Introduction
Marthamyces was described by Minter (2003) with M. emarginatus (Cooke & Massee) Minter as the type species. It was set up to accommodate species with filiform ascospores placed in Propolis (Fr.) Corda sensu Sherwood (1977) and growing on dead leaves. Marthamyces includes species with ascomata on abaxial and adaxial surface of plants throughout the world, but mainly in the tropics and subtropics (Johnston, 2006). Macroscopically, apothecia are subepidermal, orbicular to polygonal, resembling small pustules, erumpent from the substrate surface, not associated with bleaching of surrounding substrate, and lacking associated zone lines (Minter, 2003; Johnston, 2006). They are deeply immersed within the host tissue, and the surface of the hymenium often has a pruinose appearance due to the presence of small crystals amongst the paraphyses (Johnston, 2006). Microscopically, ascospores are filiform, 0- to 3-septate, with apical and basal gelatinous caps and the paraphyses have short branches near apex, tapering or swelling, tangled or with several short, finger-like projections, either often intermixed with crystals, or coated with crystals, the tips of the paraphyses sometimes being embedded in brownish gel (Sherwood, 1977; Johnston, 2006). Marthamyces is the type genus of the family Marthamycetaceae, order Chaetomellales, class Leotiomycetes of the phylum Ascomycota (Ekanayaka et al., 2019).
The genus Marthamyces has 18 described species. Of these, 13 species are registered from Australia and New Zealand, five from the Americas, three from Europe, two from Asia and one from Africa. Only two have previously been reported from Mexico, Marthamyces quadrifidus (Lév.) Minter (as Propolis quadrifida (Lev.) Mont.) from Oaxaca (Sherwood, 1977), and M. coronadoae Raymundo, R. Valenz. & Esqueda described on Fagus grandifolia Ehrh. subsp. mexicana (Martínez) A.E. Murray in the municipality of Zacualtipán, Hidalgo state (Raymundo et al., 2016). In 2019, during a visit to the Cozumel Island Biosphere Reserve, several fallen leaves of Avicennia germinans (L.) L. (black mangrove) were collected in the Punta Sur Ecological Park in mangroves. They had small pustules of erumpent apothecia of a fungus which did not correspond to any known species. The aims of this study are to describe and illustrate Marthamyces manglicola as a new species that grows on Avicennia germinans (black mangrove) on Cozumel Island and to elaborate a key to all species of the genus.
Materials and Methods
Punta Sur Ecological Park, located in the Cozumel Island Biosphere Reserve, Quintana Roo, Mexico, has an area of 1114 ha, with coastal dunes and mangroves as a principal type of vegetation (Fig. 1). Dominant plant species are Rhizophora mangle L. (Rhizophoraceae), Laguncularia racemosa (L.) C.F. Gaertn. (Combretaceae), Conocarpus erectus L. (Combretaceae) and Avicennia germinans (Acanthaceae).

Figure 1: Map of the locality of Marthamyces manglicola Raymundo, García-Martínez, Martínez-Pineda & R. Valenz.
Our specimens are deposited in the Herbarium ENCB of the Escuela Nacional de Ciencias Biológicas, Instituto Politécnico Nacional, Mexico City, Mexico. Longitude and latitude coordinates were obtained with GPS eTrex (Garmin, Olathe, USA). Colors were coded according to Kornerup and Wanscher (1978). Morphological examinations were conducted using protocols outlined by Johnston (2006) and Raymundo et al. (2016). Measurements of anatomical characters were taken from rehydrated tissues in 5% aqueous KOH and Melzer’s reagent with camera optical microscope (CX-31, Olympus, Tokyo, Japan). The macroscopic pictures were taken with a Nikon D7000 (Nikon, Tokyo, Japan) and the microscopic pictures with a Sony DSC-WX350 (Nikon, Tokyo, Japan). The morphological terms are based on the dictionary of Ulloa and Hanlin (2012).
