Introduction
Colombia is known to be one of the megadiverse countries in the world, with a great number of endemisms and a huge variety of species (Rangel-Ch., 2015). It is also the only South American country with coast on both the Atlantic and Pacific oceans. In the Atlantic, it comprises about 1600 km of coastline, with a great heterogeneity of coastal ecosystems, and climate regimes, as well as seasonal influence of local upwelling. This variety promotes a high biodiversity of marine organisms (Díaz and Acero, 2003). The macroalgal flora of the Caribbean coast of the country includes more than a third of the total species known for the tropical and subtropical western Atlantic (from North Carolina to Brazil), having less than 5% (1600 km) of the coastline from North Carolina to southern Brazil (including Caribbean Islands, 38,000 km of coastline) (Diaz-Pulido and Díaz-Ruíz, 2003; Rincón Díaz and Gavio, 2020). Of the 621 taxa currently reported for the Caribbean coast of Colombia, 351 species are red algae, which account for over 56% of the algas species of the country (Rincón Díaz and Gavio, 2020).
The International Biosphere Reserve Seaflower is the largest marine reserve in the Caribbean and one of the largest in the world. It comprises the entire Archipelago of San Andres, Old Providence and Saint Cataline, and harbours the most iconic tropical marine ecosystems, such as coral reefs and patches, rocky and sandy shore, mangrove forests and seagrass beds (Vides et al., 2016). Due to the heterogeneity of its habitats, the relatively small human population and the absence of industry and extensive agriculture, its ecosystems are rather well conserved, and for some groups of species, this area is considered a biodiversity hotspot (Acero et al., 2019; Mejía-Falla et al., 2020). Despite the great biodiversity and the conservation priorities of the International Reserve, not all groups of organisms have received the same attention, from a research point of view. Until the past decade, seaweeds were one of the least studied taxa (Albis-Salas and Gavio, 2011); recently some research has focused on macroalgal diversity of the Archipelago, and started to reveal a flora much more diverse than previously appreciated (Albis-Salas and Gavio, 2011, 2015; Ortiz and Gavio, 2012; Reyes-Gómez et al., 2013; Gavio et al., 2013, 2015; Vega-Sequeda et al., 2015; Barrera et al., 2016; Rincón-Díaz et al., 2014, 2016, 2018; Reyes-Gómez and Gavio, 2017). From these studies, the macroalgal diversity passed from 202 species registered in 2003 (Díaz-Pulido and Díaz-Ruíz, 2003) to the actual 314 taxa, including 28 Cyanophyceae, 41 Phaeophyceae, 103 Clorophyta and 142 Rhodophyta, the latter group being the most diverse (Rincón-Díaz and Ramos-Gallego, 2016; Rincón-Díaz et al., 2018).
Most of the recent additions are small filaments, with inconspicuous morphologies and difficult to detect and to identify, although they appear abundant on coral reefs and are epiphytes on larger algae or seagrasses. These findings reveal the poor knowledge on macroalgal diversity of the Archipelago and the need to continue exploring it (Rincón-Díaz et al., 2018). More species will be discovered, as formerly unexplored areas or ecosystems are sampled. The country, since 2014, has implemented a scientific exploration program to improve the knowledge on the marine ecosystems of the Archipelago, with yearly oceanographic expeditions to the islands and cays of the Archipelago.
The present study is part of a seaweed biodiversity inventory for the Seaflower Biosphere Reserve, some of the findings were collected during the Seaflower Scientific Expedition in 2019. This work aims to contribute to the knowledge of macroalgal biodiversity in the Archipelago of San Andres, Old Providence and Saint Cataline, Colombian Caribbean, adding eleven new records of red algae.
Materials and Methods
Sampling was undertaken around the islands of San Andres, Old Providence and Saint Cataline, which are part of the International Biosphere Reserve Seaflower, located in the southwestern Caribbean, Colombia (Fig. 1). The whole archipelago is volcanic in origin, and its formation is related to the San Andres Fault, in the Nicaragua Elevation. The subsidence of the volcanic basement and its cover with biogenic carbonates during the Tertiary and Quaternary originated the present atolls (Díaz et al., 1996).
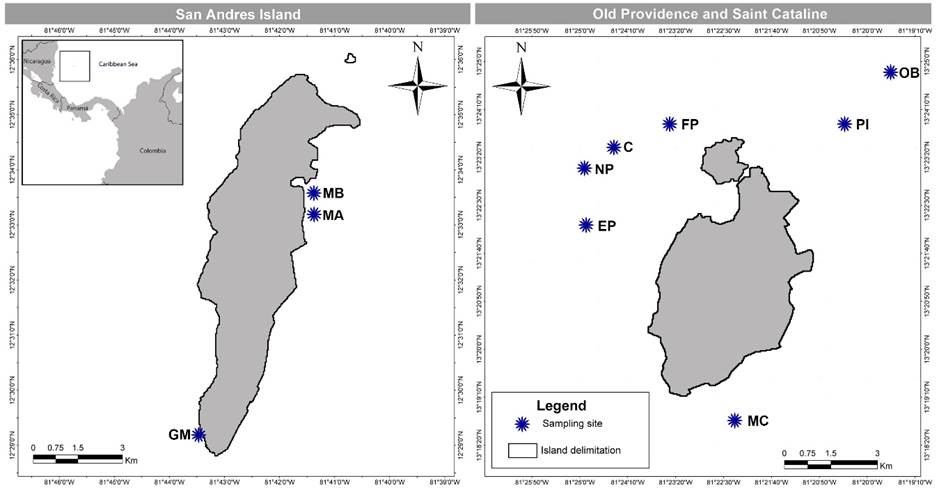
Figure 1: Map of San Andres, Old Providence and Saint Cataline Islands, Colombia, which are part of the International Biosphere Reserve Seaflower, located in the southwestern Caribbean, Colombia, with sampling sites. C=Canolly; EP=El Planchón; FP=Felipe’s Place; GM=Green Moon; MA=Mar Azul; MB=Between Mar Azul and Bahia Honda; MC=Manta’s City; NP=Nick’s Place; OB=site outside barrer reef; PI=Pinaculos.
The samples were collected around the islands in ten points covering differents ecosystems (mangroves, rocky shore, sandy shore, seagrass beds, coral reef), by SCUBA diving at depths between 0 and 37 m. Sampling was carried out between August and November 2009. Additional sampling was carried out in December 2012 and in September 2019 during the Seaflower Scientific Expedition. The algae were preserved in a 4% formalin/seawater solution and kept cool until processing. The identification was carried out using an optical microscope Olympus BX51 (Tokyo, Japan) connected to a digital camera Moticam 2300 3.0 M pixel (Hong Kong, China). Cross-sections of the specimens were made by hand with a razor blade. Images were edited using Adobe Photoshop© CS6 v. 13.0 (Adobe, 2012). To identify the species specialized literature was used (e.g., Taylor, 1960; Littler and Littler, 2000; Dawes and Mathieson, 2008). All specimens were deposited in the herbarium JIW of the Biology Department of the Universidad Nacional de Colombia, in Bogotá. Information on nomenclature and taxonomy classification was obtained from AlgaeBase (Guiry and Guiry, 2021).
Results
A total of 11 new records of red algae belonging to three orders, eight families and nine genera were identified, attached to coral or epiphytic on algae. Five species are new records for the Archipelago (*), and six are new records for Colombia (**) (Table 1).
