Introduction
Stellaria L. (Caryophyllaceae) is a genus traditionally comprising 150-200 species distributed in the temperate regions of Eurasia and North America and at the higher altitudes of tropical areas (Hernández-Ledesma et al., 2015; Tikhomirov, 2016), being most diverse in the mountains of central Asia (Sharples and Tripp, 2019). Based on recent molecular studies (Greenberg and Donoghue, 2011; Sharples and Tripp, 2019), it was shown that various species are not included in the Stellaria sensu stricto clade. In light of this new view, Stellaria s.s. should be reduced to about 120 species (Morton, 2005) to 112 (Sharples, 2019). The genus is taxonomically problematic because of its high phenotypic variability which has led to nomenclatural disorders making the identification of the various species difficult. A worldwide revision of Stellaria is still lacking. Taxonomic studies of Stellaria were mostly part of comprehensive floras: Clapham et al.,1952 (for the British Isles), Chater and Heywood, 1993 (for Europe), Wu and Ke, 1996 and Shilong and Rabeler, 2001 (for China) or Morton, 2005 (for North America). In addition, few molecular papers have been published (e.g., Greenberg and Donoghue, 2011; Sharples and Tripp, 2019; Sharples, 2019).
As part of my ongoing studies on Caryophyllaceae (e.g., Iamonico 2013, 2014, 2015, 2018; Iamonico and Domina, 2015), I here present a note about a taxon currently accepted under the genus Stellaria - Stellaria obtusa Engelm. - which, however, should be removed and recognized as a separate genus, which I name Engellaria gen. nov. (see the taxonomic treatment).
Material and Methods
The present research is based both on the analysis of the relevant literature and examination of specimens preserved at BM, CAN, CAS, CS, DOV, F, GH, HFLA, K, MIN, MO, MSC, NEBC, NY, OSC, RM, UC, US, VT, and YU (herbarium acronymsaccording to Thiers, 2021 (continuously updated)).
The distribution map was based on data from herbarium specimens and the following online sources: Hartman and Rabeler (2012), and EOL, 2020 (continuously updated). The following characters, which are relevant for the studied taxa according to literature (mainly Morton and Rabeler, 1989) and personal experience, were measured on the examined specimens (or images; see “Additional examined material”) using a millimeter ruler and an optical stereoscope Olympus SZ40 (Schönwalde-Glien, Germany): length and width of the leaves (the width was measured at the widest part of the blade), size of the seeds (both longest and shortest diameter), ratio length/width of the leaf blades, and fruit dehiscence/indehiscence.
Results and Discussion
Phylogenetic data from the literature
Greenberg and Donoghue (2011: 1642-1643) demonstrated that Stellaria is polyphyletic, highlighting that several taxa are not included in the Stellaria sensu stricto clade, but they are instead related to other genera, i.e. Geocarpon Mack., Honckenya Ehrh., Lepyrodiclis Fenzl ex Endl., Minuartia Loefl., Schiedea Cham. & Schltdl., and Wilhelmsia Rchb. The non-Stellaria species sensuGreenberg and Donoghue (2011: 1642, Fig. 2) occur, in their cladogram, in two different and not closely related clades, i.e., clade no. 10 (which corresponds to the tribes Sclerantheae Bertch. & J. Presl (see Reveal, 2011 for the correct citation of this name) and Sagineae J. Presl as recognized by Harbaugh et al. (2010)) and clade no. 15 (which corresponds to the tribe Alsineae as recognized by Harbaugh et al. (2010)). Among these divergent species, Stellaria obtusa appears to be least related to the rest of the Stellaria members, being part of the tribe Sclerantheae sensuHarbaugh et al. (2010) and sister to a clade comprising the genera Honckenya, Schiedea, and Wilhelmsia (see Greenberg and Donoghue, 2011: 1642, Fig. 2). This latter clade is sister to another well-supported clade (bootstrap value=97) including nine species. Six out of these nine latter species were later recognized as part of the following two other genera: Mononeuria Rchb. (M. cumberlandensis (Wofford & Kral) Dillenb. & Kadereit (≡ Arenaria cumberlandensis Wofford & Kral), M. glabra (Michx.) Dillenb. & Kadereit (≡ Arenaria glabra Michx.), M. minima (Mack.) Dillenb. & Kadereit (≡ Geocarpon minimum Mack.), M. nuttallii (Torr. & A. Gray) Dillenb. & Kadereit (≡ Stellaria nuttallii Torr. & A. Gray), M. uniflora (Walter) Dillenb. & Kadereit (≡ Stellaria uniflora Walter)) and Triplateia Bartl. (Triplateia moehringioides (Moç. & Sessé ex DC.) Kuntze (≡ Hymenella moehringioides Moç. & Sessé ex DC.)). The other three taxa (S. minutifolia Maguire, S. ovata Willd. ex D.F.K. Schltdl, and S. howardii Maguire) are still considered as part of the genus Stellaria but, according to Dillenberger and Kadereit (2014: 68), they form a clade (no. 4 in Dillenberger and Kadereit, 2014) which is sister to some Minuartia members. They need further study and are part of an ongoing study by the author of the present paper (Iamonico, in prep.).

