Introduction
Tropical cloud forests are heterogeneous mosaics of habitats that result in high biodiversity and endemism (Brujinzeel et al., 2010). These ecosystems are threatened by deforestation, fragmentation, land use change, and due to climate change, they are also expected to face more frequent and severe droughts (Allen et al., 2010; Bruijnzeel et al., 2010). Droughts induced by climate change will affect the performance of trees globally, but the direction (positive or negative) and magnitude of these effects will depend on the intensity of droughts and the tolerance of species to water shortage conditions (Allen et al., 2015). Considering that climate change may affect plants differently, increasing number of studies have focused on the responses of tree species and ecosystems to drought stress (Beier et al., 2012; Berry et al., 2019). The climate change responses of tree species have motivated studies on functional traits and there is growing interest in analyzing how these traits vary with increasing drought (Badano et al., 2018, 2019; Berry et al., 2019; Williams-Linera et al., 2022).
Functional traits are morphological, physiological or phenological characteristics of living organisms that directly influence their fitness (Pérez-Harguindeguy et al., 2013). The most studied functional traits of plants are linked with the morphology and physiology of their leaves (Pérez-Harguindeguy et al., 2013). Leaf traits vary with changing environmental conditions and allow plants to develop a wide range of responses (Wright et al., 2004; Pandey et al., 2009; Olguin et al., 2020). The amplitude of these responses depends on the leaf economy to capture, conserve and use light energy, water and/or nutrients (Wright et al., 2004; Reich, 2014). For instance, leaf area is related with the capture of light energy and this trait changes with the environmental conditions and the developmental stage of plants (Poorter and Rozendaal, 2008; Pérez-Harguindeguy et al., 2013). Specific leaf area (SLA), or the ratio between area and biomass of leaves, directly influences the photosynthetic capacity of plants and it is related with other attributes, such as leaf nitrogen content and growth rates (Wright et al., 2004). Chlorophyll content also plays a key role in determining the effectiveness of leaves in light capture, but it decreases with increasing irradiance (Evans and Poorter, 2001).
Furthermore, leaf area (LA), SLA and chlorophyll content are important traits for understanding vegetation responses to water stress (Valladares and Sánchez-Gómez, 2006). Plants experiencing drought exhibit reduced LA, low SLA, chlorophyll content and photosynthetic rate, and reduced growth (Valladares and Sánchez-Gómez, 2006). Leaf thickness and toughness influence shade tolerance, growth, and survival (Westbrook et al., 2011). Thickness depends on the structural properties of the leaf blade, while toughness depends on the material properties of leaves, such as cellulose and lignin content (Westbrook et al., 2011). These two traits are key components of SLA and relate with protection of leaves from herbivory and external hazards (Pérez-Harguindeguy et al., 2013). Higher leaf toughness and thickness have been associated with drier conditions and are thus proposed as potential predictors of leaf drought tolerance (Onoda et al., 2011; Pérez-Harguindeguy et al., 2013). In shade plants, leaf toughness is usually more associated with survival (reduced mortality rates) than with relative growth rates (Westbrook et al., 2011). Additionally, other experiments conducted with tree seedlings indicated that the emergence and survival rates of seedlings decreased under simulated climate change scenarios that involve increased temperature and reduced precipitation (Badano et al., 2018).
