiment began with seedlings of average height of 60.2±1.9 cm and diameter of 9.0±0.3 mm.
In addition to the 15 quadrats with the different treatments, there was a control quadrat in each block. The control consisted only of cutting the vegetation to ground level at the beginning of the experiment without sowing any A. glabra seedlings. The control quadrats were used to register if the sown seedlings had influenced the regrowth of the accompanying vegetation and allowed comparison between them and those in the treatments.
In the study region, the agricultural sowing period begins with the rainy season (June-July; E. A. Sánchez-García, personal observation). However, 2019 was an extremely dry year and there was a delay in the onset of the rains (Fig. 2), which is why the experiment began in September. From October 2019 until February 2020, the number of surviving seedlings was recorded monthly, as were their height and diameter. In addition, the vegetation that colonized each experimental quadrat was also recorded (species composition, cover, and average height of each species). Due to the mobility restrictions imposed during the COVID-19 pandemic, sampling was suspended for six months. A final sampling was carried out in September 2020.
Data analysis
Seedlings
To evaluate the main effects of the restoration technique and the pretreatment of seedlings, as well as their interaction on the proportion of surviving seedlings at the end of the experiment, a deviance analysis was carried out using a generalized linear model (GLM) with a binomial error distribution and link logit function. When the analysis detected significant differences, Tukey’s multiple comparison tests were used to determine the differences between treatments. In addition, the effect of the treatments on the change in height and diameter at the end of the experiment of the seedlings that survived was evaluated using a two-way analysis of variance (ANOVA), where the factors were restoration treatment and seedling pretreatments, and its interaction. The response variable was the difference between the initial and final height and the difference between the initial and final diameter of each seedling. Before carrying out the analysis, the values were verified to meet the residuals’ Normal distribution assumption of the test. When the analysis detected significant differences, Tukey’s test was run to determine the differences between treatments. All analyses were run in R v. 3.6.1. (R Core Team, 2019).
Vegetation
Total species richness was calculated (for the entire set of treatments), as was richness for each treatment. Each species recorded was classified based on its affinity to aquatic environments following Lot’s (2015) catalogue of flora and vegetation of Mexican wetlands. Species were classified as follows:
Aquatic: species that complete their life cycle under flood conditions where plants are submerged, partially emerging from the water, or floating on the surface. Most of these species do not survive in terrestrial environments, even for short periods.
Semi-aquatic: species that spend most of their life cycle in saturated soils but that can survive in soils with low humidity or that are dry during some time; a period in which the reproduction generally takes place.
Tolerant: these species complete most of their life cycle in a dry environment but can temporarily withstand flooding, and can even be partially submerged during the rainy season.
Terrestrial: species that complete their entire life cycle in a dry environment with no water saturation. Most of these species do not survive flooding.
In addition, the Relative Importance Value (RIV) of the plant species present in the quadrats was calculated for October 2019, February 2020, and September 2020 following the methodology of Moreno-Casasola and López-Rosas (2009). To calculate the RIV, the relative density of the species was not considered, since these are species with clonal growth, for which it is difficult to distinguish individuals. The RIV was calculated using the following formula:
Where: CRi = Relative cover of species i = (Absolute cover of species i / Sum of the absolute cover of all the species) × 100.
ARi = Relative height of species i = (Mean height of species i / Sum of the mean heights of all the species) × 100.
Cover refers to the space a single plant occupies, and is similar to its midday shade. In this study we obtained the percent cover (Kent, 2012). Additionally, the height was an estimate of the average value; the extreme values (the tallest or shortest plants) were not measured nor the height of the inflorescences (Moreno-Casasola and López-Rosas, 2009).
Cost
The cost analysis was divided into two parts: seedling pretreatment and restoration techniques, including the cost of personnel and materials for each. The cost of obtaining seedlings and their pretreatment was calculated per seedling. for the restoration techniques the cost of seedling establishment per 400 m2 was estimated. The cost of the restoration techniques and seedling pretreatments were estimated per hectare for a real-frame plantation with 625 A. glabra seedlings separated by 4 m each. Salary payments to personnel were based on local rates for an 8-hour-long workday ($12.99 USD per day).
The costs reported are representative of the years 2018-2019 under the following assumptions: 1) the initial condition of the site to be restored corresponds to a freshwater swamp dominated by A. glabra transformed into a flooded pasture dominated by an exotic grass with a low density of remaining trees; 2) there are nearby sources of propagules of A. glabra and individuals of P. sagittata; 3) livestock activities are no longer carried out within the pasture; 4) herbicides or heavy machinery were not used; 5) the workforce is local; and 6) the exchange rate is $1 USD=$19.24 MXN.