Table 1: List of new records of Rhodophyta and site of collection within the International Biosphere Reserve Seaflower, located in the southwestern Caribbean, Colombia. (MA=Mar Azul; MB=Between Mar Azul and Bahia Honda; GM=Green Moon; MC=Manta’s City; C=Canolly; FP=Felipe’s Place; NP=Nick’s Place; OB=site outside barrer reef; PI=Pinaculos; EP=El Planchón. New records for the Archipelago (*), new records for Colombia (**), specimens without information on reproductive stage (-). Collection number: BFC=N. Rincón-Díaz, HV=H. Velazquez, PVA= B. Gavio, VR=V. Reyes. Samples are deposited in the herbarium JIW.
| Taxa | MA | MB | GM | MC | C | FP | NP | OB | PI | EP | Reproductive stage | |
| Rhodophyta | ||||||||||||
| Ceramiales | ||||||||||||
| Callithamniaceae | ||||||||||||
| *Seirospora occidentalis Børgesen | x | seirosporangia | ||||||||||
| *Spyridia aculeata subsp. complanata (J. Agardh) Hommersand | x | x | (-) | |||||||||
| Ceramiaceae | ||||||||||||
| **Ceramium brevizonatum var. caraibicum H.E. Petersen & Børgesen | x | tetrasporangia | ||||||||||
| Dasyaceae | ||||||||||||
| *Dasya caraibica Børgesen | x | tetrasporangia | ||||||||||
| Rhodomelaceae | ||||||||||||
| **Wrightiella tumanowiczii (Gatty ex Harvey) F. Schmitz | x | tetrasporangia | ||||||||||
| Gelidiales | ||||||||||||
| Pterocladiaceae | ||||||||||||
| *Pterocladiella bartlettii (W.R. Taylor) Santelices | x | tetrasporangia | ||||||||||
| Rhodymeniales | ||||||||||||
| Champiaceae | ||||||||||||
| *Champia taironensis Bula-Meyer | x | tetrasporangia | ||||||||||
| Faucheaceae | ||||||||||||
| **Gloioderma iyoense Okamura | x | x | (-) | |||||||||
| Rhodymeniaceae | ||||||||||||
| **Botryocladia cf. bahamensis Ballantine & Aponte | x | (-) | ||||||||||
| **Botryocladia cf. bermudana C.W. Schneider & C.E. Lane | x | x | x | (-) | ||||||||
| *Botryocladia spinulifera W.R. Taylor & I. A. Abbott | x | x | x | (-) | ||||||||
Taxonomy
Callithamniaceae
*Seirospora occidentalisBørgesen, Botanisk Tidsskrift 30: 1-19. 1909. TYPE: DANISH WEST INDIES, no collection information (holotype: C). Fig. 2.
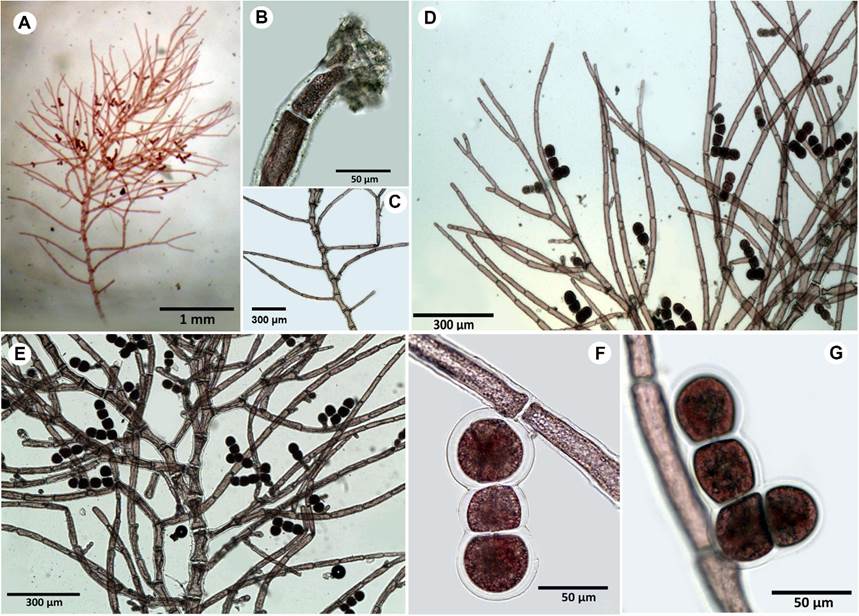
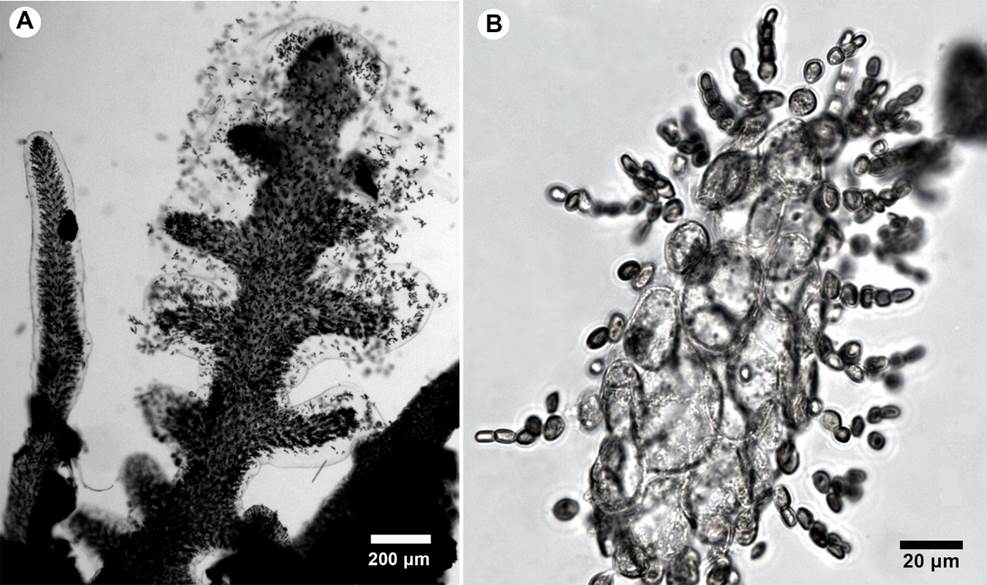
Figure 2: A-G. Seirospora occidentalis Børgesen. A. habit of a portion of the thallus; B. ecorticated rhizoidal holdfast; C. detail of the main axis; D. apex branchlets gradually reducing in size; E. seirosporangia scattered throughout the upper branches. F. seirosporangia forming simple chain; G. seirosporangia forming branched chains.
Thallus filamentous, epiphytic, about 0.5 cm tall, reddish-pink, forming delicate tufts (Fig. 2A), attached to the substratum by rhizoidal filaments forming uncorticated discoid holdfasts, rhizoids 2-4 µm diameter (Fig. 2B); cells of main axes 60-70 µm diameter, 160-180 µm long, gradually attenuating to the ultimate branchlets; terminal cells of ultimate branches much shorter, measuring 10-15 µm diameter, about 2 times as long as broad; axial cells longer above, gradually attenuating through the successive branch orders (Fig. 2C-D); branching alternate proximally, spirally arranged distally, formed from the upper distal end of axial cells, second branching dichotomous; seirosporangia scattered throughout the upper branches, sessile, arising distally from the cells of the lateral branches, forming simple o branched chains of ovoid spores, 45-60 µm diameter (Fig. 2E-G); cystocarps and spermatangia not observed.