In tropical cloud forests of Mexico, several studies used functional traits of plants in different developmental stages to measure their responses in the face of changing environmental conditions (Toledo-Aceves et al., 2017; Vergara-Gómez et al., 2020; Williams-Linera and Manrique-Ascencio, 2020). Comparisons of functional traits between juvenile and adult trees have shown that SLA varies between ontogenetic stages; however, LA and stomatal density were similar in saplings and adults (Williams-Linera and Manrique-Ascencio, 2020). Furthermore, SLA is a functional trait responsive to different light environments in cloud forest tree seedlings (Toledo-Aceves et al., 2017). Moreover, within the same species, LA and SLA vary if trees are established in different types of soil (Vergara-Gómez et al., 2020). In these cloud forests, other studies focused on functional attributes related to drought scenarios caused by climate change (Berry et al., 2019; Williams-Linera et al., 2022). In other tropical forest ecosystems, controlled experiments have been carried out exposing seedlings to low and high levels of water availability (Poorter and Markesteijn, 2008; Badano et al., 2019; Berry et al., 2019), different light levels (Saldaña-Acosta et al., 2009) or combinations of these factors (Amissah et al., 2015). Poorter and Markesteijn (2008) suggested that seedling functional traits affect survival in face of drought, indicating that the mechanisms to deal with water shortage are closely linked to the functional group of a species. Since leaf functional traits and plant performance varied into a continuum of species’ light requirements, focal tree species were selected. They were arranged along a shade-tolerance gradient in which the extremes are the light-demanding or pioneer tree species and the shade-tolerant ones (Poorter and Bongers, 2006; Poorter and Markesteijn, 2008; Poorter and Rozendaal, 2008). Light-demanding or pioneer trees tend to display higher LA and SLA as well as lower chlorophyll content, thickness and toughness, and higher relative growth rate (RGR) but lower survival rates, whereas shade-tolerant species tend to display lower LA, SLA, higher chlorophyll content, and higher thickness and toughness, lower RGR but higher survival rates (Wright et al., 2003; Poorter and Rozendaal, 2008; Kitajima and Poorter, 2010).
This study focused on determining how light environment and drought conditions induced in the field affect leaf functional traits, survival and growth of three woody species native to Mexican tropical cloud forests. Plant responses were measured under contrasting light conditions of a canopy gap and a closed forest understory. The hypothesis was that functional trait responses of plants depend on whether the focal species is an understory shade-tolerant plant, a canopy tree species belonging to advanced successional stages, or a pioneer tree able to colonize forest gaps. Overall, we expected higher variability in leaf functional traits, reduced survival, and smaller relative growth rates in the drought treatment in the closed forest understory than in gap condition. Specifically, we expected more variability in the responses of the pioneer tree than in those of the forest species.
Materials and Methods
Study site
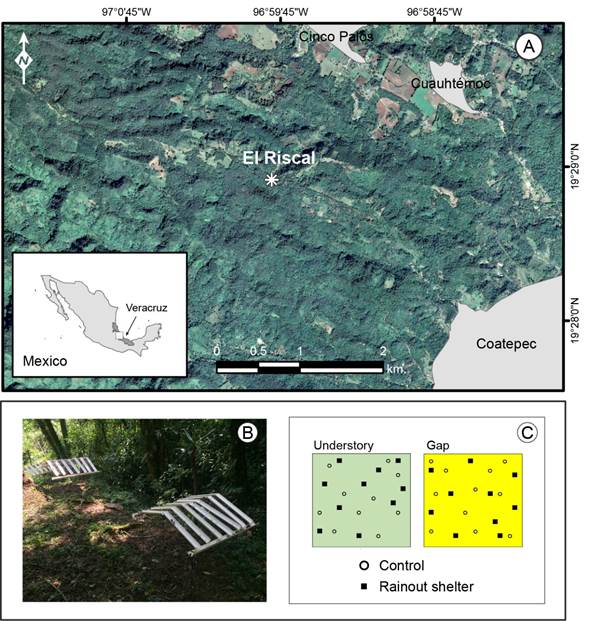
The experiment described below was conducted in the private nature reserve El Riscal, located in central Veracruz, Mexico (19°28'55.1"N, 96°59'47.6"W; 1600 m elevation; Fig. 1). Mean maximum and minimum temperatures are 23 °C and 14.4 °C, respectively, and total annual precipitation is 2105 mm (Riaño and Briones, 2015). Soil is volcanic and it has been classified as Andosol. The type of vegetation is tropical cloud forest and some common canopy tree species are Carpinus tropicalis (Donn. Sm.) Lundell, Clethra macrophylla M. Martens & Galeotti, Liquidambar styraciflua L., Quercus lancifolia Benth., Quercus xalapensis Bonpl., Styrax glabrescens Benth. and Turpinia insignis Tul. Dominant woody understory species are Eugenia capuli Schltdl., Miconia glaberrima Naudin, Ocotea psychotrioides Kunth and Palicourea padifolia (Roem. & Schult.) T.N. Taylor & Lorence (Williams-Linera, 2002). Tree species characteristic of the secondary forest are Heliocarpus donnellsmithii Rose, Leucaena diversifolia Benth., Lippia myriocephala Schltdl. & Cham., Myrsine coriacea (Sw.) R. Br. and Trema micranthum (L.) Blume (Muñiz-Castro et al., 2015).
