Introduction
The black sapote (Diospyros digyna Jacq.), a fruit belonging to the family Ebenaceae, is the most important native species the sapote fruits, which is exploited and commercialized in southern of Mexico and Central America (García-Díaz et al., 2015). In Mexico, this species is found in several tropical and subtropical areas of the country, mainly in the states of Campeche, Chiapas, Oaxaca, Veracruz and Yucatán, and the tree flowers between March and June (Morton, 1987). The sapote production reported for the year 2019 was just over 13 thousand tons, with a value of 57 million Mexican pesos and with an unknown per capita consumption (SAGARPA, 2019). The fruit is a globose berry with a persistent calyx of 7 to 12 cm in diameter, which can have different shapes: elongated cordate, oval, cordate or round (Navarrete-Zapata et al., 2020). When the fruit is immature, it has light golden-yellow flesh and is not edible due to its highly astringent taste. Upon maturity, the exocarp is bright green and the pulp becomes completely smooth and is characterized by its brown-black color, with the six to 10 seeds per fruit being encased in a transparent membrane (SAGARPA, 2003). Due to its organoleptic characteristics, the fruit is consumed fresh, and the pulp is used to make desserts (Miller et al., 1998). In addition, the genus has been associated with health benefits, e.g., it has been shown that plant extracts are related to medicinal properties against hypertension, digestion, and lowering blood sugar levels in people with type 2 diabetes, among others (Rauf et al., 2015). These properties could be related to the compounds present in fruits, which could also be present in D. digyna fruits, such as polyphenolic compounds (protocatechuic, coumaric, caffeic, ferulic and sinapic acids, and flavonols such as myricetin and quercetin), and vitamin C (Can-Cauich et al., 2017; Mannino et al., 2022). The black sapote also contains carbohydrates and minerals, being its major components sugars, polysaccharides, and organic acids such as fumaric, malic and citric acids (Jiménez-González and Guerrero-Beltrán, 2021; Mannino et al., 2022). The chemical composition of the fruit largely depends on the variety and state of maturation (Ghnimi et al., 2017). This fruit presents a climacteric behavior, and the development and ripening process is related to complex physical and chemical changes in its characteristics (Yahia and Gutierrez-Orozco, 2011). During ripening it presents changes in its enzymatic activity, softening and sweetening, as well as in its aroma and color (Arellano-Gómez et al., 2005). Despite its wide acceptance in various regional markets and its large potential as an exotic fruit, there is no information regarding the changes that take place in its physicochemical properties and antioxidant activity during the development and ripening periods. Added to the narrow difference in the color change during ripening, this does not allow to define at a glance the state of maturity of the fruit, which complicates the harvest. Therefore, the objectives of this study were to 1) evaluate the physicochemical properties and antioxidant activity during the development and ripening of D. digyna fruits, in order to know the changes of the fruit and 2) establish the appropriate time of harvest of this climacteric fruit.
Materials and Methods
Sample collection
The fruits of black sapote were obtained from Ajalpan in the state of Puebla, Mexico, located at the following coordinates: 18°23'19.7''N 97°17'54.3''W. The fruits were collected 100 days after the flowering period of the trees (date considered as zero time of the analyzes); the sampling was carried out every two weeks for eight times during the period from August 1 to December 1, 2011. During each sampling, twenty fruits from five different trees were harvested and delivered to the laboratory of the Institute of Basic Sciences of the Universidad Veracruzana (Xalapa, Veracruz, Mexico). Fruits were analyzed within 24 h of harvest, for which they were previously washed with distilled water, and selected according to their uniformity of color, size, and without apparent damage. A specimen (CIB 8988) of the collected fruits was deposited in the herbarium CIB of the Biological Research Institute of the Universidad Veracruzana (Veracruz, Mexico). Each of the analyzes was performed in triplicate.
Physical analysis
The physical properties, such as diameter and height of the black sapote fruit were immediately measured by using a digital Vernier caliper (Uline, model H-7352, Jurupa St, Ontario, California, USA) with a sensibility of 0.01 cm. The weight of each fruit was determined using an analytical balance (H&C Weighing Systems, A&D, Mod. GF-200, Columbia, South Carolina, USA).
Firmness
The firmness was analyzed using a texture analyzer TA-XT-2i (Stable Micro Systems, Lammas Road, Godalming, Surrey, UK) equipped with a 5 kg load cell. The operating conditions of the texture analyzer were a pre-test speed of 0.05 mm/s and a test and post-test speed of 0.03 mm/s. The test was carried out using the whole fruit, which was punctured at 5 mm from the top without passing completely the fruit; thus, the value of the force necessary to break out through the peel and penetrate 5 mm in the pulp was measured. The data of maximum force (N) were recorded using the Texture Expert Exceed Software (Stable Micro Systems LTD., 2006).