Figure 1: Engellaria obtusa (Engelm.) Iamonico from Western Cascades mountains Oregon, United States of America: A. habit (scale bar=1 cm); B. details of leaves (scale bar=5 mm); C. flower (scale bar=5 mm); D. fruit (scale bar=2.5 mm). Photos by T. Harvey.
Morphological data
Comparison of Stellaria obtusa with molecularly closely related genera (Honckenya, Schiedea and Wilhelmsia)
Honckenya is a monotypic genus native to the coastal areas of temperate and arctic North America, as well as northern Eurasia (Halliday, 1993; Wagner, 2005b; Sánchez Vilas, 2007: 21). The single morphologically variable species H. peploides (L.) Ehrh. is comprised of four subspecies, distinguished by habit, stem diameter, internode length, leaf shape and size, and pedicel length (Wagner, 2005b): subsp. diffusa (Hornem.) Hultén ex V.V. Petrovsky, subsp. major (Hook.) Hultén, subsp. peploides, and subsp. robusta (Fern.) Hultén (see Kurtto, 2001; Wagner, 2005b). As a whole, H. peploides is clearly a different lineage from Stellaria obtusa, displaying the following diverging characters between H. peploides and S. obtusa: size of leaves (up to 46 × 20 mm in H. peploides vs. up to 12 × 12 mm in S. obtusa), inflorescence (few-flowered inflorescence in H. peploides vs. always solitary in S. obtusa), sepals (5 in H. peploides, usually 4 in S. obtusa), petals (present in H. peploides, absent in S. obtusa), length of sepals (3.5-7 mm in H. peploides vs. 1.5-3.5 mm in S. obtusa), length and dehiscence of capsule (5-12 mm long opening by 3 valves in H. peploides vs. up to 2-3.5 mm opening by 6 valves in S. obtusa), and diameter of seeds (2-4 mm in H. peploides vs. up to 0.5-0.7 mm in S. obtusa).
Schiedea is a genus endemic to the Hawaiian Islands (Wagner et al., 2005). All Schiedea species are characterized morphologically by having various features which clearly distinguish this genus from Stellaria obtusa (Table 1). These features refer to habit (shrubs, subshrubs and vines vs. perennial herbs in S. obtusa), leaves (entire to minutely toothed vs. entire in S. obtusa), inflorescence architecture (dichasia, monochasia or panicle-like vs. flowers axillary and solitary in S. obtusa), and seed size (diameter: 0.6-1.8 mm in Schiedea vs. up to 0.5-0.7 mm in S. obtusa).
Wilhelmsia is a monotypic genus occurring in arctic northwestern North America (Canada in Northwest Territories and Yukon, and USA in Alaska) and eastern Asia (Russian Far East, Siberia) (Wagner, 2005a). The single species, W. physodes (Fisch. ex Ser.) McNeill, differs from Stellaria obtusa in several characters, i.e. pubescence (respectively, glandular pubescent vs. glabrous to finely pubescent), leaf size (2-8 × 5-15 mm in W. physodes vs. 7-12 × 2-12 mm in S. obtusa), petals (present in W. physodes, absent in S. obtusa), sepal length and colour (4.5-6 mm, often purple in W. physodes vs. 1.5-3.5 mm, green in S. obtusa), capsule width, colour and dehiscence (7-10 mm, purplish opening by 3 valves in W. physodes vs. 1-2 mm, green to pale-green opening by 6 valves in S. obtusa), and seed diameter (1.2-1.5 mm in W. physodes vs. 0.5-0.7 mm in S. obtusa) (Table 1).