Studied species
Three dominant woody species from primary and secondary cloud forests were selected for this study. These species are Eugenia capuli (Myrtaceae), a common shade-tolerant tree that inhabits the forest understory, Liquidambar styraciflua (Altingiaceae), a dominant forest canopy tree with intermediate shade-tolerance (Williams-Linera, 2002), and Trema micranthum (Cannabaceae), a pioneer tree highly abundant in secondary forests (Muñiz-Castro et al., 2015).
For the experiment, seedlings of all these species were produced from seeds in the nursery of the Botanical Garden of the Instituto de Ecologia, A.C. (INECOL), in Xalapa, Veracruz, Mexico. Plants taken to the field were 8-14 months old and their initial heights were 21.4 (SE=4.8) cm for E. capuli, 53 (13.3) cm for L. styraciflua, and 44.9 (6.7) cm for T. micranthum.
Experimental design
In the study site, two plots of 20 × 20 m were established within a forest fragment: one plot was in the forest understory 20 m away from the forest edge, and the other was in a forest gap. Before setting up the field experiment, the light environment was characterized in the center of each plot during January-February 2017. Canopy cover, recorded with a spherical densiometer (Forest Densiometers, Model-A, Arlington, USA), was about 90% in the forest understory, while vegetation cover in the gap was about 30%. The gap was composed by herbaceous plants only. Incidence of light was measured as photosynthetic active radiation between 10:00 and 14:00 h on overcast days with a pyranometer (global solar radiation, LI-COR, LI-200R, Lincoln, USA) and a datalogger (LI-COR, model LI-1400, Lincoln, USA). In the understory, light incidence fluctuated between 7.6 μmol/m²/s and 15 μmol/m²/s. In the gap, it varied between 43.5 μmol/m²/s and 44.8 μmol/m²/s.
In each plot, points were randomly selected to locate controls and rainout shelters (Fig. 1B, C). Ten rainout shelters were in the forest understory plot, and ten shelters were in the gap plot. Rainout shelters were 0.87 m height, 0.97 m width, and 1 m depth. The shelter's roof was gabled with seven parallel strips of PVC tube gutters of 7.62 cm × 46 cm, separated every 15 cm (Fig. 1B). The amount of excluded rainfall was measured during 15 rainy days in April-May 2017 with four pluviometers (Rain Gauge Tube, 140 mm, East Lansing, USA). Rainout shelters reduced rainfall approximately by 20±5%, which would represent a moderate drought according with the middle XXI-century prediction of climate change models for this region (Esperón-Rodríguez et al., 2016). The resulting experimental treatments were forest understory and gap controls (without shelters), and moderate drought (with shelters). Hereafter, treatments are referred as understory-control, understory-drought, gap-control, and gap-drought.
All plants were moved to the field to be acclimatized for two months previously to the start of the experiment. Initially, in each experimental plot we placed a total of 120 seedlings, two seedlings of each species (E. capuli, L. styraciflua, and T. micranthum) in ten controls and under ten shelters (Fig. 1C). However, due to pre-experiment mortality during the acclimation period, the experiment included a smaller number of control individuals. To guarantee no interference from water movement in the soil, the plants were placed in large plastic bags (5.68 l volume) on bricks. All plants were counted and measured every seven weeks from June 2017 to February 2018. At each monitoring time, height and basal diameter of each plant were measured with a digital vernier (Mitutoyo, model CD-6’’ ASX, Kawasaki, Japan), and four fully expanded and undamaged new leaves were collected from each plant, stored in black plastic bags, transported in a cooler to the Laboratory of Functional Ecology at INECOL, and measured in 24 hours.