Results
Seedlings
The survival of A. glabra seedlings was affected by both restoration technique and seedling pretreatment. No effect of the interaction of these factors on survival was detected (Table 1). Over time, survival was low (less than 50%). There was no period with clearly greater survival (Fig. 3). Regarding the different restoration techniques, quadrats with a raised soil level had the highest A. glabra seedling survival percent (46.2±2.4%; P=0.009, Tukey test), while the technique that consisted of planting P. sagittata had the lowest survival percent (17.5±6.4%; P<0.001, Tukey test; Fig. 3A). For seedling pretreatments, the survival of wetland seedlings was greater (41.1±3.7%) than that of nursery seedlings with or without fertilizer (26.1±5.8% and 25±6.8% respectively; P=0.003, Tukey test; Fig. 3B).
Table 1: Deviance analysis of the generalized linear model for the survival of Annona glabra L. seedlings under two factors: restoration treatment and seedling pretreatment. P is the probability that the effect of the factor is zero.
| Factor | Degrees of freedom | χ² | Degrees of freedom residuals | Residuals of χ² | P |
| Restoration technique | 4 | 24.09 | 130 | 183.97 | <0.001 |
| Seedling pretreatment | 2 | 13.11 | 128 | 170.85 | 0.001 |
| Restoration technique × Seedling pretreatment | 8 | 9.68 | 120 | 161.17 | 0.287 |

Figure 3: Average percent seedling survival ± standard error for Annona glabra L. seedlings under A) different restoration treatments over time; B) different pretreatments applied to the seedlings over time. Values with a different letter are significantly different (P<0.05). NNF = Nursery seedlings with no fertilizer; NWF = Nursery seedlings with fertilizer; Wetland = Seedling collected in the forested freshwater wetland.
Seedling height was affected by both restoration technique and seedling pretreatment, but the interaction of these factors was not significant (Table 2). More growth was recorded for seedlings grown in quadrats with raised soil (33.8±4.1 cm); and in contrast, the seedlings grown in quadrats with no modification grew the least (16.5±4.6 cm; P=0.03, Tukey test; Fig. 4A). For seedling pretreatment, significantly greater growth was recorded for the NNF (unfertilized) and NWF (fertilized) seedlings (38.8±3.9 cm and 28.2±3.3 cm, respectively) than for the wetland seedlings (12.4±3 cm; P<0.001, Tukey test; Fig. 4B), i.e., wetland seedlings grew less than half that the other treatments.
Table 2: Analysis of variance for the increase in height for Annona glabra L. seedlings that survived to the end of the experiment under two factors: restoration technique and seedling pretreatment. P is the probability that the effect of the factor is null.
| Factor | Degrees of freedom | Sum of squares | Mean squares | F | P |
| Restoration technique | 4 | 8166 | 2041.6 | 2.90 | 0.023 |
| Seedling pretreatment | 2 | 18850 | 9425.1 | 13.41 | <0.001 |
| Restoration technique × Seedling pretreatment | 8 | 2857 | 357.2 | 0.50 | 0.848 |
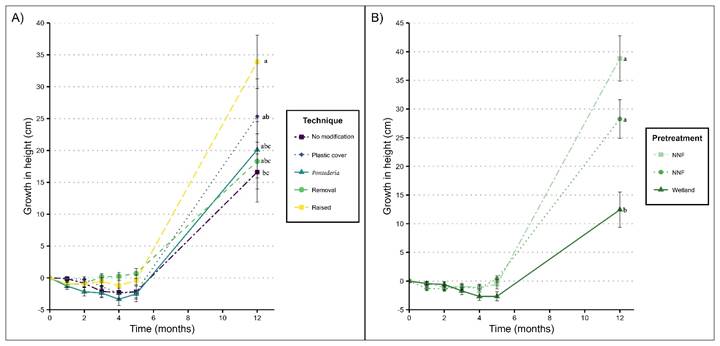
Figure 4: The average increase in height ± standard error for Annona glabra L. seedlings under A. different restoration techniques; B. different seedling pretreatments. Values with a different letter are statistically different (P<0.05). NNF = Nursery seedlings with no fertilizer; NWF = Nursery seedlings with fertilizer; Wetland = Seedling collected in the forested freshwater wetland.