Notes: the genus Seirospora Harvey is present in the western Atlantic with the taxa S. interrupta (Smith) F. Schmitz, S. occidentalis, S. purpurea M. Howe and S. viridis Aponte & D. L. Ballantine (Schneider et al., 1979; Wynne, 2017). Seirospora occidentalis is the most common and easily distinguishable species due to its ecorticated, small thallus and seirosporangia in chain, which are frequently observed (Aponte and Ballantine, 1995). In the Colombian Caribbean, S. occidentalis has been recorded for the Darien region, close to Panama (Bula-Meyer and Schnetter, 1988; Díaz-Pulido and Díaz-Ruiz, 2003). The specimens reviewed in this study agree with the description of the species offered by other studies (Børgesen, 1909; Aponte and Ballantine, 1995; García et al., 2011), except in the length of the thallus, since our specimens are smaller in size (0.5 cm vs. 1-2 cm in the original description).
Additional material examined: COLOMBIA. Old Providence, Felipe’s Place, 17.IX.2009, seirosporangia, V. Reyes 161 (JIW).
*Spyridia aculeata subsp. complanata (J. Agardh) Hommersand, University of California Publications in Botany 35: (1)-vii, 165-366. 1963. TYPE: BRAZIL. West Indies, no collection date, Agardh 19357 (holotype: LU). Fig. 3.

Figure 3: A-D. Spyridia aculeata subsp. complanata (J. Agardh) Hommersand. A. habit; B. cross section of main axis showing the large axial cell (center) surrounded by small cortical cells; C. main axis with branchlets radially arranged. D; pointed branchlet apex.
=Spyridia hypnoides subsp. complanata (J. Agardh) M. J. Wynne.
Thallus filamentous, forming shrubs, dark pink, to 3 cm high, branches in random pattern (Fig. 3A); cross-section elliptical-shaped, minor axis 373 μm long, major axis 495 μm long, cell wall 36 μm thick (Fig. 3B); surface cells arranged in alternating layers of narrow cells (10-17 μm diameter, 33-46 μm long) and wide cells (20-30 μm diameter, 30-33 μm long); branchlets corticated at nodes only; segments 30-46 μm diameter, to 36-46 μm long; nodes 39-56 μm diameter, to 16-27 μm long, cells red-brown, darker towards apices (Fig. 3C); apex pointed (Fig. 3D); reproductive structure not observed.
Notes: Spyridia aculeata subsp. complanata is a widely recorded species in the Western Atlantic, being present in Bermuda, Central America (Belize), Caribbean Island (Cuba, Lesser Antilles), North America (Florida), and South America (Brazil, Colombia, Venezuela) (Guiry and Guiry, 2021). In the Colombian Caribbean, this species (as Spyridia hypnoides subsp. complanata) has been reported for the Tayrona National Natural Park and the Darien region. Despite the absence of reproductive structures, the observed features in the present specimen match the description of the taxon (Littler and Littler, 2000).
Additional material examined: COLOMBIA. San Andres Island, Mar Azul and Bahía Honda, 13.X.2009, depth 2 m, sterile specimen found in the intertidal zone near mangroves, H. Velazquez 129 (JIW); Old Providence, Pinaculos, 14.IX.2019, depth 8 m, B. Gavio PVA027 (JIW).
Ceramiaceae
**Ceramium brevizonatum var. caraibicum H.E. Petersen & Børgesen, Dansk Botanisk Arkiv 4(7): 14-35. 1924. TYPE: DOMINICAN REPUBLIC. Beata Island, no collection date, C. H. Ostenfeld s.n. (holotype: C). Fig. 4.
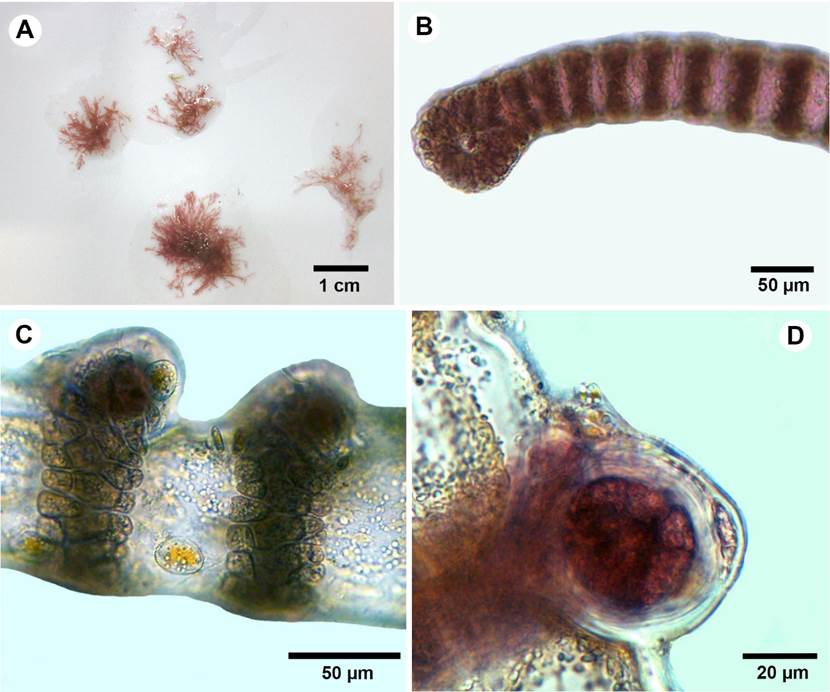
Figure 4: A-D. Ceramium brevizonatum var. caraibicum H.E. Petersen & Børgesen. A. habit; B. branch tip showing incurved apices; C. segments and nodes arrangement; D. tetrasporangia in lateral series at joints.
Thallus soft, formed by filaments entangled in clumps, light-red to red-pink in color, up to 3 cm high (Fig. 4A); branches dichotomous, apices curled inwards, laterals incurved at joints (Fig. 4B): cortication incomplete, restricted at nodes; axial cells 75-105 μm diameter, up to 127 µm long; nodes composed by heavily pigmented cells, 65-120 μm diameter, 21-63 μm long cortication arranged in three layers of cells, all irregularly rounded, layers ocasionally overlapped (Fig. 4C); tetrasporangia partially embedded, 30-40 μm diameter, adjacent to a node when present, tetrahedrally divided, one per node (Fig. 4D); carposporophytes not observed.
Notes: the genus Ceramium Roth is one of the largest genera in the Rhodophyta, with approximately 211 species distributed worldwide and 31 in the Western Atlantic (Wynne, 2017; Gavio, 2020). The main taxonomic characteristics of Ceramium are the branching pattern, tetrasporangial features and the cortication of the nodes, in particular the number of periaxial cells and their derivatives (Barros-Barreto et al., 2007). Ceramium brevizonatum var. caraibicum has a wide geographical distribution, being reported from all tropical oceans. According to Barros-Barreto et al. (2007), the morphology of C. brevizonatum var. caraibicum is similar to that of C. clarionense Setchell & N.L. Gardner and some authors have misidentified C. brevizonatum var. caraibicum from the Caribbean region with C. clarionense, based on the description and illustrations of Littler and Littler (2000). Comparing our specimen with the description of C. clarionense by Barros-Barreto et al. (2007), the tetrasporangia in C. brevizonatum var. caraibicum are smaller in size (30-40 µm) when compared with those of C. clarionense (44-90 μm). Furthermore, the tetrasporangia arrangement in C. brevizonatum var. caraibicum is always near the apex of the branch and unilateral, whereas in C. clarionense the tetrasporangia are borne on an apical branch, although they can be unilateral or in whorls at each node (Barros-Barreto et al., 2007; Fig. 11-14). Height, branchlets arrangement, pincer-like apices, entangled thallus and tetrasporangia position coincide with the description of C. brevizonatum var. caraibicum made by Børgesen (1924). A few segments showed a lower size than the original description, but most segments fit Børgesen´s measurements.