Leaf functional traits
Leaf area (LA, cm2) was measured excluding the petiole using a WinFOLIA Leaf Area Meter (software LA2400, Reagent Instruments Inc., Toronto, Canada). Leaves were oven-dried at 65 °C for 72 h and weighted to obtain their dry biomass (g). Specific leaf area (SLA, cm2/g) was calculated as the ratio between LA and leaf dry biomass. Leaf thickness (mm), toughness (Newton/cm2) and chlorophyll content (mg/cm2) were measured in two different points of each leaf. Leaf thickness was measured with a digital micrometer (Mitutoyo, Coolant proof IP65, 0.001 mm resolution, Kawasaki, Japan). Toughness was measured with a penetrometer, and it was calculated as the product of sand weight (g) needed to cause leaf rupture and area of the needle (0.043 cm2). Chlorophyll content was measured with an atLEAF chlorophyll meter (FT Green LLC, Wilmington, USA), which uses arbitrary units (SPAD units) thus a calculator included in atLEAF was applied to report chlorophyll content expressed in mg/cm2.
Survival and relative growth rate
During each survey period, each seedling was examined and classified as dead or alive to estimate survival rates. Further, data of height and basal diameter of each alive plant were used to compute relative growth rates. These values were calculated separately for each growth variable as proposed by Hunt (1990):
where G2 and G1 were the final and initial growth (cm) and t2 - t1 was the number of months between the two measurements.
Statistical analyses
For each species, survival was estimated as the number of plants alive at each treatment by the end of the study. To examine changes in seedling survival among species for the entire period we used the Kaplan-Meier survival platform (SAS, 2012). Since some of the plants survived until the end of the experiment, survivorship curves were analyzed as censored survival data. The Wilcoxon test was used to compare survival among species and treatments.
Prior to statistical analyses, functional trait variables were tested for normality using the Shapiro-Wilk test. Variables were log transformed when data normality was not met. Leaf functional trait variation of species in different conditions of light and water was determined using linear mixed-effect models, where species and treatments were used as fixed factors and individuals as a random effect factor. Differences in RGRs in height and basal diameter among species in different light conditions were examined using generalized linear models. When significant differences were found, orthogonal planned contrasts were used to detect differences among treatments. Data were analyzed using JMP v. 10.0.0 (SAS, 2012) and the statistical platform R v. 3.5.1 (R Core Team, 2018).
Results
The tree species differed significantly in all their leaf traits (Table 1; Appendix). The highest LA value was recorded in L. styraciflua, and it was followed by those of T. micranthum and E. capuli. Similar SLA values were found for L. styraciflua and T. micranthum, which were higher than those of E. capuli. Values of chlorophyll content, leaf thickness and toughness were higher in E. capuli than in the other two species (Appendix).
Table 1: Results of linear mixed models used to evaluate the effects of water restriction (control and drought treatment) in the understory and gap of a tropical cloud forest in Veracruz, Mexico. The foliar functional traits evaluated with these analyses are leaf area (LA, cm2), specific leaf area (SLA, cm2/g), chlorophyll content (mg/cm2), thickness (mm), and toughness (Newton/cm2), df=degrees of freedom, F=statistic, p=probability.