The increase in diameter of the seedlings was only affected by restoration technique (Table 3). The greatest increase in diameter was recorded in seedlings covered with plastic cover (10.7±1.0 mm; P=0.01, Tukey test); the seedlings in the quadrats with no modifications increased the least (5.8±0.6 mm; P<0.001, Tukey test; Fig. 5A). Despite not finding significant differences among seedling pretreatments, the wetland seedlings presented the greater diameter increase (7.9±0.6 mm), compared to nursery seedlings with and without fertilizer (6.2±0.6 and 6.0±0.4 cm, respectively; Fig. 5B). However, all seedlings first increased in diameter (between the third and fourth month, Fig. 5) and later started growing in height (fifth month, Fig. 4).
Table 3: Analysis of variance for the increase in diameter for Annona glabra L. seedlings that survived to the end of the experiment under two factors: restoration technique and seedling pretreatment. P is the probability that the effect of the factor is null.
| Factor | Degrees of freedom | Sum of squares | Mean squares | F | P |
| Restoration technique | 4 | 485.62 | 121.40 | 6.19 | <0.001 |
| Seedling pretreatment | 2 | 73.22 | 36.60 | 1.86 | 0.157 |
| Restoration technique × Seedling pretreatment | 8 | 94.51 | 11.81 | 0.60 | 0.774 |
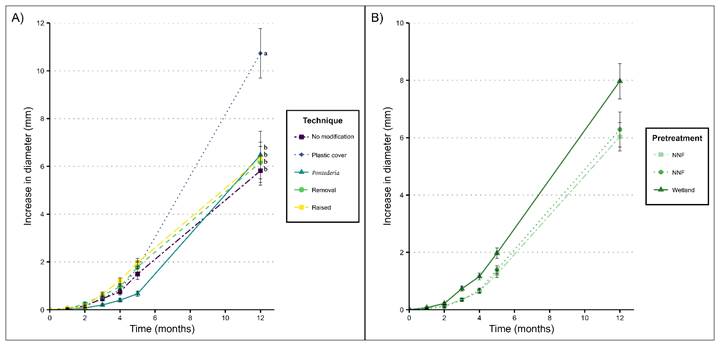
Figure 5: The average increase in diameter ± 1 standard error for Annona glabra L. seedlings under: A. different restoration techniques; B. different seedling pretreatments. Values with a different letter are statistically different (P<0.05). No significant differences were found between seedling pretreatments. NNF = Nursery with no fertilizer; NWF = Nursery with fertilizer; Wetland = Seedling collected in the forested freshwater wetland.
Vegetation
One year after beginning the restoration experiment, species richness was 40 species for the study area (Table 4). Of the species present, 62.5% were aquatic or semi-aquatic, i.e., characteristic of wetland ecosystems. The treatments with the greatest species richness were Raised soil + NWF seedlings and Raised soil + wetland seedlings, each with 30 species, followed by Pontederia + wetland seedlings with 27 species (Fig. 6). In contrast, the treatments with the lowest richness were Plastic cover + NNF seedlings, Plastic cover + wetland seedlings, and Plastic cover + NWF seedlings with 12, 14, and 17 species respectively (Fig. 6). In the control, 22 species were recorded (Table S1).
Table 4: List of species found in the quadrats in the study area, Actopan, Veracruz, Mexico, including classification of each species based on its affinity to aquatic environments following Lot’s (2015) catalogue of flora and vegetation of Mexican wetlands.
| Plant family | Species | Category |
| Annonaceae | Annona glabra L. | Aquatic |
| Alismataceae | Echinodorus paniculatus Micheli | Aquatic |