A key of all described species to the genus Marthamyces is presented as a tool to differentiate the new species from the other species. The identification key can be applied globally given the broad distribution of the host (Avicennia L.) in different parts of the world. The following literature was used to elaborate this key: De Notaris (1845), Sherwood (1977), Johnston (1986, 1991, 2006), Gu et al. (2015), Raymundo et al. (2016), Crous et al. (2019), Johnston and Park (2019).
Results
Taxonomy
Ascomycota
Letiomycetes
Chaetomellales
Marthamycetaceae
Marthamyces manglicola Raymundo, García-Martínez, Martínez-Pineda & R. Valenz., sp. nov. Figs. 2, 3.

Figure 2: Marthamyces manglicola Raymundo, García-Martínez, Martínez-Pineda & R. Valenz. A. ascomata growing on leaves of Avicennia germinans L.; B. gregarious apothecia on abaxial surface; C. gregarious apothecia on adaxial surface; D. apothecia with exposed hymenium; E. polygonal hypothecia; F. quadrangular and triangular apothecia; G. pruinose hymenium.
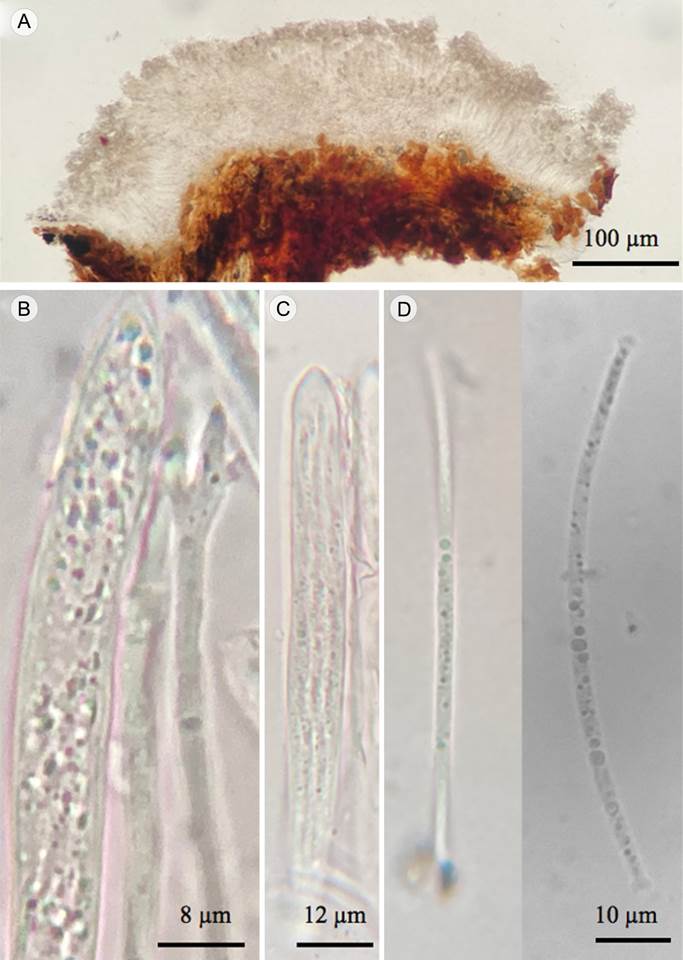
Figure 3: Marthamyces manglicola Raymundo, García-Martínez, Martínez-Pineda & R. Valenz. A. apothecium; B. asci and paraphyse apex; C. asci; D. ascospores.
TYPE: MEXICO. Quintana Roo, municipio Cozumel, Reserva de la Biosfera Isla de Cozumel, Parque Ecológico Punta Sur, 28°16'48''N, 86°58'44.39''W, 16.X.2019, T. Raymundo 8324 (holotype: ENCB!); Mycobank: MB839088.
Marthamyces manglicola differs from M. quadrifidus in having apothecia opening by 3-5 pruinose and yellowish white to pale yellow flaps, a hymenial surface greyish blue to dull blue in young specimens, fading to bluish white to pale blue or pale yellow in mature apothecia; paraphyses swelling slightly near apex, with several short, finger-like projections and ascospores 50-60 × 1-2.4 µm, straight to slightly curved, hyaline, 0- to 1-septate.