Table 1: Morphological comparison between Engellaria Iamonico and the related genera Honckenya Ehrh., Schiedea Cham. & Schltdl., and Wilhelmsia Rchb.
| Engellaria Iamonico | Honckenya Ehrh. | Schiedea Cham. & Schltdl. | Wilhelmsia Rchb. | |
| Habit | perennial herbs | perennial herbs | shrubs, subshrubs and vines | perennial herbs |
| Leaf succulence, size (mm, length × width), and margins | not succulent, 7-12 × 2-12, entire | succulent, 4-46 × 0.5-20, often crenulate | succulent, 10-240 × 1-110, to minutely toothed | not succulent, 5-15 × 2-8, serrulate or toothed at least along the distal half of the blade |
| Inflorescence | solitary flowers | few-flowered or solitary flowers | dichasia/monochasia or panicle-like | solitary flowers |
| Petals (number) | absent | mostly 5 | absent | 5 |
| Sepals length (mm) and colour | 1.5-3.5, green | 3.5-7, green | 2.0-12.0, often purple | 4.5-6.0, purplish |
| Styles (mm) | 0.3-0.5 | 1-2 | up to 11 | 2.5-3 |
| Capsule size (mm, length × width), colour, and dehiscence | 2-3.5 × 1-2, pale-green, opening by 6 valves | 5-12.0 × 5-10, green to yellowish, opening by 3 valves | 1.5-12 × 0.5-6, green (sometimes with apex purple), opening by 4-11 valves | 8-10 × 7-10, purplish, opening by 3 valves |
| Seed diameter (mm) | 0.5-0.7 | 2.0-4.0 | 0.6-1.8 | 1.2-1.5 |
Comparison of Stellaria obtusa with apetalous Stellaria sensu stricto members
Stellaria obtusa, an apetalous species, displays a unique morphology among the apetalous Stellaria species (S. crispa Cham. & Schltdl., S. dicranoides (Cham. & Schltdl.) Fenzl (Fenzl’s species is here accepted as a member of Stellaria according to the treatment by Morton (2005) who, however (see also Harbaugh et al., 2010: 195), reported that S. dicranoides is of uncertain generic position and it could be placed under the genus Arenaria L.), S. media L. s.l., S. pallida (Dumort.) Crép., S. irrigua Bunge (= S. umbellata Turcz. according to Sharples and Tripp, 2019) (Table 2)).
Table 2: Morphological comparison between Engellaria obtusa (Engelm.) Iamonico, and the apetalous members of Stellaria L. Concerning S. media (L.) Vill. the description refers to the apetalous forms.
| Engellaria obtusa (Engelm.) Iamonico | Stellaria crispa Cham. & Schltdl | Stellaria dicranoides (Cham. & Schltdl.) Fenzl | Stellaria irrigua Bunge | Stellaria media (L.) Vill. | Stellaria pallida (Dumort.) Crép. | |
| Habit | perennial, creeping | perennial, forming mats | perennial, forming cushions | perennial, erect or forming clumps | annual/biennal, prostrate to ascending | annual, prostrate |
| Stem | glabrous (rarely hairy) | glabrous | glabrous | glabrous | pubescent all around or with 1-2 lines of trichomes | one line of hairs below each node |
| Leaf shape and size (length width, mm) | ovate, 7.0-12.0 × 2.0-12.0 | broadly elliptic to ovate, 4.0-25.0 × 2.0-15.0 | oblanceolate to obovate, 3.0-5.0 × 1.0-1.5 | elliptic or lanceolate, 1.0- 10.0 × 1.0-3.0 | ovate to broadly elliptic, 10.0- 20.0 × 5.0-10.0 | ovate to elliptic, 3.0-15.0 × 1.0- 7.0 |
| Inflorescence | flowers solitary | flowers solitary | inflorescence few-flowered or flowers solitary | inflorescence usually few- flowered | inflorescence 5- to many-flowered | inflorescence 3- to many-flowered |
| Sepal | obtuse, glabrous, with obscure veins | acute to acuminate, glabrous, with 3 prominent veins | acute, glabrous, with 1 prominent vein | obtuse, glabrous, with 3 prominent veins | obtuse, pubescent, with obscure veins | acute, pubescent, with obscure veins |
| Styles (mm) | <0.5 | ~ 1.0 | ~ 1.0 | ~ 0.25 | 0.5-1.0 | 0.2-0.5 |
| Capsules | globose (length ~ width) | ovoid (length >1.5 times width) | ovoid (length ~ 1.5 times width) | conic (length ~ 1.5 times width) | ovoid-pyriform (length ~ 1.5 times width) | ovoid (length ~ 1.5 times width) |
| Seeds (mm) | 0.5-0.7 | 0.7-1.0 | 1.0-1.1 | 0.5-0.7 | (0.8)1.0-1.3 | 0.5-0.9 |
Stellaria crispa usually has longer leaves (4-25 mm vs. 7-12 in S. obtusa), acute to acuminate sepals with 3 prominent veins (vs. obtuse with obscure veins in S. obtusa), styles that are about 1 mm long (vs. <0.5 mm long in S. obtusa), and ovoid capsules that are more than 1.5 times as long as wide (vs. globose).