| Alismataceae | Limnocharis flava (L.) Buchenau | Aquatic |
| Araliaceae | Hydrocotyle bonariensis Lam. | Aquatic |
| Asclepiadaceae | Asclepias curassavica L. | Semi-aquatic |
| Asteraceae | Eclipta prostrata (L.) L. | Aquatic |
| Asteraceae | Pluchea odorata (L.) Cass. | Tolerant |
| Asteraceae | Morphospecies 1 | - |
| Combretaceae | Laguncularia racemosa (L.) C.F. Gaertn. | Aquatic |
| Commelinaceae | Commelina erecta L. | Terrestrial |
| Convolvulaceae | Ipomoea tiliacea (Willd.) Choisy | Semi-aquatic |
| Cucurbitaceae | Melothria pendula L. | Terrestrial |
| Cyperaceae | Cyperus articulatus L. | Aquatic |
| Cyperaceae | Cyperus digitatus Roxb. | Aquatic |
| Cyperaceae | Cyperus ochraceus Vahl | Tolerant |
| Cyperaceae | Eleocharis geniculata (L.) Roem. & Schult. | Semi-aquatic |
| Cyperaceae | Eleocharis interstincta (Vahl) Roem. & Schult. | Aquatic |
| Cyperaceae | Eleocharis mutata (L.) Roem. & Schult. | Aquatic |
| Fabaceae | Dalbergia brownei (Jacq.) Urban | Aquatic/Semi-aquatic |
| Fabaceae | Enterolobium cyclocarpum (Jacq.) Griseb. | Terrestrial |
| Fabaceae | Mimosa pigra L. | Terrestrial |
| Heliconiaceae | Heliconia latispatha Benth. | Terrestrial |
| Lemnaceae | Spirodela polyrhiza (L.) Schleid. | Aquatic |
| Marantaceae | Thalia geniculata L. | Aquatic |
| Onagraceae | Ludwigia octovalvis (Jacq.) P.H. Raven | Semi-aquatic |
| Passifloraceae | Passiflora biflora Lam. | Terrestrial |
| Poaceae | Echinochloa pyramidalis (Lam.) Hitchc. & Chase | Aquatic |
| Poaceae | Hymenachne amplexicaulis (Rudge) Nees | Aquatic |
| Poaceae | Leersia hexandra Sw. | Aquatic |
| Poaceae | Morphospecies 2 | - |
| Poaceae | Morphospecies 3 | - |
| Polygonaceae | Polygonum hydropiperoides Michx. | Semi-aquatic |
| Pontederiaceae | Pontederia sagittata C. Presl | Aquatic |
| Scrophulariaceae | Bacopa monnieri (L.) Wettst. | Aquatic |
| Solanaceae | Solanum campechiense L. | Semi-aquatic |
| Solanaceae | Solanum diphyllum L. | Terrestrial |
| Typhaceae | Typha domingensis Pers. | Aquatic |
| Verbenaceae | Lippia nodiflora (L.) Michx. | Tolerant |
| Not identified | Morphospecies 4 | - |
| Not identified | Morphospecies 5 | - |
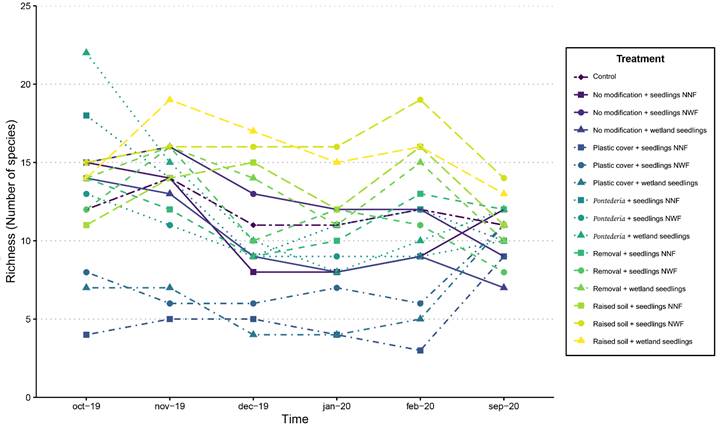
Figure 6: Species richness in the control and 15 experimental restoration treatments in the first 12 months after starting the restoration treatments.
The highest number of species was recorded for the Raised soil restoration technique (34 species), followed by Soil removal and Pontederia with 31 and 30 species, respectively (Fig. 7). Richness was lowest for the Plastic cover technique and the one with No modifications (22 species; Fig. 7).
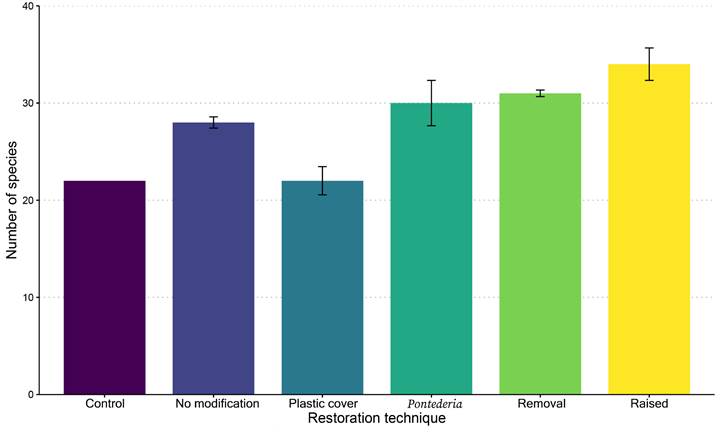
Figure 7: Average species richness ± standard error for the five restoration techniques and the control for October 2019 to September 2020.