Apothecia 0.7-1 mm diameter, on abaxial and adaxial surfaces of dead leaves, subepidermal, resembling small pustules, visible initially as round brown patches on the leaf surface, becoming raised as ascomata mature, erumpent from the substrate surface, not associated with bleaching of host tissue, without zone lines, polygonal, some triangular, quadrangular to rhomboid, others irregular in shape, superficial layer brown to beige in young apothecia, covering the hymenium, opening by 3-5 prominent, irregular, pruinose and yellowish white (4A2) to pale yellow (4A3) flaps; hymenium somewhat depressed below level of the substrate, pruinose, greyish blue (22B4) to dull blue (22D5) in young specimens, fading to bluish white (22A2) to pale blue (22A3) or pale yellow (4A3) in several parts of mature apothecia, perimeter line absent; paraphyses up to 90 µm long and 2 µm diameter, swelling slightly near apex, up to 3 µm diameter, with several short, finger-like projections, coated with crystals at the apex, crystals soluble in KOH; asci 65-80 × 6-8 µm, subcylindric to subfusoid, tapering at apex, wall undifferentiated at the apex, 8-spored; ascospores parallel to subparallel in the ascus, 50-60 × 1-2.4 µm, filiform, straight to slightly curved on release, colorless in KOH, thin walled, 0- to 1-septate, apical and basal gelatinous caps, globose, 2-2.5 µm diameter, gelatinous caps soluble in KOH.
Habit and habitat: growing on fresh fallen leaves and dead leaves of Avicennia germinans in mangrove forest.
Etymology: referring to the common name in Spanish (manglar) of habitat where the species is growing.
Additional material examined: MEXICO. Quintana Roo, municipio Cozumel, Reserva de la Biosfera Isla de Cozumel, Parque Ecológico Punta Sur, 28°16'48''N, 86°58'44.39''W, 16.X.2019, T. Raymundo 8323 (ENCB), 8338 (ENCB), 8341 (ENCB).
Taxonomy notes: Marthamyces manglicola is characterized by its polygonal to irregular apothecia, the young apothecia erumpent from a brown covering layer, opening by 3-5 prominent irregular and yellowish white to pale yellow flaps, and by its straight to slightly curved on release and 0- to 1-septate ascospores.
Key to the world species of Marthamyces based on literature
1a. Apothecia growing on several Monocots: Carex L. and Ficinia Schrad. (Cyperaceae), Dendrobium Sw. (Orchidaceae), Andropogon L. and Panicum Lam. (Poaceae), Phormium J.R. Forst. & G. Forst (Xanthorrhoeaceae) ...................................................................................... 2
1b. Apothecia growing on several Eudicots and some Magnoliids: Avicennia L. (Asterides, Acanthaceae), Aetoxicum Ruiz & Pav. (Superasterids, Aetoxicaceae), Fissistigma Griff. (Magnoliids, Annonaceae), Arctostaphylos Adans. (Asterids, Ericaceae), Fagus L. and Quercus L. (Rosids, Fagaceae), Eucalyptus L´ Hér. and Metrosideros Banks ex Gaertn. (Rosids, Myrtaceae), Olea L. (Asterids, Oleaceae), Hakea Schrad. and Orites R. Br. (Eudicots, Proteaceae) ..…....…..........…….....…… 6
2a. Apothecia opening by single longitudinal slits ….....…….. Marthamyces desmoschoeni (P.R. Johnst.) Minter
2b. Apothecia opening in several radial or irregular slits … 3
3a. Only growing on Carex firma Host. from Austria ……..........… Marthamyces foliicola Nograsek & Matzer
3b. Species growing on Andropogon, Dendrobium, Panicum or Phormium .….............................................……..…… 4
4a. Apothecia round, opening by radial slits, growing on Dendrobium from New Zealand ….............................................. Marthamyces dendrobi (P. R. Johnst.) Minter
4b. Apothecia not round, opening by irregular slits, growing on Andropogon, Panicum or Phormium from USA and New Zealand ………...........………………..…….………………. 5
5a. Apothecia narrow elliptical to elongate, no flaps ruptured, hymenium pale blue, 37-42 × 2-3 µm, 1-septate, straight, on Andropogon and Panicum from USA …... Marthamyces culmigenus (Ellis & Langlois) P. R. Johnst.