Additional material examined: COLOMBIA. San Andres Island, Mar Azul, depth 2 m, 4.X.2009, tetrasporic, H. Velazquez 192 (JIW).
Dasyaceae
**Dasya caraibicaBørgesen, Dansk Botanisk Arkiv 3: 305-368. 1919. TYPE: VIRGIN ISLANDS. No collection information (holotype: C). Fig. 5.
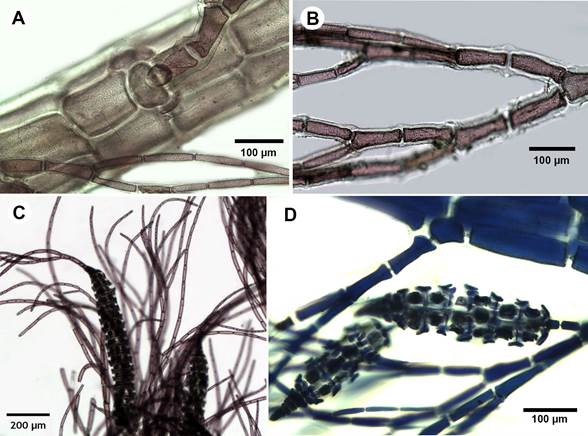
Figure 5: A-G. Dasya caraibica Børgesen. A. pseudolateral filaments and cortication; B. dichotomous branching in monosiphonous branchlets, acute angle; C. tetrasporangial stichidia with apical filaments; D. stichidium with seven fertile segments and tetraspores.
Thallus erect, to 2.5 cm tall, dark rosy-purple, arising from discoidal holdfasts; main axis to 700 µm in diameter proximally, decreasing to 180 µm distally, partially covered by rhizoidal cortication, with a small axial cell surrounded by five pericentral cells, 90-125 µm diameter; incomplete cortication developed on polysiphonous branches; pseudolaterals monosiphonous, dichotomically branched, spirally distributed, tapering, with cells shorter and thicker at base (75 µm diameter × 17 µm long), longer and thinner upwards (10 µm diameter × 32.5 µm long) (Fig. 5A-B); tetrasporangial stichidia single, borne on two-celled pedicels, composed of 4-9 fertile segments, 100-110 µm diameter, 310-1000 µm long at maturity; sporangia ovoid, 25-30 µm diameter, tetrahedrally divided, four per fertile segment (Fig. 5C-D); gametangia and cystocarps not observed.
Notes: the genus Dasya C. Agardh includes red algae with a hairy and delicate appearance and rigid to flaccid texture. The genus currently includes over 90 species distributed in sub-polar, temperate and tropical seas (Guiry and Guiry, 2021). Díaz-Pulido and Díaz-Ruíz (2003) reported ten species of Dasya for the Caribbean Colombian: D. antillarum (M. Howe) A.J.K. Millar, D. baillouviana (S.G. Gmelin) Montagne, D. collinsiana M. Howe, D. corymbifera J. Agardh, D. harveyi Ashmead, D. mollis Harvey, D. ocellata (Grateloup) Harvey, D. punicea (Zanardini) Meneghini, D. rigidula (Kützing) Ardissone, and D. spinuligera Collins & Hervey; two of them have been recorded for the International Biosphere Reserve Seaflower: D. collinsiana (Ortiz and Gavio, 2012) and D. rigidula (Gavio et al., 2015). The taxonomy of Dasya is very difficult, and its diversity is still far from being satisfactorily understood. Anatomical and morphological interspecific differences are scarce, and several species show high intraspecific variability (Pena-Martín et al., 2014). According to Børgesen (1919), D. caraibica measures up to 20 cm, which is huge in size, compared to our sample (up to 2.5 cm). The description coincides with the incomplete cortical layer developed upon younger branches and limited to a single or a few strands descending in the furrows between the pericentral cells, but their size is much smaller than that described by Børgesen (300-400 µm vs. up to 125 µm). Børgesen described Acrochaetium opetigenum Børgesen as a species commonly observed on D. caraibica, which was not the case in our specimen. Another difference is the depth at which the species was found: Børgesen dredged the species from a depth of 30-50 m, while our species was found at 16 m depth. Although Børgesen's specimen was sterile, López-Piñero and Ballantine (2001 described the tetrasporangial stichidium, which coincides in shape and size with our samples. Dasya antillarum described in Littler and Littler (2000) presents similar stichidia in shape. However, the size of the thallus is much smaller in our sample, the color described is different (brown in D. antillarum), the cortication is heavy in the main axes, and the stichidia are longer in our sample (300-500 µm long in D. antillarum vs. 310-1000 µm long in our samples).
Additional material examined: COLOMBIA. Old Providence, El Planchón, 18.X.2009, depth 16 m, tetrasporangia stichidia, epiphytic on Corallinaceae, V. Reyes 139 (JIW).
Rhodomelaceae
**Wrightiella tumanowiczii (Gatty ex Harvey) F. Schmitz, Berichte der deutsche botanischen Gesellschaft 11: 212-232. 1893. TYPE: WEST INDIES. No collection information (holotype: possibly TCD). Fig. 6.

Figure 6: A-D. Wrightiella tumanowiczii (Gatty ex Harvey) F. Schmitz. A. cross section of main axis showing four pericentral cells and thick cortication; B. main axis with spines (arrow) and spirally disposed monoosiphonous filaments; C. tetrasporangia spirally disposed.
Thallus erect, bushy, straw-yellow to bright red-pink; branching alternate to spiral; main axes to 750 µm diameter, with four pericentral cells, 200-225 µm diameter, completely corticated by small cells, which reach 45 µm diameter (Fig. 6A), branches to 210 µm diameter, numerous, repeatedly branched above, gradually tapering with numerous short spines and uniseriate branchlets; spines 46-115 µm diameter at base, to 57-185 µm long, irregularly arranged (Fig. 6B); uniseriate branchlets tapering to 15 µm diameter, cells 50-150 µm long; tetrasporangia spherical, 50-75 µm diameter, in spiral series on uniseriate branchlets (Fig. 6C); cystocarps not observed.