Color
The color of the fruit was analyzed using a colorimeter (ColorFlex V1-72, Hunter Lab, Reston VA, USA), evaluating the L* (lightness), a* (green to red) and b* (blue to yellow) parameters. In addition, hue (H°, shade of the color), chroma (purity of a given hue) and total color change (ΔE) were calculated according to the following equations (Loayza et al., 2021):
Respiration rate
The respiration rate was determined via a gravimetric method in glass jars described by Saltveit and Sharaf (1992), analyzing the production of carbon dioxide (CO2). Rate of CO2 production was calculated in closed 10 l containers and reported as the amount of mg of CO2 produced per hour, wich was calculated by the following equation:
Where, V 1 is the volume of the 0.1 N KOH solution, N 1 is the normality of KOH; V 2 is the volume of HCl 0.2 N spent, N 2 is the normality of HCl; W is the weight of the sample, T is the time that the fruit remained inside the chamber. Results were expressed as mgCO2 kg-1h-1.
Physicochemical and proximate composition
The percentages of moisture, ashes, and pH were determined through methods described by the AOAC (2005). The protein content was determined according to Kirk et al. (2005) by the Kjeldahl method using a micro-Kjeldahl distiller (Labconco, Kansas City, Missouri, USA).
Maturity index
The maturity index has been widely used as a criterion of maturity in fruits, and it is calculated as the ratio of total soluble solids content to total titratable acidity (TSS/TTA). Therefore, the soluble solids were evaluated using a digital refractometer (model HI96800, Hanna Instruments, Rhode Island, USA), for which the fruit pulp was extracted, ground, and filtered to obtain a juice, a sample of 0.1 ml of juice was placed on the refractometer. Another sample was diluted by adding 10 ml of distilled water and the titratable acidity was evaluated (Muharfiza et al., 2017).
Determination of bioactive compounds
Preparation of the sample for analysis of bioactive compounds
The lyophilized sample of pulp (1 g) was mechanically homogenized with 10 ml of bidistilled water in a manual blender and sonicated in an Ultrasonic bath (Rochester Industrial Services, Branson model 2510, Rochester, New York, USA) for 30 min and agitated in a horizontal shaker at room temperature (24 °C) for 1.5 h. Then, the sample was centrifuged (Hettich, Mod. Universal 32R, Föhrenstr, Tuttlingen, Germany) at 2200 g for 15 min. The supernatant was removed, and the residue was re-extracted twice with 10 ml of a mixture of methanol: HCl 0.1N (1:1, v/v). The three supernatants were pooled and brought to a final volume of 100 ml with the same solvent used in the last two extractions. This extract was prepared in triplicate and used for the analysis by triplicate of bioactive compounds and antioxidant activity.
Vitamin C
The vitamin C content was determined according to Juárez-Trujillo et al. (2018) by using a second-order derivative spectrometric method. For this, a calibration curve was generated with different concentrations of ascorbic acid (3.5-17.6 mg/ml), the absorbance was evaluated in the range of 250-350 nm using a spectrophotometer (8453; Agilent Technologies, Santa Clara, California, USA). The results were expressed as mg equivalents of ascorbic acid per gram of sample.
Polyphenols
Total polyphenol content was determined in the extract according to Singleton et al. (1999) with slight modifications according to Juárez-Trujillo et al. (2018). For this, 30 µl of extract and 30 µl of Folin-Ciocalteu reagent (Sigma-Aldrich, San Louis, Missouri, USA) were added to a well of a microplate, which was incubated for 2 min at 40 °C. Then 240 µl of Na2CO3 were added to the mixture; once the mixture was stirred, it was incubated for 20 min at 40 °C. After this time, the absorbance was measured at 765 nm on a spectrophotometer (Cary 100 UV-Visible, Varian Analytical Instruments, Walnut Creek, California, USA). The results were expressed as mg GAE/g, by generating a calibration curve in a range of 0-2 mg/g.
The concentration of tannins was determined using a method proposed by Lastra et al. (2000). For this determination, 1 ml of extract was mixed with 2 ml of distilled water, 0.5 ml of Folin-Denis reagent (Sigma-Aldrich, San Louis, Missouri, USA), and 0.5 ml of an aqueous solution of sodium carbonate (8.5% w/v) and completed to 25 ml with distilled water. After 20 min incubation at room temperature, the absorbance at 760 nm was determined in a spectrophotometer (Varian, Cary 100 UV-Visible, Varian Analytical Instruments, Walnut Creek, California, USA). The results were expressed as % of tannins with the help of a tannic acid calibration curve (0-2 mg/g).
Carotenoid content
The carotenoid content was evaluated according to Ghafoor et al. (2020) with some modifications. For this, 1 g sample was macerated with 10 ml of acetone, until exhaustion. The extract was filtered and placed in a separatory funnel, to which 5 ml of petroleum ether was added and mixed. Subsequently, 15 ml of distilled water was added, observing the formation of the ethereal layer. The lower layer was re-extracted with 5 ml of ether and 15 ml of water. This washing step was repeated three times, then it was diluted until reaching 50 ml of petroleum ether into a volumetric flask. Finally, the extract was read at 450 nm in a spectrophotometer (Cary 100 UV-Visible, Varian Analytical Instruments, Walnut Creek, California, USA). Total carotenoids were expressed as mg/100g of fruit and calculated according to the following equation:
Where, L is the absorbance of the sample, A is the volume of the flask (50 ml), and M is the sample weight.