Stellaria media is a highly variable species. The forms having flowers without petals differ from S. obtusa by the following characters: stems pubescent all around or with 1-2 lines of hairs (vs. stems glabrous (rarely hairy)), larger leaves (10-20 × 5-10 mm vs. 7-12 × 2-12 mm in S. obtusa), inflorescences 5- to many-flowered (vs. flowers solitary in S. obtusa), sepals pubescent (vs. glabrous), larger seeds ((0.8)1-1.3 mm vs. 0.5-0.7 mm in S. obtusa), capsule shape (ovoid-pyriform vs. globose in S. obtusa).
Stellaria pallida is an annual species (S. obtusa is perennial), with stems with 1 line of hairs for each node (vs. glabrous (rarely hairy) in S. obtusa), narrower leaves at average (0.9-7 vs. 2-12), 3- to many-flowered inflorescences (vs. flowers solitary in S. obtusa), and an ovoid capsule (vs. globose in S. obtusa).
Stellaria irrigua has ± elliptic or lanceolate leaves, 1-10 × 1-3 mm (vs. ovate, 7-12 × 2-12 mm in S. obtusa), 3- to many-flowered inflorescences (vs. flowers solitary in S. obtusa), with bracts (vs. bractless), sepals with 3 prominent veins (vs. obscure veins in S. obtusa), and conic capsule (vs. globose).
Taxonomic treatment
Based on the discussion above about molecular data and morphology of Stellaria obtusa and the related taxa, I here propose a new monotypic genus in the Caryophyllaceae. See Table 1 for a comparison with the similar genera.
Engellaria Iamonico gen. nov.
TYPE: Engellaria obtusa (Engelm.) Iamonico (basionym: Stellaria obtusa Engelm.).
Engellaria includes perennial herbs which display entire leaves, solitary flowers, usually 4 sepals (rarely 5), green, 1.5-3.5 mm long, petals absent, styles 0.3-0.5 mm long, capsule dehiscent by 6 valves, pale-green, 2-3.5 mm long, 1-2 mm wide, seeds 0.5-0.7 mm in diameter.
Distribution: see the description of Engellaria obtusa.
Etymology: my first idea was to dedicate the new genus to George Engelmann (1809-1884), an American physician and botanist who first described Stellaria obtusa, the single member of Engellaria. However, the name Engelmannia (derived from the surname Engelmann) has been published four times (Engelmannia A. Gray ex Nutt. (in 1840), Engelmannia Klotzsch (in 1841), Engelmannia Torr. & A. Gray (in 1842), Engelmannia Pfeiff. (in 1845)) and my proposal would be illegitimate (later homonym according to the Art. 53.1 of the ICN, see Turland et al., 2018; note that also the Klotzsch’s, Torrey & Gray’s and Pfeiffer are homonyms and therefore illegitimate). So, with the aim to maintain “Engelmann” in the generic epithet, I created a name which results from a merge of “Engelmann” (Engel-) and “Stellaria” (-laria).
Proposed vernacular name: Engelmann’s starwort.
Species richness: a monotypic genus comprising the species Engellaria obtusa (Engelm.) Iamonico.
Chorology: see Engellaria obtusa.
Inclusion of Engellaria in the diagnostic key of Caryophyllaceae sensu Rabeler and Hartman (2005): on the basis of the generic diagnosis above and the description of Engellaria obtusa (below), I here propose a subkey for the apetalous members belonging to the American Caryophyllaceae genera characterized by having leaves without stipules, sepals free, and fruits a capsule (steps 25-29 of the diagnostic key from Rabeler and Hartman (2005)):
1a. Capsule cylindric with 8 or 10 valves; sepals usually 4-5 ................................................................................... 2
1b. Capsule ovoid or globose with up to usually 6 valves; sepals usually 4-6 ...................................................... 3
2a. Capsule valves usually 8; styles usually 4 .................................................................................... Moenchia Ehrh.
2b. Capsule valves usually 10; styles usually 5 ....................................................................................... Cerastium L.
3a. Sepals 4(-5) .................................................................. 4
3b. Sepals 5(-6) .................................................................. 5
4a. Capsule valves 4-5; styles 4-5 ........................... Sagina L.
4b. Capsule valves 6; styles 3(-4) ......... Engellaria Iamonico
5a. Capsule valves or teeth 2 times the number of styles .. 6
5b. Capsule valves or teeth usually equal in number of styles ................................................................................... 7
6a. Styles 3(5); capsule valves (3, 4) 6 (8, 10); seeds usually yellowish to brown, smooth or tuberculate when shiny .................................................................... Stellaria L.
6b. Styles 3; capsule valves 6; seeds dark brown or black, always shiny, smooth or obscurely tuberculate ................................................................................ Arenaria L.
7a. Stamens 5 ......................................... Mononeuria Rchb.
7b. Stamens 10 ............................................. Sabulina Rchb.
Engellaria obtusa (Engelm.) Iamonico, comb. nov. Fig. 1.