| Functional trait | Treatment | Species | Species × Treatment | ||||||
|---|---|---|---|---|---|---|---|---|---|
| df | F | p | df | F | p | df | F | p | |
| Leaf area | 3 | 10.08 | <0.001 | 2 | 46.03 | <0.001 | 6 | 8.55 | <0.001 |
| SLA | 3 | 0.183 | 0.91 | 2 | 7.86 | <0.001 | 6 | 2.128 | 0.055 |
| Chlorophyll | 3 | 3.08 | 0.03 | 2 | 524.3 | <0.001 | 6 | 29.21 | <0.001 |
| Leaf thickness | 3 | 14.72 | <0.001 | 2 | 28.41 | <0. 001 | 6 | 2.387 | 0.03 |
| Leaf toughness | 3 | 1.94 | 0.126 | 2 | 187.82 | <0.001 | 6 | 6.49 | <0.001 |
Leaf area, chlorophyll content and thickness differed among combinations of light conditions (understory and gap) and water levels (control and drought), whereas SLA and toughness did not differ among these treatments (Table 1; Fig. 1). Overall, LA was smaller in the understory than in the gap, but no effects of drought were found on this variable. Chlorophyll content was lower under induced drought conditions in both the understory and the gap, while leaf thickness was lower in the gap-drought than in the other treatments (Table 1; Fig. 1).
Except for SLA, all other leaf traits significantly differed between control and drought at the two light conditions (Table 2). However, the direction of the effect of drought varied across species. Leaf area increased with drought for E. capuli in the gap and for T. micranthum in the forest understory, whereas it decreased with drought for L. styraciflua in both the forest understory, and the gap (Table 2; Appendix). In the forest understory, chlorophyll content of E. capuli and L. styraciflua did not differ between control and drought plots, but values of this response variable decreased with increasing drought for T. micranthum. In contrast, chlorophyl content in the gap decreased with drought for E. capuli and L. styraciflua, while it did not differ between control and drought for T. micranthum (Table 2; Appendix). No effects of drought were found on leaf thickness in the understory for any species. Nevertheless, leaf thickness decreased with drought for E. capuli and L. styraciflua in the gap, while no effects of increasing drought were found for T. micranthum in this habitat (Table 2; Appendix). Toughness of E. capuli and L. styraciflua did not differ between control and drought in the forest understory, but toughness increased with drought for T. micranthum seedlings transplanted in this habitat. In the gap, toughness decreased with drought for E. capuli and L. styraciflua, while no differences between treatments were found for T. micranthum (Table 2; Appendix).
Table 2: Results of orthogonal contrast used to evaluate the effect of water restriction (control and drought treatment) on the functional traits variation of the three woody plant species A) in the understory and B) in the gap of a tropical cloud forest in Veracruz, Mexico. The direction of the effect is indicated as increase or decrease from control to drought (p<0.05), or no effect (p>0.05), df=degrees of freedom, t=statistic, p=probability.
| A) Understory | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Eugenia capuli Schltdl. | Liquidambar styraciflua L. | Trema micranthum (L.) Blume | ||||||||||
| Direction | df | t | p | Direction | df | t | p | Direction | df | t | p | |
| Leaf area | No effect | 111 | -0.21 | 0.83 | Decrease | 111 | -2.21 | 0.029 | Increase | 111 | 2.06 | 0.041 |
| SLA | No effect | 111 | 0.21 | 0.84 | No effect | 111 | -1.83 | 0.069 | No effect | 111 | -1.32 | 0.19 |
| Chlorophyll | No effect | 111 | -1.53 | 0.13 | No effect | 111 | 1.75 | 0.08 | Decrease | 111 | -12.6 | < 0.001 |
| Thickness | No effect | 111 | 0.45 | 0.64 | No effect | 111 | -0.26 | 0.79 | No effect | 111 | -0.87 | 0.386 |
| Toughness | No effect | 111 | 0.57 | 0.57 | No effect | 111 | -1.1 | 0.2 | Increase | 111 | 2.73 | 0.007 |
| B) Gap | ||||||||||||
| Leaf area | Increase | 111 | 5.069 | < 0.001 | Decrease | 111 | -2.02 | 0.046 | No effect | 111 | -1.111 | 0.27 |