Antioxidant activity
The antioxidant activity was studied from the fruit extract, previously described, which was filtered and lyophilized, for which the reducing power and the DPPH (2,2-diphenyl-1-picrylhydrazyl) free radical scavenging capacity were evaluated. The reducing power was determined according to Yen and Chen (1995), where 0.25 ml of a sample with a concentration of 1 mg/ml was mixed with 2.5 ml of sodium phosphate buffer 200 mM (pH 6.6) and 2.5 ml of potassium ferricyanide (1% v/v). The mixture was incubated for 20 min at 50 °C in a water bath. After incubation, 2.5 ml of an aqueous solution of trichloroacetic a°cid (10%, w/v) was added and the mixture was centrifuged (Hettich, Model Universal 32R, Föhrenstr, Tuttlingen, Germany). Then, 5 ml of supernatant was added to 5 ml of distilled water and 1 ml of ferric chloride. The absorbance was read at 700 nm. Finally, the reducing power was reported as the absorbance obtained from each sample and was compared to the reducing power of ascorbic acid.
The antioxidant activity was also evaluated by the scavenging DPPH free radical according to Liyana-Pathirana et al. (2006). For this, 0.1 ml of the sample at a concentration of 1 mg/ml was added to 2.9 ml of DPPH solution and kept protected from the light. Samples were read at 517 nm after 10, 20, and 30 min in a spectrophotometer (Cary 100 UV-Visible, Varian Analytical Instruments, Walnut Creek, California, USA). A control with 0.1 ml of methanol was prepared simultaneously.
Statistical analyzes
For the treatment of results, an analysis of variance (ANOVA) was carried out to verify that the assumptions of normality and homogeneity of variances were met, using Statistica v. 10 (StatSoft Inc, 2011). Comparisons among the samples were performed by a post hoc Tukey analysis with α 0.05. A Pearson correlation was performed to establish the relationship between the different variables.
Results
Physical analyzes
The changes in the physical characteristics during the development and ripening of the fruit are shown in Table 1. It is observed that the weight increased significantly (p<0.05) over time, multiplying its size more than 6 times, reaching a final weight of 499.98 g. On the other hand, the height increased significantly (2.18 times) reaching 9.6 cm at the end of the study. In the case of the diameter, it reached 11.4 cm; however, the increase was significant until week 10. No significant differences were found in height and diameter during the last three samplings.
Table 1: Physicochemical parameters of Diospyros digyna Jacq. samples during on-tree growing. Data have mean standard deviation. Different letters within the same row mean significant difference (p<0.05, n=10). Samples of the fruits were collected three months and nine days after the flowering period of the trees. L*=lightness, a*=green to red, b*=blue to yellow.
| Weeks | ||||||||
| Parameter | 0 (1 Aug) | 2 (16 Aug) | 4 (30 Aug) | 6 (13 Sep) | 8 (27 Sep) | 10 (11 Nov) | 12 (25 Nov) | 14 (1 Dec) |
| Physical parameters | ||||||||
| Weight (g) | 72.52±9.60a | 97.6±5.60b | 161.2±11.90c | 200.9±5.95d | 251.9±21.10e | 341.5±6.80f | 356.9±9.20g | 499.98±4.40h |
| Height (cm) | 4.05±0.13a | 4.40±0.65a | 5.76±0.27b | 5.60±0.18b | 8.00±0.27c | 7.86±0.85cd | 8.15±0.75cd | 9.60±0.95d |
| Diameter (cm) | 5.61±0.45a | 6.5±0.50b | 7.4±0.40c | 8.1±0.25d | 9.9±0.30e | 10.5±0.75ef | 10.9±0.95ef | 11.40±0.75f |
| Color parameters of the pericarp | ||||||||
| L* | 51.51±0.09a | 51.52±0.10a | 48.62±0.14b | 47.56±1.50bc | 45.97±1.90c | 45.91±0.98cd | 42.43±2.90de | 39.68±0.26e |
| a* | -15.95±0.19a | -15.95±0.20a | -14.61±0.17b | -14.49±0.25b | -14.72±0.17b | -13.83±0.97bc | -13.29±0.24c | -13.06±0.51c |
| b* | 35.13±0.75a | 35.13±0.66a | 36.35±0.19b | 35.73±0.44ab | 38.73±0.15c | 39.67±0.78d | 39.64±0.19d | 41.55±0.90e |
| Hue angle | 155.58 ±0.05a | 155.58±0.05a | 155.58±0.18a | 158.10±0.20b | 157.94±0.36b | 160.79±0.29c | 161.48±0.90cd | 162.55±0.90d |
| Chroma | 38.56±0.03a | 38.50±0.03a | 39.25±0.90a | 38.52±0.08a | 41.44±0.18b | 41.99±0.77bc | 41.77±0.10c | 43.53±0.03d |
| Physicochemical and proximate composition | ||||||||