≡ Stellaria obtusa Engelm., Bot. Gaz. 7(1): 5. 1882. TYPE: UNITED STATES OF AMERICA. Colorado, Anthracite Ck (Creek), Colorado, 1881, T. S. Brandegee s.n. (lectotype designated here: UC120055! (image available at UC, 2021a); isolectotypes: GH00037991!, GH00037990! (image available at HUH, 2021a), NY00353081! (image available at NYBG, 2021a), YU001676! (image available at YU, 2021)).
≡ Alsine obtusa (Engelm.) Rose, Contr. U.S. Natl. Herb. 3(9): 569. 1896.
= Stellaria washingtoniana B.L. Rob., Bot. Gaz. 25(3): 166-167. 1898. TYPE: UNITED STATES OF AMERICA, Alder wood, Upper Valley of the Nesqually, 18.VI.1895, O. D. Allen 157 (lectotype designated by Holmgren and Holmgren, 2012 GH00037993! (image of the lectotype available at HUH, 2021b); isolectotypes: BM000884641! (NMH, 2021), CAN171422, CAS5630! (CAS, 2021a), CS106217! (CS, 2021), DOV0008406! (DOV, 2021), F0053353F! (F, 2021a), FV0053354F! (F, 2021b), GH00037994!, plant on the left (HUH, 2021c), K000723568! (K, 2021), MIN1002528! (JSTOR, 2021a), MO2196020! (TROPICOS, 2021), MSC0092930! (JSTOR, 2021b), NY353086! (NYBG, 2021b), RM0002200! (RM, 2021a), US00611211!, and US00103238! (both at US, 2021a), VT053289! (JSTOR, 2021c)).
≡ Alsine washingtoniana (B.L. Rob.) A. Haller, Cat. N. Amer. Pl. (ed. 2): 4. 1900.
= Alsine viridulaPiper, Contr. U.S. Natl. Herb. 16(5): 207. 1913. TYPE: UNITED STATES OF AMERICA. Idaho, ridges south from Wiessners Peak (Mount Wiessner), 1700 m, 28.VII.1896, J. B. Leiberg 1396 (holotype: US00103237! (image available at US, 2021b); isotypes: CAS0000308! (CAS, 2021b), F281494! (F, 2021c), GH00037575! (HUH, 2021d), NY342333! (NYBG, 2021c), OSC04665! (OSC, 2021), RM0002118! (RM, 2021b), UC154382! (UC, 2021b)).
≡ Stellaria viridula (Piper) St. John in St. John & Warren, Prelim. List Pl. Kaniksu Nat. For. 1: 6. 1925.
= Alsine obtusa (Engelm.) Howell, Fl. N. W. Amer. 1: 83. 1897, nom. inval. (isonym, Art. 6.3 Note 2 of the ICN, Turland et al., 2018).
Perennial herbs, rhizomatous (rhizome usually white), creeping (hemicryptophytes); stems prostrate, often matted (not forming cushions), branched, (3-)5-20(-25) cm long, with internodes as long as or longer than the leaves, usually glabrous; leaves sessile or shortly petiolate with blades ovate (7-9(-12) × 2-12 mm (sizes are given as length × width through the text)), glabrous (ciliate near the base), shiny, margins entire and ciliate in the proximal part, base rounded or cuneate, apex acute; flowers solitary, axillary, without bracts, 1.5-2 mm in diameter; pedicels spreading (in fruiting stage), 3-12 mm long, glabrous; sepals usually 4 (rarely 5), with 3(-5), green with obscure dark-green veins, ovate-lanceolate, 1-1.2-(1.5) × 1.5-3.5 mm, glabrous, apex obtuse, margins scarious, narrow (3-4 times narrower than the width of the blade); stamens 8(-10); petals absent; styles usually 3, 0.3-0.5 mm long; ovary 1-loculed at maturity (rarely 3-loculed in young individuals); ovules numerous; capsules pale-green, globose, 2-3.5 × 1-2 mm, about 2 times the length of the sepals, opening by 6 valves; seeds grayish-black or dark-brown, elliptic, 0.5-0.7 mm in diameter, finely reticulate.
Vernacular names: blunt-sepaled starwort (E-Flora BC, 2020), Engelmann’s starwort (here proposed), Rocky Mountain starwort (Morton, 2005; Hartman and Rabeler, 2012), Rocky Mountain chickweed (Hassler, 2020).
Habitat: moist areas in woods, shaded edges of creeks, slopes; altitude: 300-3400 m a.s.l.