| SLA | No effect | 111 | 0.634 | 0.52 | No effect | 111 | -0.37 | 0.71 | No effect | 111 | -1.726 | 0.09 |
| Chlorophyll | Decrease | 111 | -2.37 | 0.019 | Decrease | 111 | -3.89 | 0.0002 | No effect | 111 | -0.154 | 0.88 |
| Thickness | Decrease | 111 | -5.79 | < 0.001 | Decrease | 111 | -2.03 | 0.044 | No effect | 111 | -0.892 | 0.37 |
| Toughness | Decrease | 111 | -2 | 0.047 | Decrease | 111 | -4.29 | <0.001 | No effect | 111 | 1.801 | 0.07 |
Survival curves differed among tree species (Wilcoxon=87.02, p<0.0001). Survival was higher for E. capuli, followed by L. styraciflua and T. micranthum. Eugenia capuli displayed statistically similar survival curves across treatments (Fig. 2A), while L. styraciflua displayed higher survival in the gap-drought treatment (Fig. 2B) and the highest survival of T. micranthum was found under gap conditions (Fig. 2C). Overall, there were differences among treatments. The lowest and the highest survival were recorded in understory-control and gap-control, respectively. Intermediate survival values were found in both the understory-drought and gap-drought treatments (Wilcoxon=58.29, p<0.0001).

Figure 2: Leaf functional traits (mean±1 SE) in controls and water restriction plots located in the forest understory and gap of a tropical cloud forest in Veracruz, Mexico. The functional traits are: A. leaf area (LA, cm2); B. specific leaf area (SLA, cm2/g); C. chlorophyll content (mg/cm2); D. leaf thickness (mm); E. leaf toughness (Newton/cm2). Different superscript letters indicate significant differences between treatments (p<0.05).
Growth rates of the seedlings were compared between forest understory and gap for the drought treatment only. This was because of insufficient number of seedlings at control plots survived until the end of the experiment. In the understory and gap, T. micranthum had the highest RGR in terms of height (RGRh, Χ2 2, 28=6.06, p=0.048; Fig. 3A), whereas E. capuli in understory had the highest RGR in terms of basal diameter (RGRd, Χ2 2, 28=14.28, p=0.0008; Fig. 3B).

Figure 3: Survival (%) of A. Eugenia capuli Schltdl.; B. Liquidambar styraciflua L.; C. Trema micranthum (L.) Blume in controls and water restriction plots located in the forest understory and gap of a tropical cloud forest in Veracruz, Mexico. Different letters following the curve denote significant differences between treatments (p<0.05).
Discussion
Although water restriction was moderate, representing ca. 20% less precipitation, tree seedlings displayed variation in leaf functional traits, seedling survival, and RGR. Functional traits have been used as an approach to evaluate plant species performance in different light conditions (Poorter and Bongers, 2006; Saldaña-Acosta et al., 2009; Toledo-Aceves et al., 2017), water availability levels (Amissah et al., 2015) and soil conditions (Vergara-Gómez et al., 2020). Leaf functional traits of each studied species responded accordingly to the functional group they belong in a continuum of light requirements (Poorter and Bongers, 2006), where trees may have different leaf traits responses depending on water availability (Poorter and Markesteijn, 2008; Berry et al., 2019). Trade-offs have been widely reported, but some studies have found that traits and survival of tropical forest tree seedlings respond independently to drought and shade (Amissah et al., 2015).
The results indicated that moderate drought significantly affects most leaf traits at forest gaps, particularly in E. capuli and L. styraciflua, but not in T. micranthum. In the present study, smaller leaves occurred in L. styraciflua when exposed to drought in both light conditions (Fig. 4). This leaf size reduction has been reported as a short-term strategy that plants display during unusual stress events such as reduced precipitation or experimental drought (Evans and Poorter, 2001; Meier and Leuschner, 2008; Poorter and Rozendaal, 2008).