| Moisture (%) | 84.35±1.10a | 84.8±0.70a | 81.7±1.50bc | 81.6±0.50b | 81.2±0.90bc | 81.5±0.70bc | 79.4±1.60c | 79.75±1.90bc |
| Protein (%) | 0.70±0.03b | 0.70±0.03b | 0.68±0.01b | 0.69±0.06b | 0.87±0.10a | 0.90±0.09a | 0.43±0.01c | 0.42±0.01c |
| Ash (%) | 0.24±0.13a | 0.39±0.04ad | 0.48±0.02b | 0.53±0.10bc | 0.54±0.07bc | 0.57±0.03c | 0.64±0.12c | 0.48±0.08bcd |
| pH | 5.7±0.02a | 6.17±0.18b | 6.17±0.08b | 6.56±0.50c | 5.88±0.17ade | 6.48±0.50bcd | 5.66±0.83abde | 5.70±0.04ae |
| Soluble solids (°Bx) | 2.00±0.03e | 2.77±0.23d | 3.67±0.58c | 3.73±0.47c | 5.17±0.29a | 5.57±0.28a | 5.83±0.58a | 4.60±0.01b |
| Total acidity (%) | 0.14±0.03a | 0.12±0.02a | 0.10±0.02ab | 0.09±0.02ab | 0.09±0.01b | 0.09±0.05ab | 0.09±0.01b | 0.09±0.01b |
| Maturation index | 14.23±0.23f | 22.98±0.01e | 36.31±0.10d | 40.55±0.80c | 55.02±0.90b | 61.42±0.89a | 59.96±0.90a | 60.00±0.67a |
| Respiration rate | ||||||||
| Respiration rate (mg CO2kg-1 h-1) | 1061.10±0.01a | 586.86±0.10b | 490.20±2.10c | 423.05±0.05d | 312.97±0.32g | 350.86±0.10e | 318.59±0.00f | 303.58±0.63h |
Color
Table 1 shows the parameters obtained in the color of the pericarp, where the initial values were 51.51, -15.95 and 35.13 for L*, a* and b*, respectively. The L* parameter decreased significantly during the pre-harvest period; in contrast, the a* and b* parameters increased over the 14 weeks, indicating a loss of greenness, and an increasing yellowness. Figure 1 shows the color change of black sapote during the 14 weeks evaluated. This was also confirmed by the total color change (ΔE) after 14 weeks with a value of 13.76. Chroma parameter also increased significantly from week eight, so there is a relationship concerning the maturity of the fruit.
Physicochemical and proximate composition
The majority component in fruits, such as the black sapote, is water. So, regarding the moisture content of the black sapote fruit, a decrease in the parameter was observed with respect to the sample collection time, reaching a value of 79.75% in week 14 (Table 1).
Changes in protein content are a common feature of fruit ripening. In this case, a significant decrease in the protein content of the fruits was found over the 14-week period, from 0.70% to 0.42%. Conversely, the ash percentage increased from 0.24 to 0.48% during the pre-harvest period. In the case of pH, its initial value was 5.7, reaching a value of 6.48±0.50 at week 10. However, at week 14 the pH decreased again to 5.7.
In this research, the black sapote presented a significant increase (p<0.05) from 2 to 4.6 °Bx, which was mainly associated with an increase in the sugar content of the fruit during the 14 weeks of analysis. On the other hand, the titratable acidity, another important chemical characteristic, decreased from 0.14 to 0.09% over the 14 weeks (Table 1).
Maturity index
The present study showed an increasing trend from an initial value of 14.23 to 61.42 of maturity index by week 10, which then remained the same for the next four weeks, thus indicating the beginning of the harvest period of the fruit and its commercial maturity.
Respiration rate
A decrease in CO2 production was observed from 1061.10 to 303.58 mg CO2 kg-1h-1 at 14 weeks (Table 1). However, the respiration rate of the black sapote did not show a characteristic climacteric peak. Nevertheless, the difference in change in respiration rate was smaller in the last two weeks, suggesting the start of commercial maturity or harvest time, which is physically characterized by the presence of a yellow zone around the peduncle of the fruit and a raised calyx at the base of the fruit (Fig. 2).
Firmness
Figure 3 shows a representative image of one of the replicas, showing the fracturability when penetrating the peel and its subsequent increase until reaching the maximum hardness at 5 mm of penetration, which refers to the pulp. Table 2 shows the values of the fracturability of the peel and the maximum hardness attained in the fruit pulp with respect to the degree of maturity. The fracturability of the peel decreased 36% from an initial value of 2.185 to 1.409 N. The peel showed significant changes in texture values (p≥0.05) over time.