Notes: Wrightiella F. Schmitz is a genus with only two species currently recognized: W. blodgettii (Harvey) F. Schmitz and W. tumanowiczii. The latter has been recorded in Atlantic Island (Bermuda), North America (Florida, Mexico, North Carolina, Texas), Caribbean Island (Bahamas, Cuba, Greater and Lesser Antilles, Virgin Island), South America (Brazil) and South West Asia (Bangladesh) (Guiry and Guiry, 2021). According to Børgesen (1919), this species is easily distinguished by its polysiphonal thallus with four pericentral cells, short spines scattered on branches and uniseriate branchlets raising on branches. Furthermore, the tetrasporangia arising in spiral series at the upper end of uniseriate branchlets is another characteristic feature. In the same description, Børgesen mentioned that Falkenberg considered the two taxa as different forms of the same species. For Børgesen the different appearance of his plant was due to its occurrence in deep water (dredged at 18-27 m depth), suggesting wide morphological plasticity. According to Schneider and Searles (1991), these taxa are likely to be conspecific. Our specimen presents all characteristics (vegetative and reproductive) of W. tumanowiczii, as shown by Børgesen (1919) and Littler and Littler (2000).
Additional material examined: COLOMBIA. Old Providence, El Planchón, 18.X.2009, tetrasporangia, V. Reyes 137 (JIW).
Pterocladiaceae
*Pterocladiella bartlettii (W.R. Taylor) Santelices, Journal of Applied Phycology 10: 242. 1998. TYPE: HAITI. Saint Louis du Sud, 13.V.1941, H. H. Bartlett 17960 (INA). Fig. 7.
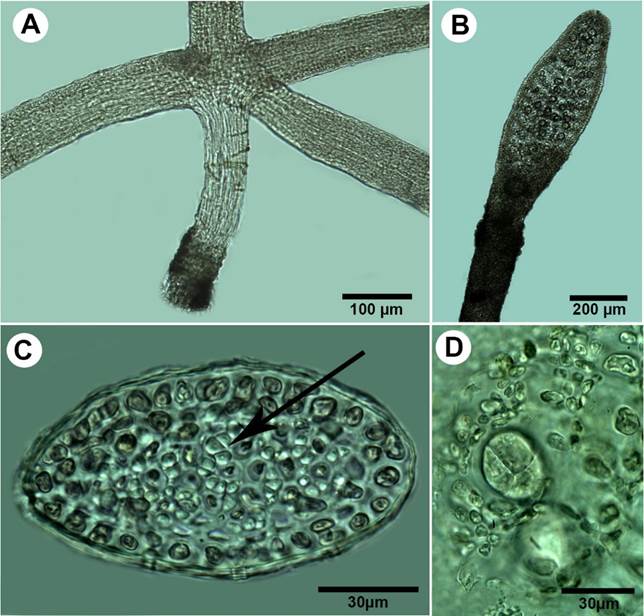
Figure 7: A-D. Pterocladiella bartlettii (W.R. Taylor) Santelices. A. cylindrical stolon with peg-like rhizoidal holdfast; B. branch with tetrasporangial stichidia; C. transverse section of erect axes showing cortical cells and rhizines (arrows) restricted to the central medulla; D. transverse section of cruciate tetrasporangial sorus.
Thalli up to 0.4 cm high, purple to yellowish in colour; erect axes arising from terete stolons 65-90 µm in diameter, attached to the substratum by peg-like rhizoidal holdfasts (Fig. 7A); branches cylindrical at base and semicompressed distally, irregular, up to three orders of branching, mostly at right angles from the main axis; branches linear-ligulate, tapering into acute apices with one apical cell, mostly constricted at the base even when bearing lateral ligulate tetrasporangial stichidia (Fig. 7B); erect axes to 155 µm in diameter, with one to two layers of cortical cells, outermost layer anticlinally oriented; rhizines abundant, translucent, surrounding the small medullary cells (Fig. 7C); tetrasporangial stichidia terminally placed on ligulate branches and branchlets; tetrasporangia rounded, cruciate divided to 25 µm diameter (Fig. 7D); reproductive gametophytes not observed.
Notes: Pterocladiella B. Santelices & Hommersand was proposed to accommodate species previously assigned to Pterocladia J. Agardh that have features such as intercalary carpogonia directed towards both surfaces of the thallus, nutritive filaments growing centripetally and forming a virtually solid cylinder around the central axis, and cystocarps usually attached to one side of the cystocarp floor with chains of carposporangia on the remaining three sides (Santelices and Hommersand, 1997). The genus currently includes 24 species (Guiry and Guiry, 2021) distributed in tropical, subtropical, and temperate regions. Molecular studies carried out since 1997 supported the monophyly of Pterocladiella (Bailey and Freshwater, 1997; Freshwater and Bailey, 1998; Iha et al., 2017). Seven species of Pterocladiella are recorded for the tropical and subtropical Western Atlantic, based on morphological or molecular studies (Wynne, 2017). Pterocladiella bartlettii has been recorded for the tropical and subtropical western Atlantic, but it has recently been observed also in Southeast Asia from Malaysia and Singapore, and is the most common Pterocladiella species in Brazil (Iha et al., 2017). Its characteristic transversal sections through both terete and flattened portions of branches show numerous rhizines among the central medullary cells. Its small size and its thinner branches are some of the characters that may distinguish this taxon from other Pterocladiella species (Thomas and Freshwater, 2001; Iha et al., 2017).
Additional material examined: COLOMBIA. Old Providence, Manta’s City, depth 11 m, 16.X.2009, reproductive with tetrasporangia in stichidia, epiphytic on Corallinaceae algae, V. Reyes 213 (JIW).
Champiaceae
*Champia taironensis Bula-Meyer, Caldasia 19: 83-90. 1997. TYPE: COLOMBIA. Parque Natural Nacional Tairona (PNNT), costa Caribe, no collection date, Bula-Meyer R-242A (COL). Fig. 8.
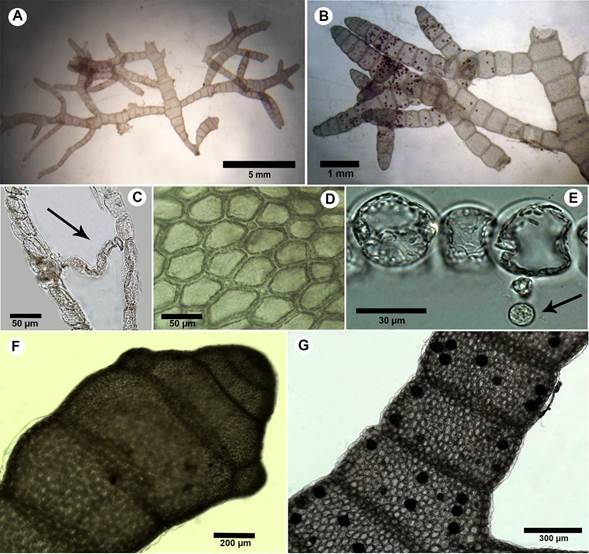
Figure 8: A-G. Champia taironensis Bula-Meyer. A. habit of specimen showing the holdfast and main axis; B. branching habits with anastomosed pair; C. longitudinal section showing single-layered septa between nodes (arrow); D. superficial view of cortical cells, E. longitudinal section showing gland cell (arrow); F. branch arising from the septum; G. axial segments with tetrasporangia.
Thallus reddish-brown to pink, slightly gelatinous, soft, prostrate and spreading; upright axes arising from small discoidal holdfasts up to 1.9 cm height, axes slightly compressed, divided into segments by single-layered septa (Fig. 8A); segments shorter than broad in all portions, more pronounced in younger portions; slightly constricted at septa, 450-1150 µm diameter; branches opposite, occasionally alternate (Fig. 8B); single-layered septa present internally at nodes, septal cells rectangular, 13-18 μm broad, 18-25 µm long (Fig. 8C); external cortical cells small, 25-37 µm diameter, 20-70 µm long, deciduous hairs absent (Fig. 8D); inner cortical cells produce spherical gland cells 7.5-10 μm diameter, facing the interior cavity (Fig. 8E); branches arise at nodes (Fig. 8F); tetrasporangia scattered under the cortex of internodal segments, tetrahedral, spherical, 50-65 μm diameter (probably immature) (Fig. 8G); cystocarps and spermatangia not observed.