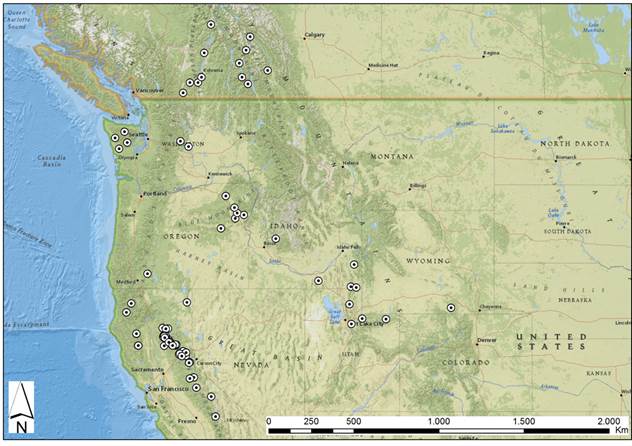
Chorology (Fig. 2): endemic to western North America in Canada (British Columbia, Alberta), and USA (California, Colorado, Idaho, Montana, Oregon, Utah, Washington, Wyoming).
Chromosome number: 2n=26, 52, c. 65, c. 78 (Hartman, 1971; Morton and Rabeler, 1989).
Conservation status: I prefer not carrying out the IUCN assessment of Engellaria obtusa, since I think that further distribution data are needed. As a consequence, this species is to be considered as DD (Data Deficient) according to the IUCN criteria (IUCN, 2016).
Typification of Stellaria obtusa: Engelmann (1882: 5) validly published the name Stellaria obtusa with a diagnosis (in English), the provenance (“Western Colorado on the tributaries of Gunnison River, alt. 9000 to 10,000 feet”), the habitat (“in damp grounds”) and the collector (“T. S. Brandegee”).
I found pertinent specimens at GH (barcodes 00037990, and 00037991), NY (barcode 00353081), UC (barcode 120055), and YU (barcode 001076), but no sheets at MO (where Engelmann would have written the description of S. obtusa) were traced. Therefore, this strengthens the case for selecting a lectotype from material at another herbarium. All five specimens are part of T. S. Brandegee’s collection and were collected by him in 1881 in “Anthracite Ck, Colorado” (as reported on the original labels). Anthracite Creek (= Ck) is a river in Gunnison County. The data on the labels match Engelmann’s protologue of Stellaria obtusa, the plants were collected before 1882 (year of collection 1881) and the morphology matches Engelmann’s diagnosis. The GH, NY, UC, and YU specimens are part of the original material used by Engelmann (1882: 5) to propose the new species. Since the UC specimen appears to be the best preserved, I here designated it as the lectotype of the name Stellaria obtusa, whereas the GH, NY, and YU specimens are isolectoypes (Arts. 9.3 and 9.4 of the ICN, Turland et al., 2018). The abovementioned specimens bear recently printed labels. The UC specimen mentions “NEOTYPE of: Stellaria obtusa Engelm. Bot. Gaz. (Crawfordsville) 7: 5. 1882. = Stellaria obtusa Engelm. Det.: Richard K. Rabeler 1986 Michigan State University Herbarium (MSC)”. The GH and NY ones bear “ISONEOTYPE of: Stellaria obtusa Engelm. Bot. Gaz. (Crawfordsville) 7: 5. 1882. = Stellaria obtusa Engelm. Det.: Richard K. Rabeler 1986 Michigan State University Herbarium (MSC)”, and the YU bears “ISOTYPE A DUPLICATE OF THE HOLOTYPE Stellaria obtusa Engelm. Bot. Gaz. 7: 5. 1882”. No published paper, in which a neotypification by R. K. Rabeler was proposed, was traced and, as a consequence, the statements occurring on the printed labels of GH, NY, and UC specimens cannot be considered as an effective typification. Moreover, since original material exists, a lectotypification is required (Arts. 9.3 and 9.4 of the ICN, see Turland et al., 2018), whereas a neotypification is appropriate “if no original material exists” (see Art. 9.8 of the ICN). The “isotype” indication on the YU specimens cannot be retained neither, since the holotype was not cited by Engelmann (1882: 5) (see Art. 9.1 of the ICN, Turland et al., 2018, and the considerations given by McNeill, 2014).
Note on the type of Alsine viridula: Alsine viridula was validly published by Piper (1913: 207) through a detailed diagnosis followed by data regarding the provenance, habitat, collector, date of collection, and herbarium (“Type in the U.S. National Herbarium, no. 249940, collected on ridges south from Wiessner Peak, Idhao, July 28, 1895, by J. B. Leiberg (no. 1396). Growing in springy places in canyons, altitude 1700 meters. Also collected along rivulets in woods, altitude 1400 meters, in the Blue Mountains, Columbia County, Washington, July 1896, C. V. Piper, no. 2328”).