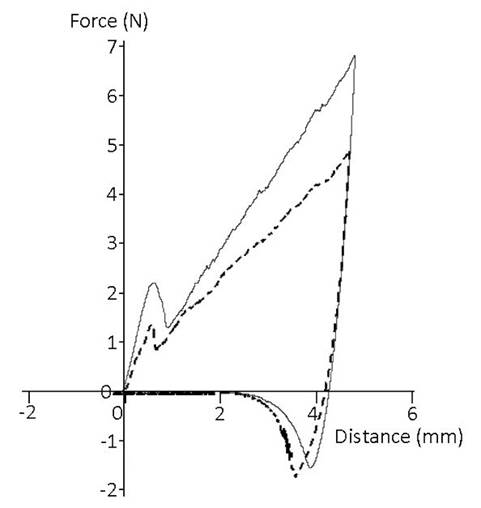
Figure 3: Stress-strain graphs obtained from a texture analyzer at time 0 (―) and time 14 (- -) in the pre-harvest period of Diospyros digyna Jacq.
Table 2: Fracture strength of the peel and maximum force obtained at 5 mm within black sapote Diospyros digyna Jacq. pulp at different stages of maturity. Values in the same column with different letters indicate significant differences, Tukey (p<0.05). The results show the average of three trials ± SD.
| Time (weeks) | Peel (N) | Pulp (N) |
| 0 | 2.185 ± 0.02g | 7.459 ± 0.06d |
| 2 | 1.748 ± 0.06f | 7.293 ± 0.16d |
| 4 | 1.659 ± 0.02e | 6.467 ± 0.37c |
| 6 | 1.615 ± 0.01d | 6.608 ± 0.22c |
| 8 | 1.527 ± 0.02c | 6.348 ± 0.33c |
| 10 | 1.473 ± 0.27abcdef | 5.620 ± 0.33b |
| 12 | 1.456 ± 0.02b | 5.101 ± 0.13a |
| 14 | 1.409 ± 0.00a | 5.356 ± 0.43ab |
On the other hand, the maximum hardness obtained at 5 mm in the fruit pulp was approximately 7.459 N at week zero and decreased to 5.356 N by week 14, so there was a decrease of 29%. However, no significant differences were found in the maximum hardness between week 10 and 14.
Bioactive compounds
Figure 4 shows the changes in the profile of vitamin C, polyphenols, tannins and carotenes, compounds with antioxidant properties at different stages of fruit growth. Figure 4A shows the increase in vitamin C concentration during fruit development (from 60.60 to 369.140 mg/100 g). The production of vitamin C followed two patterns: first, the vitamin C content varied from 60.60 mg/100 g (week zero) to 124.93 mg/100 g (week 10), following a zero-order kinetic with a constant speed of k=-9.75 (r=0.8314); and second, after week 10, there was a significant increase in the content of vitamin C to 369.14 mg/100 g (week 14), with k=-103.09 (r=0.9378).
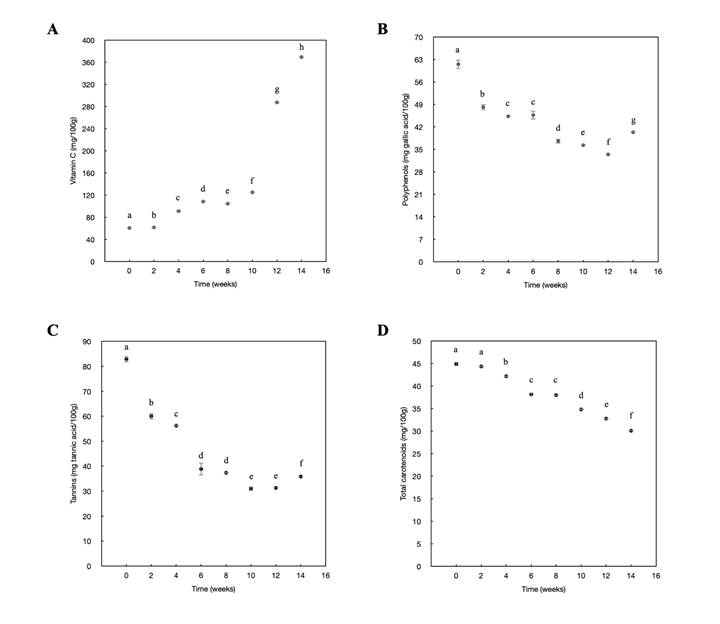
Figure 4: Changes during the pre-harvest period in Diospyros digyna Jacq. A. vitamin C; B. polyphenols; C. tannins; D. total carotenoids.
In this research, during the development of black sapote, the polyphenol content exhibited an initial value of 61.54 mg/100 g, reaching its greatest decrease in week 12 with a value of 33.46 mg/100 g (Fig. 4B). Tannins (Fig. 4C) showed 57% decrease in concentration at the end of the analysis (week 14). However, in week 10, the lowest value was observed, which coincides with other parameters evaluated in this manuscript, such as the week of the beginning of the harvest period and commercial maturity. Regarding carotenoids, the black sapote exhibited a decrease in its concentration during its development (from 44.34 to 30.06 mg/100 g) (Fig. 4DD. So, the lowest value was found in week 14 (30.06 mg/100 g).