Notes: the genus Champia Desvaux (Rhodymeniales, Champiaceae) currently contains 45 species worldwide distributed (Guiry and Guiry, 2021). Of these, nine taxa are known in the western Atlantic from cold temperate to tropical environments (Wynne, 2017). In the Colombian Caribbean, eight species of Champia have been reported to date, but only three of them are recorded for the Seaflower International Biosphere Reserve: C. parvula (C. Agardh) Harvey var. postrata L.G. Williams (Gavio et al., 2015), C. salicornioides Harvey (Ortiz and Gavio, 2012), and C. vieillardii Kützing (Díaz-Pulido and Díaz-Ruíz, 2003). According to Schneider et al. (2018), it is possible that reports of C. compressa Harvey and C. vieillardii in warm waters of the western Atlantic correspond to C. hasselbringii C.W. Schneider & G.W. Saunders. The species C. compressa and C. vieillardii have been repeatedly reported for several localities of the Caribbean Sea, despite their type localities being located in South Africa and New Caledonia, respectively. The two species names have been used separately as well as interchangeably in many reports (Schneider et al., 2018), but the numerous records of C. compressa in tropical areas should be attributed to C. vieillardii (Bula-Meyer, 1997). Champia taironensis shares similar morphological characters with C. compressa, C. hasselbringii and C. vieillardii, which have flattened or compressed axes and similar branching patterns. The observed specimen of C. taironensis arises from a discoid holdfast forming an erect habit, its distal segments are shorter than broad (rectangular shape) and do not present deciduous hair in the distal part. All these characters differ from C. hasselbringii, which shows barrel-shape distal segments that are markedly pitched at the septa and the presence of deciduous hair. Champia compressa is attached by a stolon base with several haptera, has sparsely pinnate, opposite or alternate branches, while C. taironensis presents a single holdfast and branching frequently tripinnate and regularly opposite. On the other hand, C. vieillardii has several small holdfasts to attach to the substrate and its tetrasporangia are small (37-44 μm diameter), compared with the tetrasporangia (50-65 μm diameter, probably immature) observed in C. taironensis.
Recent molecular studies on specimens from the western Atlantic have revealed new cryptic species previously considered as C. parvula (C. Agardh) Harvey: C. farlowii M.K. Griffith, C.W. Schneider & C.E. Lane. from New England, USA, Champia insularis C.W. Schneider & G.W. Saunders from Bermuda, and Champia puertoricensis Lozada-Troche & D.L. Ballantine from Puerto Rico. These authors suggest the need to carry out molecular studies on the specimens currently identified as C. parvula and C. parvula var. postrata in the Greater Caribbean Sea (Griffith et al., 2017; Schneider et al., 2018). Champia parvula var. postrata has been frequently collected in San Andres and Old Providence Islands, and it has been reported in Quitasueño Bank (Gavio et al., 2015); it is the most common Champia species in the Seaflower International Biosphere Reserve.
To date, C. taironensis had been recorded only in its type locality, the Tayrona National Natural Park near Santa Marta, at 12-23 m depth, epiphytic on Udotea occidentalis A. Gepp & E.S. Gepp (Bula-Meyer, 1997; 2001). Bula-Meyer (2001) described C. taironensis as an annual species, with higher frequency and density during the rainy season. This is the first report of C. taironensis outside its type locality.
Additional material examined: COLOMBIA. Old Providence, Manta’s City, depth 11 m, 16.X.2009, tetrasporic specimen, V. Reyes 219 (JIW).
Faucheaceae
**Gloioderma iyoense Okamura, Icones of Japanese algae 7, 19-48. 1934. TYPE: JAPAN. Otateba, Ehime Prefecture, no collection date, K. Okamura s.n. (SAP). Fig. 9.
Thallus postrate, soft, gelatinous, complanate, whitish-pink, 1 cm tall (Fig. 9A); branches alternate to almost opposite, 200 µm wide, apices rounded, with gelatinous transparent cover very visible (Fig. 9B); medulla composed of 3-4 layers of polygonal cells, 23-29 × 33-40 µm; cortex formed by anticlinal filaments of small cells, 3-5 µm diameter, 6-9 µm long, pseudodichotomously branched; reproductive structures not observed.
Notes: the genus Gloioderma J. Agardh encompasses only four species currently accepted: Gloioderma atlanticum Searles, G. australe J. Agardh, G. halymenioides (Harvey) J. Agardh and G. iyoense Okamura (Guiry and Guiry, 2021). Only G. atlanticum and G. iyoense have been reported for the Western Atlantic, while the other two species are restricted to the Pacific Ocean (G. australe) and Indo-Pacific (G. halymenioides). Gloioderma atlanticum, originally described from North Carolina, has wider branches than G. iyoense, 2 mm vs. 1 mm wide (Schneider and Lane, 2007). Norris (1991) suggested that these two species (as Gloiocladia atlantica (Searles) R.E. Norris and Gloiocladia iyoensis (Okamura) R.E. Norris) might be conspecific, and molecular analysis should be carried on to confirm or reject his hypotesis. Due to the small size and narrow branches of our specimen, it fits the description of G. iyoense. This species has been reported in the Western Atlantic, in Bermuda (Schneider and Lane, 2007), Venezuela (Gómez et al., 2013) and Cuba (Moreira González et al., 2017).
Additional material examined: COLOMBIA. Old Providence, Pinnacles, depth 14 m, 11.IX.2019, vegetative thallus, B. Gavio PVA048 (JIW). San Andres Island, Green Moon, depth 10 m, 12.XII.2012, vegetative thallus, N. Rincón-Díaz BFC009 (JIW).
Rhodymeniaceae
**Botryocladia cf. bahamensis Ballantine & Aponte, Cryptogamie, Algologie 23: 123-130. 2002. TYPE: BAHAMAS. Lee Stocking Island, 21.VIII.1994, D. L. Ballantine 4904, 198086 (US). Fig. 10.