I located eight relevant specimens at CAS (barcode 0000308), F (barcode 281494), GH (barcode 00037575), NY (barcode 342333), OSC (barcode 04665), RM (barcode 0002118), UC (barcode 154382), and US (barcode 00103237). All these specimens bear the following original label: “PLANTS OF NORTHERN IDAHO. REGION OF THE COEUR D’ALENE MOUNTAINS. Spring places in canyons Ridges south from Wiessner’s Peak alt. 1700 m No. 1396 John B. Leiberg collector, July 28, 1895”. The information on this label matches information reported by Piper (1913: 207) in the protologue. Moreover, the morphology of the exsiccata also matches Piper’s diagnosis. According to the considerations by McNeill (2014); Piper’s (1913) statement “Type in the U.S. National Herbarium, no. 249940” clearly indicates the author had the intention to identify the US specimen as the holotype. As a consequence, all other seven specimens found are isotypes (Arts. 9.1 and 9.5 of the ICN, Turland et al., 2018).
According to the current concept of Stellaria (Morton, 2005; Sharples and Tripp, 2019), all the specimens of original material found are identifiable as Engellaria obtusa and, as a consequence, Alsine viridula can be synonymized with it.
Additional examined material: names of plants are given in alphabetical order. Specimens, under each species, are given in alphabetical order of the names of the countries and chronological order of the dates of collection.
Engellaria obtusa: UNITED STATES OF AMERICA. Colorado, Rio Blanco Co., Flat-Tops Wilderness, Marvine Creek, trail between trailhead and Slide Lake, locally abundant along the edge of trails, in aspen forest with abundant tall herbs, 11.VI.1988, W. A. Webere 17890 (NY03328683). Idaho, Caribou Co., Grays Range, Gravel Creek Campground, 22 airline miles, north-northeast of Soda Springs, 6700 ft, 19.VII.1971, N. H. Holmgren 5518 (NY00482982). Cassia Co., Mt. Independence, vicinity of Independence Lake, Picea engelmannii, Abies lasiocarpa, Pinus flexilis forest, 09.VIII.2008, J. F. Smith 7732 (NY01264791). Franklin Co., moist soil under willows in headwaters of Logan River, 2 miles north of Utah state line, 8000 feet, 15.VII.1958, L. C. Anderson 1348 (NY00482990); Franklin Co., Wasatch Range, Bear River Range, along Beaver Creek on the south side of the Egan Basin Road, 11.7 km (7.3 miles) north of U.S. Highway 89, among willows in moist ground, 8100 ft, 21.VII.2010, N. H. Holmgren 16267 (NY01207811). Washington, Lake Cushman, deep woods, in clayey ground, VIII.1895, C. V. Piper 2238 (GH00283957, syntype of the name Stellaria washingtoniana).
Honckenya peploides: CANADA. Baffin Island, 1978, P. Wood s.n. (BM001050332). UNITED KINGDOM. England, Mablethorpe, 12.VI.1893, F. A. Lees s.n. (BM013414807). Humberston, Marine sand, 02.VI.1905, E. A. Woodruffe-Peacock s.n. (BM013414808). UNITED STATES OF AMERICA. Massachusetts Plymouth, Plymouth, 24.VIII.1935, C. Darling s.n. (GH00698566). New Hampshire Rye, Rye, 10.III.1959, S. K. Harris 21019 (NEBC00698555). New York, Suffolk Co., 28.VIII.1920, W. C. Ferguson s.n. (NY3266682).
Stellaria crispa: CANADA. British Columbia, Gordon Head, Victoria, woods, 6.VIII.1921, F. W. Hunnewell 7705 (GH01755808). Hoheae Island Kyuquot, sea level, 29.VI.1958, J. W. Eastham 129 (GH01755809). UNITED STATES OF AMERICA. Idaho, about Lake Waha, Nez Perces Co., 2000-3500 feet, 25.VI.1896, A. A. and E. G. Heller 3367 (P05437041); 12 miles west of Salmon, under alders and birches along small creek entering on south side of Salmon River, 12.VII.1945, J. H. Christ and W. W. Ward 14685 (NY03317726); South Fork of the Boise River, road from Featherville, W of Mann Creek, seep, slope along road, 24.VI.2009, J. F. Smith 8131 (NY03317710). Washington, Mount Adams, 15.VIII.1882, T. Howell s.n. (P05437044).
Stellaria dicranoides: UNITED STATES OF AMERICA. Alaska, Cape Lisburne. W. Eskimoland, 1849, B. C. Seeman s.n. (K000723558); Healy, 1922, J. P. Anderson 1626 (NY03317779); Mt. Distin, 04.VII.1938, J. P. Anderson s.n. (GH01755881); Mt. Austin, 04.VII.1938, J. P. Anderson 3771 (NY03317778); vicinity of the Upper Kurupa River Valley, about 3000 ft, 02.VI.1952, A. R. Hodgdon 8106 (GH01755882); Mt. Copper, NW slope to summit of Mt. Eielson, 10.VII.1956, L. A. Viereck 1194 (GH00293224).