Antioxidant activity
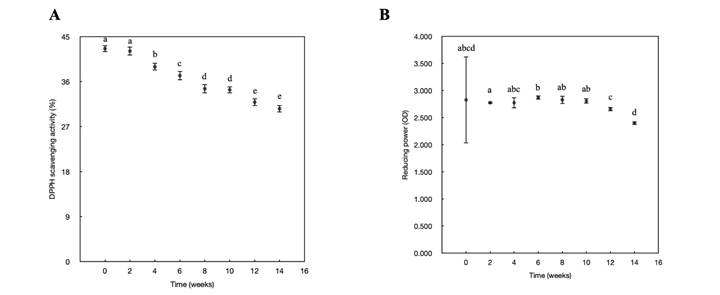
Figure 5A shows that the DPPH scavenging activity of the unripe black sapote decreased from 42.60% to 30.56% from week 0 to week 14. In contrast, the reducing power of the black sapote showed no significant difference (p≥0.05) throughout the 14-week pre-harvest period (Fig. 5B).
Multivariate analysis of Diospyros digyna
Pearson correlation was used to indicate the relationship between the physicochemical and antioxidant properties with the maturity of the black sapote (Table 3). Significantly strong (p<0.01) positive correlations have been observed. Such is the case of the respiration rate with the broken peel (r2=0.986), the total acidity (r2=0.948), tannins (r2=0.967), and polyphenols (r2=0.961). That could establish that a higher rate of respiration promotes the formation of bioactive compounds such as polyphenols and tannins, as well as organic acids, which would explain the increase in total acidity. In the same way, the color parameters a* (r2=0.909), b* (r2=0.909), and the hue angle (r2=0.945) showed a positive correlation with the maturity index during fruit development. The L* parameter showed a significantly stronger positive correlation with carotenoids (r2=0.973) and DPPH scavenging (r2=0.975). A strong positive correlation between DPPH scavenging and the contents of total carotenoids (r2=0.979) was observed, which indicates that when the fruit is unripe on the tree, these components contribute to the antioxidant activity of the fruit, as well as polyphenols (r2=0.842) and tannins (r2=0.878). A significant negative correlation (p<0.01) was found between the maximum force required to penetrate the peel (r2=-0.919) and pulp (r2=-0.935) and the maturation index.
Table 3: Correlations between variables during the pre-harvest period of black sapote Diospyros digyna Jacq. Values in bold are significantly different from zero with a significance level of p<0.01. L*=lightness, a*=green to red, b*=blue to yellow.
| Variables | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 |
| 1. Respiration rate | 1 | ||||||||||||||
| 2. Soluble solids (ºBrix) | -0.890 | 1 | |||||||||||||
| 3. Total Carotenes | 0.765 | -0.824 | 1 | ||||||||||||
| 4. Total acidity | 0.948 | -0.831 | 0.703 | 1 | |||||||||||
| 5. Tannins | 0.967 | -0.920 | 0.853 | 0.935 | 1 | ||||||||||
| 6. Polyphenols | 0.961 | -0.961 | 0.779 | 0.874 | 0.945 | 1 | |||||||||
| 7. DPPH scavenging | 0.812 | -0.886 | 0.979 | 0.756 | 0.878 | 0.842 | 1 | ||||||||
| 8. Vitamin C | -0.500 | 0.560 | -0.882 | -0.388 | -0.573 | -0.544 | -0.844 | 1 | |||||||
| 9. Maturity index | -0.909 | 0.965 | -0.920 | -0.859 | -0.944 | -0.931 | -0.959 | 0.679 | 1 | ||||||
| 10. Broken peel | 0.986 | -0.874 | 0.829 | 0.915 | 0.958 | 0.950 | 0.864 | -0.627 | -0.919 | 1 | |||||
| 11. L* | 0.714 | -0.773 | 0.973 | 0.645 | 0.780 | 0.739 | 0.975 | -0.935 | -0.880 | 0.800 | 1 | ||||
| 12. a* | -0.778 | 0.829 | -0.961 | -0.753 | -0.836 | -0.791 | -0.953 | 0.854 | 0.909 | -0.838 | -0.949 | 1 | |||
| 13. b* | -0.716 | 0.825 | -0.932 | -0.600 | -0.754 | -0.764 | -0.945 | 0.833 | 0.909 | -0.777 | -0.936 | 0.885 | 1 | ||
| 14. Hue angle | -0.784 | 0.866 | -0.973 | -0.720 | -0.833 | -0.815 | -0.980 | 0.856 | 0.945 | -0.842 | -0.967 | 0.977 | 0.963 | 1 | |
| 15. Maximum force in pulp | 0.780 | -0.903 | 0.920 | 0.769 | 0.897 | 0.811 | 0.914 | -0.649 | -0.935 | 0.783 | 0.831 | -0.878 | -0.835 | -0.891 | 1 |
Discussion
Morphological and physicochemical properties
Weight, height, and diameter are physical characteristics associated with maturity and are considered primary indicators in fruit harvest (Shen et al., 2019). Therefore, knowing the changes in the physical characteristics of the fruit such as the black sapote allows the producer to harvest at the right time, avoiding losses and having the necessary time for its commercialization. The results obtained showed an increase in the values of each characteristic during its development, which were higher than those reported for the persimmon fruits (Diospyros kaki Thunb. and D. kaki cultivar Rubi) (Lucena Cavalcante et al., 2007; Del Bubba et al., 2009), and those described for black sapote by Corral-Aguayo et al. (2008), who obtained a maximum weight of 179.97±17.34 g for black sapote that was at its overripe stage. Likewise, firmness is another parameter that changes during maturation. In this sense, the results showed a decrease in the fracturability and maximum hardness during maturation, which may be related to changes in cell structure. Therefore, as the fruit ripens, compounds such as pectin and protopectin are separated from the cellulose and solubilized in the water of the fruit, which leads to a softening of the tissue, and therefore a decrease in firmness (Del Bubba et al., 2009; Chang et al., 2017; Xue et al., 2020; Jiménez-González and Guerrero-Beltrán, 2021). As mentioned in the results, a significant negative correlation was found between the maximum force required to penetrate the peel, pulp and the maturation index, noting that the higher the maturity index, the lower the force that will be required to penetrate. This, in addition to the results mentioned above, suggests that, based on these parameters, the fruit could be harvested from week 10, since no significant differences are observed in the maximum penetration force.