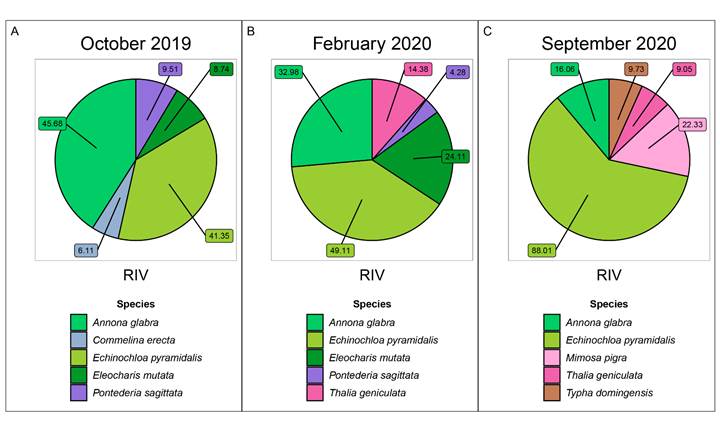
One month after starting the experimental restoration treatments (October, 2019), the species with the highest average RIV in all treatments were A. glabra (45.6±8.2), E. pyramidalis (41.3±4.6), P. sagittata (9.5±0.4), Eleocharis mutata (L.) Roem. & Schult. (8.7±0.4) and Commelina erecta L. (6.1±0.7; Fig. 8). In February 2020 (five months after starting the experiment), the species with the highest average RIV recorded were E. pyramidalis (49.1±4.1), A. glabra (32.9±10.0), E. mutata (24.1±2.0), T. geniculata (14.3±1.5) and P. sagittata (12.6±4.2; Fig. 8). One year after starting the restoration treatments (September 2020), the species with the highest average RIV were E. pyramidalis (88.02.3), Mimosa pigra L. (22.3±1.9), A. glabra (16.6±1.3), T. domingensis (9.7±0.8) and T. geniculata (9.0±1.5; Fig. 8). Table S2 shows more details about RIV’s values and individual treatments.
Cost
The economic cost of the different restoration techniques we tested varied. The technique with the lowest cost per hectare was No modification at $1136.95 USD, while the technique with the highest cost was that of Raised soil level at $2535.4 (Table 5). Regarding the economic cost per seedling, the wetland seedlings pretreatment (seedlings collected from the freshwater swamp) had the lowest cost per seedling at $1.99 USD (Table 6). The economic cost per seedling was greater under the nursery pretreatments with and without fertilizer at $2.69 and $2.49 USD, respectively (Table 6).
Table 5: Estimate of the economic cost per restoration technique and per hectare for each technique (only labor and materials were considered, not including seedlings). Cost in dollars (USD). Time and cost estimated for 400 m2.
| Technique | Activity / materials | Time (hours) | Cost ($) | Total ($) | Total per hectare ($) |
| No modification | Cutting vegetation | 16 | 25.98 | 649.68 | 1136.95 |
| Planting seedlings | 12 | 19.49 | 487.26 | ||
| Plastic cover | Cutting vegetation | 16 | 25.98 | 649.68 | 1640.46 |
| Setting up the plastic | 10 | 16.24 | 406.05 | ||
| Planting seedlings | 12 | 19.49 | 487.26 | ||
| Plastic sheet | - | 3.89 | 97.45 | ||
| Pontederia | Cutting vegetation | 16 | 25.98 | 649.68 | 1989.66 |
| Planting seedlings | 12 | 19.49 | 487.26 | ||
| Transplanting Pontederia | 21 | 34.10 | 852.71 | ||
| Soil Removal | Cutting vegetation | 16 | 25.98 | 649.68 | 1624.22 |
| Soil removal and mixing | 12 | 19.49 | 487.26 | ||
| Planting seedlings | 12 | 19.49 | 487.26 | ||
| Raised the soil level | Cutting vegetation | 16 | 25.98 | 649.68 | 2535.40 |
| Soil raised 20 cm above ground level to form a mound | 33 | 53.59 | 1339.98 | ||
| Planting seedlings | 12 | 19.49 | 487.26 | ||
| Plastic mesh to retain soil | - | 2.33 | 58.47 |
Table 6: Estimate of the economic cost per seedling in the different pretreatments and the economic cost of carrying out reforestation with 625 Annona glabra L. seedlings using the different pretreatments as part of the ecological restoration activities on one hectare of freshwater swamp. Seedlings were irrigated two days a week for eight months. Cost in dollars (USD).