Stellaria media: CAMEROON. Northwest Region Bui Elak Summit of Mount Oku, 10.VI.1996, S. Cable 3083 (K000337575). FRANCE. 78 Yvelines, Poigny-la-Forêt, env. de Rambouillet, Poigny, 13.X.1055, P. Jovet s.n. (P02599326). GERMANY. Bayern, Lkr. Garmisch-Partenkirchen, S Bad Kohlgrub, TK 8332/3, Bergwiesen, Fichtenwaldrand, 1484 m, 31.VII.2001, R. Willing and E. Willing 14599D (B-10-0070647). ITALY. Lazio, Rome Province, Appia Antica Regional Park, “Acquedotti” Park, walkways, 03.V.2020, D. Iamonico s.n. (HFLA). UNITED KINGDOM. Kew Gardens, Birdcage Walk, Kew Green, in grass at the base of a lime tree (zone 118), 23.VI.2008, T. A. Cope RBG115 (K000914072). UNITED STATES OF AMERICA. Wisconsin, Grant Co., along railroad, at Walnut Street, 28.IV.2012, M. Nee 59060 (NY03328371).
Stellaria pallida: ITALY. Lazio, Rome Province, Appia Antica Regional Park, “Acquedotti” Park, walkways, 03.V.2020, D. Iamonico s.n. (HFLA). MALAWI. Southern Region, Zomba District, Zomba Plateau, near Ku Chave Inn, 1530 m, weed on Hotel rock garden, 14.III.1970, R. K. Brummit 9178 (P05138272). POLAND. Silesiaca, Breslau, an der Chaussee nach Hundsfeld, 14.VI.1993, C. G. Baenitz s.n. (US03610250). UNITED KINGDOM. Kew Gardens, Birdcage Walk, Kew Green, in grass at the base of a lime tree (zone 118), 10.IV.2013, T. A. Cope RBG564 (K000914610). UNITED STATES OF AMERICA. Florida, Nassau Co., along base of walls of Fort Clinch, Fort Clinch State Park, 29.III.1982, D. S. and H. B. Correll 53453 (NY03328715). Ohio, Wyandot Co., 4.5 km NE of Upper Sandusky, Indian Mill, small park opposite the Mill, flood plain of Sandusky River, 06.V.2012, M. Nee 59115 (NY03328714).
Stellaria irrigua (= S. umbellata): RUSSIA. Aksu et Sarchan, 1841, G. S. Karelin and I. P. Kirilov 1305 (K000723680); In muscosis rupium summarum alpium Alatau ad fontes fluviorum Aksu et Sarchan, 1842, G. S. Karelin and I. P. Kirilov 1305 (K001327006). UNITED STATES OF AMERICA. California, 7 miles north of ft Bidwell, Warner mts., 8.VII.1955, R. J. Wetherby 1618 (NY00453086). Montana, Glacier National Park, open rocky slope, along the trail from Many Glacier Hotel to Piegan Pass, 11.VIII.1919, P. C. Standley 17485 (US03607539). Utah, Wasatch Co. Uinta Mountains, 18.VIII.1998, N. H. Holmgren 13411 (NY01192094). Wyoming, Gunnison Co., Northern Gunnison Basin, West Elk Wilderness, along North Smith Fork Creek, ca 12 air mi ENE of Crawford, 28.V.1998, K. J. Taylor 7464 (GH01845069).
Wilhelmsia physodes: NORTH AMERICA. 1849 (HOOKERIANUM HERBARIUM 1867), s. coll. s.n. (K000742026). CANADA. Edmund’s Island at Rampant House on the Yukon - Alaska border, scattered in Aspen woods, 06.VII.1951, C. C. Loan 551 (GH01624268); Yukon, Ogilvie Mts., river flats along Dempster Rd. Mile 57, 2500-4200 ft, 09.VII.1966, R.T. Polsid 162 (GH01624269); Nisling River Valley, 2700 ft, sand and gravel river bar, 23.VII.1980, W. J. Cody and J. H. Ginns 28277 (NY03329338). RUSSIA. Magadan, regio Tschukotschi, alluvium, 05.VIII.1966, V. Petrovsky 5625 (P06673352). UNITED STATES OF AMERICA. Alaska, railroad embankment, Cantwell, 18.VIII.1939, A. Nelson and R. A. Nelson 4207 (NY03329354).











 nueva página del texto (beta)
nueva página del texto (beta)