5b. Apothecia irregular in shape, splitting irregularly, flaps not defined, hymenium pale yellow to greenish yellow, ascospores 55-78 × 3.5-4.5 µm, 1-septate, straight to slightly curved, on Phormium tenax J. R. Forst. & G. Forst. from New Zealand ........................................................................... Marthamyces harakeke P.R. Johnst.
6a. Ascospores 0- to 1-septate ……………...………………………. 7
6b. Ascospores 3- to 5-septate ………………………...…..……… 16
7a. Ascospores straight to slightly curved ……………….….….. 8
7b. Ascospores curved, sigmoid or coiled ………..…………... 14
8a. Paraphyses simple, not branched, becoming clavate …….…....……. Marthamyces johntonii Crous & Carnegie
8b. Paraphyses branched at the apex …………………………….. 9
9a. Paraphyses swelling near the apex, with several short finger-like projections ….....................................…... 10
9b. Paraphyses tapering toward the apex, with several short narrow branches at apex …...................................... 11
10a. Apothecia round to irregular, hymenium dark green, without crystals, opening by 2-3 irregular white flaps, ascospores (65-)75-85(-90) × 2-2.5 µm, on Orites acicularis (R. Br.) Roem. & Schult. from Tasmania ……………................…… Marthamyces oritis P.R. Johnst.
10b. Apothecia polygonal to irregular, hymenium bluish white to pale blue, pruinose by the crystals, opening by 3-5 yellowish white to pale yellow flaps, ascospores 50-60 × 1-2.4 µm, on Avicennia germinans L. from Mexico ………....…… Marthamyces manglicola Raymundo, García-Martínez, Martínez-Pineda & R. Valenz.
11a. Growing on Eucalyptus L. Her., flaps dark brown, 3-5 in the apothecia ………………………...................................………..... Marthamyces emarginatus (Cooke & Massee) Minter
11b. Growing on Metrosideros, flaps white to pale yellow, (2-)3-4(-5) in the apothecia …………….................….... 12
12a. Apothecia angular to elongate …….............................................……..….......…. Marthamyces renga P. R. Johnst.
12b. Apothecia orbicular to angular, not elongate ……...… 13
13a. Hymenium white, ascospores 45-85 × 2-3 µm, growing on M. robusta A. Cunn. from New Zealand …...………………... Marthamyces metrosideri P. R. Johnst.
13b. Hymenium pale yellow to grey, ascospores 50-70 × 1.5-2 µm, growing on M. collina (J. R. Forst. & G. Forst.) A. Gray from the Cook Islands …..........................................................… Marthamyces maccormackii P. R. Johnst.
14a. Ascospores (95-)110-135(-160) × 2-2.5 µm .....................................................… Marthamyces hakeae P. R. Johnst.