Color is another physical characteristic that showed a relationship with the ripening of the fruits. Color change in fruit is one of the most striking phenomena associated with ripening, since in most cases this makes it possible to identify the appropriate harvest time (Gross et al., 1984). Nonetheless, for the black sapote, the slight change in color of the pericarp makes it difficult to know when it should be harvested, since when the fruit is immature its color is clover green, and when it ripens its color is forest green with small brown spots (Jiménez-González and Guerrero-Beltrán, 2021). On the contrary, the color of the pulp changes from yellow to black and thus offers an indicator of ripeness. However, for this research, only the color of the pericarp was evaluated since it is the only color that could be evaluated before being harvested. Hue angle and chroma were two parameters monitored in this study, since it has been established that hue angle is the best indicator of color changes during fruit development, while chroma indicates color intensity (Mikulic-Petkovsek et al., 2015). As mentioned above, both parameters increase over time during the development and ripening of black sapote, indicating an increase in the intensity of the green color coinciding with the change from clover green to forest green (Jiménez-González and Guerrero-Beltrán, 2021).
When the black sapote is harvested in its maturity stage, it is usually a climacteric fruit since it produces high amounts of carbon dioxide (CO2) and ethylene (C2H4), which makes this fruit highly perishable. A production of 367.3 mg of CO2 kg-1h-1 has been reported even after six days of storage (Arellano-Gómez et al., 2005). Therefore, refrigerated storage is recommended in order to increase shelf life and decrease the rate of ripening. However, the CO2 production in this study (Table 1) was evaluated during development, which in this case showed a decrease over the 14 weeks. So, it did not show the climacteric peak, at least not during the 14-week period of our study. However, the last two weeks there was a change in respiration, physically characterized by the presence of a yellow zone around the peduncle, which suggests that the fruit could be harvested in week 10. The values obtained in this test were similar to those obtained by Arellano-Gómez et al. (2005) (367.3 mg CO2 kg-1h-1) and higher than those reported for persimmon (46 mg CO2 kg-1h-1), which could indicate that black sapote is more perishable than persimmon, despite the fact that both fruits are climacteric (Dussán-Sarria et al., 2008).
Water is the major component of black sapote, and as its content decreases, the size and weight of the fruit increases, which may be due to the fact that, during development, water is mainly used to produce pectic substances, carbohydrates, and other components of the pulp (Chávez-Reyes and Arana-Errasquin, 2006; Corral-Aguayo et al., 2008; Conesa et al., 2020). The mineral content is a changing condition during maturation of the fruits (Marles, 2017) in the case of black sapote, an increase was observed during its development due to an accumulation of minerals. It is well known that black sapote is rich in calcium (Morton, 1987), so it could be an important source of this, even better than fruits such as banana, coconut, kiwi, and papaya (Yahia et al., 2011). Phosphorus and iron can also be found in significant quantities (Jiménez-González and Guerrero-Beltrán, 2021).
On the other hand, within the content of black sapote are organic acids, which decrease during the development of the fruit, because they are used in the respiration of the fruit and are converted into sugars (Wills et al., 2007). Therefore, acidity and solid soluble content are considered as indicators of fruit maturity. Acidity in a sensory sense affects the acceptance of the fruit, e.g., fruits with acidity levels from 0.08 to 1.95 can be classified as mild-tasting fruits and therefore are easily accepted by consumers (Paiva et al., 1997). As the black sapote is found within this classification, it will not present problems of acceptance. Soluble solids content also defines the acceptance of fresh fruit since consumers generally prefer sweeter fruits. An increase in soluble solids is observed, which may also be due to the degradation of polysaccharides into simple sugars (Wills et al., 2007). Soluble solids have been reported from 14 to 22 °Brix in black sapote (Moo-Huchin et al., 2014; Navarrete-Zapata et al., 2020); however, in this study the soluble solids reached a maximum value of 5.83 °Brix in week 12. This difference may be since the Brix degree values of the fruits reported by other authors were in different stages of maturity. Total titratable acidity and soluble solids content are related to the maturity index. This criterion is useful for determining harvest time. Besides, this parameter is the most important since it determines the flavor of the products (Muharfiza et al., 2017).