| Pretreatment | Activities / Materials | Time (hours) per seedling | Cost ($) | Total cost per seedling ($) | Total cost per hectare ($) |
| Nursery without fertilizer | Collecting seedlings | 0.15 | 0.24 | 2.49 | 1557.22 |
| Planting seedlings in plastic bags | 0.3 | 0.48 | |||
| Watering | 1.02 | 1.65 | |||
| Plastic bags | - | 0.10 | |||
| Nursery with fertilizer | Collecting seedlings | 0.15 | 0.24 | 2.69 | 1684.17 |
| Planting seedlings in plastic bags | 0.3 | 0.48 | |||
| Watering | 1.02 | 1.65 | |||
| Applying fertilizer | 0.128 | 0.20 | |||
| Plastic bags | - | 0.10 | |||
| Wetland | Collecting seedlings | 0.32 | 0.51 | 1.99 | 1246.58 |
| Planting seedlings in plastic bags | 0.3 | 0.48 | |||
| Watering | 0.48 | 0.77 | |||
| Plastic bags | - | 0.20 |
Discussion
Seedlings
One of the most vulnerable stages is the seedling stage. This is mainly due to the high degree of fragility of seedlings under adverse environmental conditions, since even small decreases in their biomass can lead to their death (Facelli, 2008). Particularly in wetland systems, seedlings are subjected to high levels of stress due to the low oxygen levels caused by long periods of flooding (Mitsch and Gosselink, 2015), and there is often strong competition with other species, particularly herbaceous ones (Gómez-Aparicio, 2009; Sánchez-Luna et al., 2022). At the restoration site, after the planting of A. glabra seedlings, the water level reached about 60 cm (the maximum level in two years), which meant a high stress level for the seedlings. The restoration technique where the soil level was raised had the highest survival of A. glabra seedlings in this study, likely because the water level and the duration of flooding or soil saturation were reduced, resulting in less stress on the seedlings from excess water. When soil is flooded, the water displaces the oxygen of the porous spaces, encouraging anaerobiosis, which promotes changes in the physicochemical soil conditions lowering the value redox’s potentials, among others (Mitsch and Gosselink, 2015). In various types of swamps, including mangroves, mounds are naturally formed by organic debris, the growth of dominant tree roots, or large fallen trees. These mounds protrude above the surface of the water and are usually sites for the germination and establishment of seedlings of various species (Cronk and Fennesy, 2001). The technique in which P. sagittata individuals were planted could have led to competition for space and resources between A. glabra seedlings and P. sagittata plants, which is reflected in the lowest percent survival of the five restoration techniques. It has been reported that the presence of neighboring plant species in wetland restoration areas has negative effects on survival (Gómez-Aparicio, 2009; Sánchez-Luna et al., 2022), as is the case with P. sagittata. It is important that, in addition to planting, restoration actions reduce competition from herbaceous plants to increase the probability of survival of the planted seedlings.
Regarding the pretreatment of seedlings, the wetland seedlings had a higher percent survival than the nursery seedlings, which can be explained by their greater height and diameter at the time of sowing (i.e., greater age and vigor). In addition, the wetland seedlings were collected from two freshwater swamp sites, and so were pre-adapted to the environmental conditions of the restoration site. However, those seedlings also had to spend a brief period in the nursery, for adapting to the greenhouse bags and ensuring that all the seedlings, when sown, had been under similar previous conditions. There are no studies on the presence of seedling banks of this species in freshwater swamps, though personal observations (P. Moreno-Casasola) indicate that few seedlings remain from one year to the next, especially in rainy years. Young plants of different sizes are not found, unlike P. aquatica, which does form seedling banks that cover a wide range of sizes.
The establishment and survival of plant species depend on water and nutrient supply, so plants must grow continuously, expanding the area of the surface that interacts with the environment (Crang et al., 2018). The increase in seedling height and diameter for those that survived 12 months after sowing had similar trends among treatments. The greatest increase in height was recorded for seedlings where the soil was raised above ground level. In this technique, the stress caused by the flood was lower, which was not only reflected in seedling survival, but also in highest species richness and height increase. Vertical increase is an important factor in the ecological strategy of plants as it guarantees access to light (Falster and Westoby, 2003; Moles et al., 2009), and in wetlands environments could help adaptation and survival of seedlings. Also, during the successional process, acquiring greater height than neighboring plants confers a competitive advantage through prior access to light (Westoby et al., 2002). Regarding the pretreatment of seedlings, a greater increase in vertical growth was recorded in nursery seedlings than in wetland seedlings. The seedlings in the No modification technique grew the least. The absence of any type of intervention in this technique led to the highest recorded RIV for the grass E. pyramidalis, which could have influenced the growth of the A. glabra seedlings. It has been reported that on restoration sites it is important to control grasses to guarantee favorable environmental conditions such as soil moisture, light, and nutrient availability, so that the seedlings can grow better (Grossnickle and Ivetić, 2017). According to Crang et al. (2018), height increase occurs in stems, leaves, roots, and flowers, and is one of the two main types of growth, which is called primary growth, where the plants tend to grow tall first. Because the wetland seedlings were taller, they grew more slowly than the nursery seedlings did; nonetheless, they showed the highest survival of all the treatments.