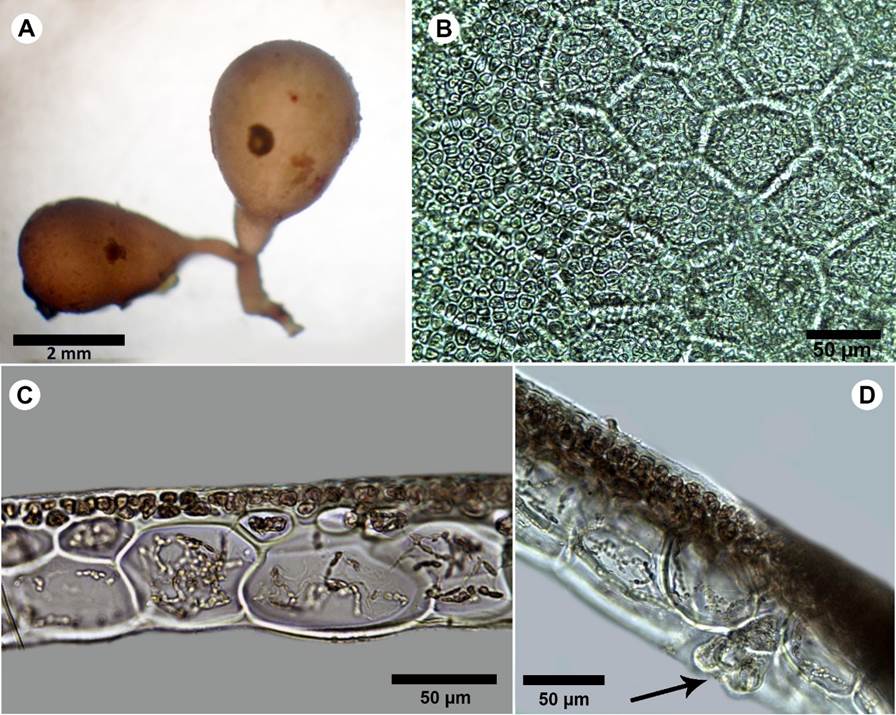
Figure 10: A-D. Botryocladia cf. bahamensis Ballantine & Aponte. A. habit; B. outer cortex of vesicle wall in surface view; C. cross-sections of vesicle walls; D. gland cells (arrow).
Plants brownish to rosy-red, to 6 mm tall, attached by small discoid holdfasts, giving rise to short simple stipes bearing two vesicles; vesicles obovoid to obpyriform, 2-3 mm diameter; 3-4 mm long; stipes short, terete, less than 1 mm diameter, solid (Fig. 10A), vesicle walls consisting of 2-3 layers, innermost cells of the medulla 50-60 µm diameter, 60-90 µm long, polygonal in surface view and complete cortication (Fig. 10B-C); gland cells one to four, obovoid to pyriform, 18-20 µm diameter, 20-30 µm long (Fig. 10D); reproductive structures not observed. In some parts the specimen showed different coloration, maybe due to endophytes or disease.
Notes: seven species of Botryocladia have been recorded in the Colombian Caribbean: B. monoica Schnetter, B. enteromorpha (Harvey) W.E. Schmidt, Lozada-Troche, D.L. Ballantine & Fredericq (as Chrysymenia enteromorpha Harvey), B. occidentalis (Børgesen) Kylin, B. panpefussiana Ganesan and Lemus, B. pyriformis (Børgesen) Kylin, B. shanksii E.Y. Dawson and B. spinulifera W.R Taylor & I.A. Abbott (Díaz-Pulido and Díaz-Ruíz, 2003). The present finding is the first record of B. cf. bahamensis outside its type locality. The species of the Botryocladia complex rarely have a single defining characteristic as appearing in B. spinulifera, where the spine-like projections from the cortical matrix make the taxon easily identifiable. Our specimen presents the most representative vegetative morphological characters of B. bahamensis; however, the size of the vesicles is much smaller (9 mm) compared to that described in B. bahamensis (25 mm). According to Wilkes et al. (2006), the difficulty in using vegetative morphology for inferring taxonomic relationships in the Rhodymeniales has been discussed in the past by several authors, who consider that reproductive characters should have a much greater weight. Unfortunately, reproduction has not been well characterized for many species of Botryocladia. Furthermore, it is not always possible to observe reproductive structures, as in our case. With these considerations, we tentatively assign the specimen to the taxon B. bahamensis. Fertile specimens, along with molecular data, should elucidate the identity of this organism.
Additional material examined: COLOMBIA. Old Providence, Mantas City, depth 11 m, 16.X.2009, vegetative thallus, V. Reyes 67 (JIW).
**Botryocladia cf. bermudana C.W. Schneider & C.E. Lane. Phycologia 47: 614-619. 2008. TYPE: BERMUDA. Hunt Island, Port Royal Bay, 11.V.1949. W. R. Taylor and A. J. Bernatowicz 49-1724 (MICH). Fig. 11.
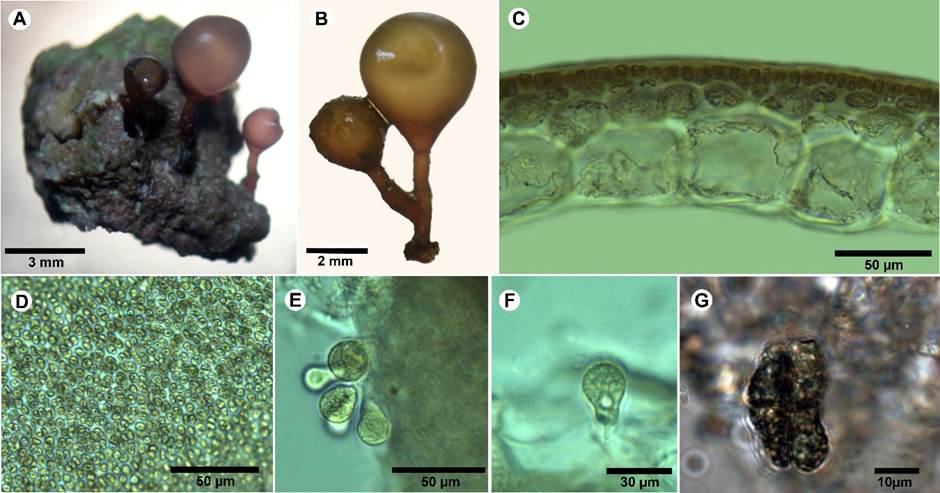
Figure 11: A-G. Botryocladia cf. bermudana C.W. Schneider & C.E. Lane. A. habit of specimen on rock; B. small plants with one or two spherical to subspherical vesicles on short stipes; C. cross-sections of vesicle walls; D. outer cortex of vesicle wall in surface view; E. gland cells; F. pyriform gland cell; G. cruciate tetrasporangia.
Plants brownish to rosy-red, to 10 mm tall, attached by small discoid holdfasts, giving rise to stipes bearing one to three vesicles (Fig. 11A); vesicles obpyriform to obovoid, to 2-6 mm diameter, 2-5 mm long (Fig. 11B); stipes terete and branched, up to 1.5 mm diameter, solid; vesicle walls consisting of 3-5 layers, cells of the medulla 65-100 µm thick, 80-140 µm long in section, polygonal in surface view with large and small cells intermingled, vesicle cortex of 1-2 layers (Fig. 11C); complete cortication (Fig. 11D); gland cells 1-4, pyriform to obovoid shape, to 20 µm diameter, 25 µm long (Fig. 11E-F); tetrasporangia in scattered discrete sori in the outer cortex, spherical to ellipsoidal, cruciately divided, 20 µm diameter, 30 µm long (Fig. 11G); spermatangia and cystocarp not observed; growing on rock, at depth of 9-10 m.