Chemical compounds and antioxidant properties
Diospyros digyna Jacq. fruits are known for their nutraceutical and pharmacological properties, derived from the bioactivity of secondary metabolites such as flavonoids, phenolic acids, among others (Martínez-Las Heras et al., 2017; Ramírez-Briones et al., 2019). One of the major bioactive components found in the black sapote is vitamin C, which contributes to the antioxidant activity (Corral-Aguayo et al., 2008). In this study, the production of vitamin C during fruit development showed two stages: in the second stage the increase was much higher than during the first stage, which coincides with a constant respiration rate, and with the conversion of substances into vitamin C in fruits. The phenolic compounds have also been associated with the antioxidant activity of fruits. Among the phenolic compounds reported in black sapote are trans-cinnamic acid, sinapic acid, caffeic acid, coumaric acid, p-hydroxybenzoic acid, myricetin, ferulic acid, ellagic acid and catechin, epicatechin and quercetin (Can-Cauich et al., 2017; Mannino et al., 2022). In this research, a decrease in the content of polyphenols (33.46 mg/100 g) was observed after 12 weeks of development. This value was found below that reported by Moo-Huchin et al. (2014) (158 mg/100 g). Meanwhile, tannins are high molecular weight polyphenolic compounds. Its presence causes the astringency of immature fruits, for which the changes in the tannin profile play an important role in determining the harvest time (Maitera et al., 2018). Therefore, in this case, a decrease in the concentration of tannins was observed, with 10 weeks being the time with the lowest content, and with a decrease of 57% at the end of the study (week 14), which coincides with other parameters evaluated in this research, such as the week of the beginning of the harvest period and commercial maturity. This decrease has also been observed in fruits of the same genus such as D. kaki, known as persimmon (Del Bubba et al., 2009). The carotenoids showed a decrease in concentration during their development, which could be associated with the presence of active oxygen produced in the chloroplasts during photosynthesis or by the action of lipoxygenase enzymes (Seymour et al., 1993).
Black sapote fruits have a large amount of phenolic compounds and carotenoids that have already been shown to have antioxidant capacity. This coincides with the correlation analysis, where a positive correlation between L* parameter, carotenoids and DPPH was observed. This could be interpreted as the carotenoids contributing to the intensification of luminosity in the fruit, which could increase the capacity of scavenging the DPPH. However, previous studies have established that antioxidant activity may depend on the harvest season and maturity of the fruit (Arellano-Gómez et al., 2005; Chávez-Reyes and Arana-Errasquin, 2006; Corral-Aguayo et al., 2008; Ramírez-Briones et al., 2019). So, the reducing power and DPPH radical scavenging were evaluated in order to demonstrate the ability to donate electrons of the compounds of black sapote, which could scavenge free radicals and reactive oxygen species, and also reduce the valence of elements to their lowest valence. There are different mechanisms by which antioxidant activity is evaluated; the reducing power is based on the transfer of a single electron. While the DPPH radical scavenging assay is based on the transfer of a hydrogen atom, it is thus assumed in this method that the demonstrated antioxidant activity is equivalent to its ability to donate an electron (Shahidi and Zhong, 2015). In this study, a reduction in the antioxidant activity evaluated with both methods was observed, possibly due to the decrease in compounds such as polyphenols, tannins, and mainly carotenoids, which contribute to antioxidant activity (Chen et al., 2008) of black sapote, according to the correlation results.
Conclusions
The physicochemical and antioxidant properties of the black sapote fruit undergo modifications while the fruit continues to develop on the tree and ripening, like any fruit. However, the lack of studies and its characteristic color change that remains imperceptible to the human eye have not allowed to establish the best harvest time. Among the main characteristics of the ripening of a fruit is the formation of bioactive compounds. Our results showed that the antioxidant activity is mainly provided by carotenoids, polyphenols, and tannins. In addition, together with the changes observed in the firmness and decrease in respiration, among other parameters, it can be concluded that the fruits could be harvested in week 10 of the analysis, enabling to take advantage of the components such as vitamins, minerals, bioactive compounds found in the D. digyna fruits, which is considered the most important species of sapote fruits, harvested and consumed in Mexico (García-Díaz et al., 2015). However, it is an under valued product because there are few studies, despite being associated with benefits for health. Therefore, it is advisable to continue researching this fruit.











 nueva página del texto (beta)
nueva página del texto (beta)