In plants, stem diameter is related to mechanical support and their capacity to absorb water and minerals (Crang et al., 2018). In this study, the stems of the seedlings began to increase in diameter before they increased in height, and the increase in diameter was only affected by restoration technique. The seedlings in the Plastic cover technique had the greatest increase. The plastic cover reduced competition with other plant species and was effective in reducing the abundance of exotic grasses, such as E. pyramidalis, as also observed by López-Rosas et al. (2010). However, during the dry season, it can also reduce the amount of water that seeps into the soil and increase the temperature around the seedlings. It has been reported that under stressful conditions, such as greater evapotranspiration and less precipitation, plants allocate more resources to increasing diameter than to growing in height (Vizcaíno-Palomar et al., 2017), which could explain why the seedlings’ diameter increased under this technique. Although no significant differences were found among seedling pretreatments, there was a greater increase in the diameter of the wetland seedlings. In most of the seedlings under this pretreatment, the appearance of hypertrophic lenticels was detected, which has been reported as a morphological and physiological adaptation in both the seedlings (Núñez-Elisea et al., 1999; Mielke et al., 2005) and adult plants (Yáñez-Espinosa and Terrazas, 2001) of A. glabra in the face of flood conditions. The greater increase in the diameter of wetland seedlings is related to the appearance of a greater number of lenticels compared to nursery seedlings. In addition, increasing diameter is a type of secondary growth in plants (Crang et al., 2018) that occurs after the plant has reached height enough to access light. Given this, and the fact that at the time of sowing the wetland seedlings were taller than the nursery seedlings, the increase in diameter (secondary growth) was favored in wetland seedlings.
Vegetation
A total of 40 species were found in the study area, the vast majority herbaceous, except for Laguncularia racemosa (L.) C.F. Gaertn., A. glabra, and Enterolobium cyclocarpum (Jacq.) Griseb. The latter is a tree species found in medium-height tropical forests (Niembro-Rocas et al., 2010). Eight seedlings were recorded in October 2019, before the rise in water level occurred in the area; however, none were able to establish. Freshwater swamps form a continuous gradient with the mangroves, so the dispersal and establishment of L. racemosa in these sites is a recurring phenomenon. However, the process of succession, a year after starting the experimental restoration treatments, was determined by herbaceous species. The number of species found in this study represents 42.1% of the number of species reported for marshes on the coastal plain of central Veracruz (Moreno-Casasola et al., 2010). Most of the species present in our study area (62.5%) are aquatic or semi-aquatic species that are adapted to flood conditions. Many of these species are dominant in wetlands, such as A. glabra, L. racemosa, P. sagittata, E. paniculatus, and T. geniculata, which disperse their seeds by water during the flooding season, so the area has a high potential for regeneration. Species richness in the present study was higher than that reported for other ecological restoration efforts in coastal wetlands (a marsh and a swamp) of Veracruz (López-Rosas et al., 2010; Sánchez-Luna, 2018).
In general, the species with the highest RIV in the study area were E. pyramidalis, followed by A. glabra and M. pigra. Echinochloa pyramidalis, a grass, increased its RIV from 41.3 in October 2019 to 88 in September 2020. The recovery capacity of this grass in the experimental restoration quadrats is related to the invasion potential of exotic grasses. These grasses can compete with local species, reducing the availability of water and nutrients, and thus decrease the percentage of germination and establishment of local species (D’Antonio and Vitousek, 1992). In restoration areas, pasture species harm other species (Gómez-Aparicio, 2009). For example, E. pyramidalis has high biomass and root production (López-Rosas et al., 2005) and when this grass is introduced into marshes, it is capable of dominating the wetland (López-Rosas et al., 2006). One year after starting the experiment, the RIV of E. pyramidalis was lower in the Plastic cover (83.6) and Raised soil (78.1) techniques. In contrast to what happened with E. pyramidalis, the RIV of A. glabra decreased from 45.6 in October 2019 to 16.6 in September 2020. This decrease in RIV was due to the low percent of survival of A. glabra seedlings. The techniques with the highest RIV for A. glabra one year after starting the experiment were No modification (23.2) and Soil removal (20.4). However, the RIV of the M. pigra shrub increased considerably: it was absent in October 2019, due to the cutting previous to the planting, but in February and September 2020 its RIV rose to 6.3 and 22.3, respectively. The rapid increase in its RIV may be due to its invasive behavior. This shrub is very aggressive and is considered invasive in northern Australia, Southeast Asia, and some regions of Africa (Braithwaite et al., 1989; Lonsdale, 1993; Shanungu, 2009). Its presence in these areas significantly reduces the diversity of plant and animal species (Paynter, 2005; Shanungu, 2009). In Veracruz, this species has been recorded in degraded wetlands (Moreno-Casasola, 2008), suggesting that it is an opportunistic or ruderal type of species. The techniques with the highest RIV for M. pigra one year after starting the experiment were Plastic cover (30.8) and Raised soil (24.9). The plastic used deteriorated due to the rain and water, allowing some plant species such as M. pigra to germinate. In addition, M. pigra is classified as a terrestrial species (though it is frequent in flooded pastures), so raising the level of the soil favored its establishment. Among other species with high RIVs, native hydrophytic species such as T. domingensis and T. geniculata were present.