Figure 4: Relative growth rate in A) height and B) basal diameter of Eugenia capuli Schltdl., Liquidambar styraciflua L. and Trema micranthum (L.) Blume subjected to water restriction in the understory and gap of a tropical cloud forest in Veracruz, Mexico. Different superscript letters denote significant differences between species (p<0.05).
Although SLA has been reported as the most responsive trait to different light environments in cloud forest tree species (Saldaña-Acosta et al., 2009; Toledo-Aceves et al., 2017; Williams-Linera and Manrique-Ascencio, 2020), in the present study it was found that SLA did not change in response to drought or light conditions. These results are consistent with other studies that reported that not all species are equally affected by drought (Berry et al., 2019; Williams-Linera et al., 2022) or response to different light levels (Saldaña-Acosta et al., 2009; Buajan et al., 2017; Olguin et al., 2020). For instance, Saldaña-Acosta et al. (2009) found partial responses where only five species of cloud forests had the largest SLA at low light levels. Olguin et al. (2020) compared the plasticity of seedlings of two tree species acclimated to full sun and canopy shade, finding that light-demanding species displayed similar SLA in both light conditions and suggesting that they did not have the ability to acclimate the leaves to increase light absorption under shade. Buajan et al. (2017), in an evergreen-broadleaved forest from China, studied the effect of light in medium and large canopy openings and reported a negative correlation between light intensity and SLA, with SLA increasing with decreasing light in small and medium size openings.
Overall, chlorophyll content decreased with increasing drought, but it also showed a trend to decrease at the gap. Plants experiencing drought usually exhibit reduced chlorophyll content to reduce photochemical stress (Valladares and Sánchez-Gómez, 2006). Further, decreases in chlorophyll have also been related with increases in irradiation (Evans and Poorter, 2001). In E. capuli and L. styraciflua, chlorophyll content decreased in gap and drought, while T. micranthum showed higher chlorophyll content in the understory. Therefore, changes in the direction of the effect of light intensity and drought on this leaf trait seems to respond according to the functional group to which the tree species belong.
In the drought treatment, leaf thickness and toughness decreased in the gap for E. capuli and L. styraciflua. Conversely, the pioneer species did not change leaf thickness in response to the treatments, and toughness varied in the understory only. Leaf thickness is a trait that changes with light exposure and among plant functional groups (Pérez-Harguindeguy et al., 2013). Also, toughness is an important part of the shade tolerance strategy characterized by a long leaf lifespan, high survival rates and slow growth rates during the regeneration phase (Kitajima and Poorter, 2010).
Survival and growth together gave a picture of the variability among the studied tree species. As expected, the shade-tolerant species E. capuli had the highest survival, whereas the pioneer light-demanding tree T. micranthum displayed the lowest survival in both the gap and the understory. Greenhouse studies have also demonstrated that tree seedlings can survive prolonged drought at shade than at elevated light conditions (Amissah et al., 2015; Berry et al., 2019), while field studies indicated that seedling survival under dry conditions would be higher in understory habitats than in gaps (Amissah et al., 2015), as occurred in this study. The RGR indicated differences of species in response to moderate drought. The shade-tolerant species had the highest RGRd, while T. micranthum had higher RGRh and lower RGRd; this response of taller and thinner is a way to intercept more light with less carbon investment under sunny conditions (Wright et al., 2004). A similar trend has been reported in other shade-intolerant species, which are taller under full sun than at understory habitats, while shade-intermediate species reach higher height under the canopy than in sunny condition (Olguin et al., 2020).
In summary, this work pointed out that plant traits responded to light condition, but drought also affects functional traits when light environment change. The study of only three species cannot be conclusive of forest light conditions and moderate drought responses, and this is a shortcoming of the study that is acknowledged. Additional studies should include several species of functional groups to support any of these patterns. In conclusion, the results of this study indicate that functional traits of shade-tolerant and intermediate tolerant species shift less under a moderate drought than those of a pioneer tree as long as their light environment is maintained.











 nueva página del texto (beta)
nueva página del texto (beta)