14b. Ascospores 60-74(-80) × 1.2-2.4 µm …..…….………….. 15
15a. Apothecia triangular to pentagonal, occasionally elliptical, hymenium white, opening by 3-5 grey flaps, ascospores 60-72 × 1.2-1.6 µm, on Fissistigma from China ………..… Marthamyces chinensis Y. R. Lin & H. L. Gu
15b. Apothecia orbicular to polygonal, opening by 4-5 brownish beige flaps, hymenium pale grey to pale yellow, ascospores 60-74(-80) × 1.6-2.4 µm, on Fagus grandifolia subsp. mexicana from Mexico ....… Marthamyces coronadoae Raymundo, R. Valenz. & Esqueda
16a. Ascospores 5-septate, 75-105 × 2.5-3 µm, on Olea from Italy .............. Marthamyces panizzei (De Not.) Minter
16b. Ascospores 3-septate, 50-75 × 1.5 µm, on Aetoxicum, Arctostaphylos and Quercus from Chile, Sweden and USA .............................…………………………………………. 17
17a. Apothecia angular, hymenium pale gray ..........................................….. Marthamyces quadrifidus (Lev) Minter
17b. Apothecia orbicular, hymenium white to pale yellow …………………….................................................……….. 18
18a. Growing on Quercus, ascospores 50-65 × 1.5 µm …...…. Marthamyces quercifolius (Cooke & Ellis) Minter
18b. Growing on Arctostaphylos uva-ursi (L.) Spreng., ascospores 60-75 × 1.5-2.0 µm ……….................................……………..….…….. Marthamyces phacidioides (Fr.) Minter
Discussion
The principal features that can distinguish species of Marthamyces are color and presence of pruinose material in flaps of the ascomata, color of the hymenial surface and size, shape and number of septa of ascospores. In addition, we consider that the plant with which each Marthamyces species is associated can be an important ecological character to separate species. Besides, other authors who have described species of Marthamyces have found a narrow range of hosts (Gu et al., 2015; Raymundo et al., 2016; Johnston and Park, 2019). Marthamyces coronadoae and M. emarginatus are close to M. manglicola by having 0- to 1-septate ascospores but differ by ascospore size and associated plant identity; the first species has larger ascospores (60-75 × 1.6-2.4 µm) and grows on Fagus L. (Raymundo et al., 2016), while in the second ascospores are (60-)65-75(-85) × 2(-2.5) μm and the fungus grows on Eucalyptus (Sherwood, 1977; Johnston, 2006). Other American species are M. phacidioides (Fr.) Minter, M. quercifolius (Cooke & Ellis) Minter and M. quadrifidus, but they are separated by 3-septate and larger ascospores (Sherwood, 1977; Cabarroi-Hernández et al., 2014).
Species of Marthamyces have been quoted as biotrophs (Gu et al., 2015; Johnston and Park, 2019) and saprobes (Minter, 2003; Raymundo et al., 2016) and always as foliicolous fungi. Ascomata of Marthamyces manglicola were found on freshly fallen leaves. Hyde and Soytong (2008), Promputtha et al. (2010) and De Silva et al. (2016) suggest that this is evidence of an endophytic life strategy, growing on living leaves, followed by the production of sporophores as saprobes after leaf death, during decomposition. For mangroves this process is very important because they generate a large amount of litter which fungi can decompose into simpler organic matter which is then exported to the neighboring ecosystem such as seagrasses and coral reefs.
Another important aspect is the host identity of the associated plant. Marthamyces species show a preference for colonizing particular plants (Johnston and Park, 2019). Although M. manglicola was found in a mangrove forest with four mangrove species: Avicennia germinans, Rhizophora mangle L., Laguncularia racemosa C.F.Gaertn. and Conocarpus erectus L. (Téllez-Valdés et al., 1989), it was found only on A. germinans. The factors likely to determine host specificityare physicochemical characteristics of the leaves, such as the amount of tannins, degree of lignification and the presence of salt. Compared with other mangrove species, A. germinans is known to have less tannins than R. mangle and more salt than the other taxa mentioned, due to its ability to exclude salt through leaf glands (Clough, 2013). Marthamyces manglicola can therefore be considered a halotolerant and able to tolerate conditions particular to mangrove forest such as waterlogging and high solar radiation.
Conclusions
Most Marthamyces species have been reported from temperate and tropical forest (Minter, 2003; Johnston and Park, 2019). Marthamyces manglicola is the first species of this genus found in mangrove ecosystems, and is manglicolous. This is important because only around 300 species of manglicolous fungi are known globally (Raghukumar, 2017). Marthamyces manglicola is presented as a new species growing on fresh fallen and dead leaves of Avicennia germinans in mangrove forests in Cozumel Island.











 nova página do texto(beta)
nova página do texto(beta)