Cost
The difference in the cost of nursery seedlings and wetland seedlings was due to the shorter time that the latter spent in the nursery. A shorter time in the nursery means less expense caring for, and watering, the seedlings. The estimated cost per seedling of A. glabra in this study ($1.99-2.69 USD) was lower than the cost per seedling of P. aquatica reported by Sánchez-Luna et al. (2022) in restoration work on a floodplain freshwater swamp in northern Veracruz. Estimates of the cost of the restoration techniques were $1136.95 USD to $2535.40 USD per hectare. The lowest economic cost per hectare was for the No modification technique, because it only involved cutting the grass and planting seedlings. Raising the ground level had the highest estimated cost, owing not only to the need to purchase tools and materials, but particularly to the labor required. This technique has also been used successfully in the ecological restoration of mangroves in the Yucatan Peninsula (Teutli-Hernández et al., 2020). According to the results, the most expensive approach per hectare is the Raised soil treatment + NWF seedlings at $4219.57 USD/ha, and the least expensive is the No modification + wetland seedlings at $2383.53 USD. The cost of ecologically restoring a wetland that was converted to cropland has been estimated at $2319 USD/ha (Peh et al., 2014), and is similar to the least expensive of our restoration treatments. In addition, the costs of the different experimental treatments are lower than those estimated for the freshwater swamp of P. aquatica in Veracruz, where cutting the grass and planting seedlings was calculated at $3551 USD/ha, and applying mulch or a Plastic cover and planting seedlings was estimated at $5106 USD/ha and $9824 USD/ha, respectively (Sánchez-Luna et al., 2022). Due to the high cost of ecological restoration projects, other techniques have been implemented, such as directly sowing seeds in the field to avoid paying for seedling transport and make the construction of nurseries for the seedlings unnecessary, which also reduces the cost of field labor; as much as 64% can be saved using these approaches (Freitas et al., 2019).
Conclusions
It is necessary to optimize the initial reforestation conditions to get better results during restoration. The survival of A. glabra seedlings was highest under the technique of Raised soil level and using seedlings from the freshwater swamp. This technique also favored an increase in height of the seedlings. The increase in diameter was greater with the Plastic cover technique. The RIV of E. pyramidalis was lowest with the techniques of Raised soil and using a Plastic cover, while the RIV of A. glabra was highest with the No modification and Soil removal techniques.
There is a lot of experience in the restoration of mangrove swamps, but much less in restoring freshwater swamps. Restoration efforts are costly and often take place in hard-to-reach areas and under complex working conditions. Therefore, it is important to develop and apply better techniques to ensure greater seedling survival and the success of the restoration.
To optimize the survival and growth of seedlings, the accompanying vegetation and the economic cost of different restoration techniques must be taken into account. In future ecological restoration projects in the freshwater swamps dominated by A. glabra in flooded pastures, we recommend raising the soil 20 cm above ground level in the transplant areas and covering the mounds with dark material before planting. Our results indicate that these techniques could minimize the stress on the seedlings caused by flooding, thereby increasing their probability of survival, and reducing the cover of the exotic grass and other invasive species. In addition, for planting this study suggests that seedlings be collected from a nearby fragment of freshwater swamp. The use of nursery seedlings is not recommended because they have a lower percent survival; nor is the use of fertilizer recommended since it did not lead to any improvement in survival or growth. Restoration efforts should be started after the nortes season when the soil is saturated with water, the seeds naturally germinate and the seedlings establish.
Given the alteration and degradation coastal wetlands are undergoing, and their ecological and economic importance, it is crucial to continue conducting ecological restoration experimental studies that allow us to understand which restoration techniques are the most effective and economical.











 nueva página del texto (beta)
nueva página del texto (beta)