Notes: according to the description of Botryocladia bermudana (Schneider and Lane, 2008), our specimen presents the size and characteristics of typically wild B. bermudana plants, which generally grows in rock crevices or overtopped by larger algae on the coral reef. The species is small (less than 1 cm tall) and develops one or two spherical to subspherical vesicles atop short stipes. Usually, this species is found as scattered, cryptic individuals, but it has also been collected in Bermuda Aquarium (Schneider and Lane, 2008). In this artificial environment lacking herbivores and fed by the waters of Harrington Sound, plants grow larger when compared with the plants in coral reef environments. The same authors consider that there are no obvious features that are wholly unique to B. bermudana, but a combination of differences in a suite of characters separates it from all other species in the genus. The specimens reviewed in this study agree with most of B. bermudana features; however, the size of the medullary cells is smaller in our sample (65-90 µm diameter × 85-120 µm long) than in the original description of B. bermudana (90-120 µm diameter × 60-150 µm long). Likewise, the size of the tetrasporangia differs, being slightly smaller in our sample (30 µm × 20 µm), than in the original description (24-30 µm × 26-48 µm). The size of these features in our samples falls in the smaller range of the original circumscription. Molecular studies are required to help discern and clarify the diversity of Botryocladia species present in the International Biosphere Reserve Seaflower.
Additional material examined: COLOMBIA. Old Providence, Mantas City, depth 9-10 m in a coral cavity, 16.X.2009, vegetative thallus, V. Reyes 8 (JIW), V. Reyes 246 (JIW). Old Providence, Canolly, 17.X.2009, vegetative thallus, V. Reyes 6 (JIW).
*Botryocladia spinulifera W.R. Taylor & I.A. Abbott. British Phycological Journal 8: 409-412. 1973. TYPE: AMERICAN VIRGIN ISLANDS. Grass Point, St Croix, 21.I. 1973, W. H. Adey 11407 (MICH). Fig. 12.
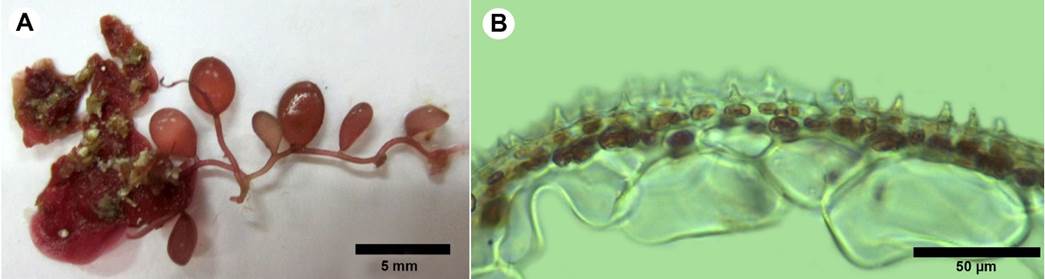
Figure 12: A-B. Botryocladia spinulifera W.R. Taylor & I.A. Abbott. A. habit; B. cross-section of vesicle walls showing continuous cell arrangement with spiny projections form the cortical matrix.
Plant dark rose-red, creeping, to 15 mm tall, attached by small discoid holdfasts; blades in vesicular shape, spherical to obovoid, 2.5 mm diameter, 3.7 mm long (Fig. 12A); stolon solid, cylindrical, 337 µm diameter, stalk small to absent; vesicules walls consisting of 2-3 layers, central cavity filled with mucilage, medullary cells 37-79 µm diameter, 28-39 µm long, surface cells round, 11-13 µm diameter with spiny projections, spines 4-9 µm high (Fig. 12B); gland cells not observed; reproductive structure not observed.
Notes: the genus Botryocladia (J. Agardh) Kylin is found in tropical, subtropical, and warm-temperate seas, occurring most commonly in deep subtidal habitats. Their thalli consist of small, swollen vesicles, borne either alone or in small groups on a solid, erect axis (Wilkes et al., 2006). To date, there are 17 recognized species of Botryocladia in the tropical and subtropical Western Atlantic (Wynne, 2017; Schmidt et al., 2017). Botryocladia spinulifera was described by Taylor and Abbott (1973) from the West Indies. This species is easy to recognize by its spiny projections on the surface. Ballantine (1985) considers that despite its distinctive morphology, B. spinulifera has been found only rarely in the Caribbean, a fact probably related to its cryptic habit. The specimens were found growing on rock under the algal canopy, so the alga is not visible without scraping the seaweeds above it.
Additional material examined: COLOMBIA. Old Providence, Canolly, depth 13-15 m, 17.X.2009, epiphytic on Valonia sp. and Peyssonnelia sp., vegetative specimens, V. Reyes 219 (JIW); Old Providence, Nick’s Place, depth 37 m, 18.X.2009, vegetative specimens, V. Reyes 114 (JIW); Old Providence, site outside barriers reef, depth 14 m, 11.IX.2019, vegetative specimens, B. Gavio PVA066 (JIW).
Discussion
According to Luypaert et al. (2020), the state of marine biodiversity in the Anthropocene is complex. At the global level, taxonomic assessments in the marine domain are very incomplete. The scientific exploration program implemented in the Seaflower Biosphere Reserve contributes to obtain data from poorly studied areas of the Caribbean basin. A complete taxonomic list not only allows us to know the diversity present in place but also to observe changes in species composition over time. Taxonomic lists become important reference points in the context of climate change, where the occurrence of major hurricanes (such as Iota in Old Providence and Saint Cataline occurred in December 2020) can lead to a significant increase in opportunistic macroalgae and loss of species of ecological importance. It is currently not known whether these taxa are new entries in the phycological flora of the Archipelago, or they have been neglected in previous studies. However, exotic species such as Griffithsia capitata Børgesen (Rincón-Díaz et al., 2016) and possibly Wrangelia gordoniae K.E. Bucher, D.L. Ballantine, C. Lozada-Troche & J.N. Norris (Rincón-Díaz et al., 2018) have been reported in the past few years in the Archipelago. Further studies will fill the gaps in understanding this biodiversity and the changes species are facing.
Most of the specimens reported here present smaller sizes than observed at other localities, yet they are reproductive. These reproductive structures are relevant for the correct taxonomic identification of red algae. At the same time, they show that these small-sized specimens are fully grown thalli. To date, it is not clear if particular ecological conditions promote early maturation in small thalli, or if this characteristic underlines the presence of cryptic taxa in the Archipelago. This should be analyzed using molecular data and specialized ecological studies. Furthermore, detailed studies are required to understand the distribution, population dynamics and status of rare or uncommon taxa. Considering the unprecedented pressures seaweeds are facing, including coastal pollution and human-induced global warming, it is critical to reinforce our knowledge of seaweed biodiversity (Mantri et al., 2020).
The complete macroalgal checklist of the Seaflower Biosphere Reserve reaches, at present, 325 taxa: 153 Rhodophyta, 41 Phaeophyceae, 103 Chlorophyta, 28 Cyanophyta. According to Diaz-Pulido and Diaz-Ruíz (2003), the Archipelago of San Andres and Old Providence corresponded to the third geographic sector with the greatest diversity of algae in the Colombian Caribbean, preceded by Tayrona National Park (364 taxa) and the Darien region (217 taxa). With the research carried out in the past 10 years, the number of registered taxa has increased from 202 to 325, which represents a 62% increase in the knowledge of diversity and places the Archipelago in the second most diverse region in the Colombian Caribbean, accounting for more than half (52%) of the macroalgal species of the country. The great marine extension of the Archipelago (about 180,000 km2), together with the diversity of ecosystems and the limited direct human impact, are the main reason of the high diversity of marine algae in the area.
Biosphere Reserves are considered learning laboratories and have been established for the sustainable use of their resources; to accomplish this goal, their biodiversity should be well understood. According to Pino-Del-Carpio et al. (2014), management plans in Biosphere Reserves may be improved if alternative sources of information as the Global Biodiversity Information Facility index (GBIF) database and scientific literature are consulted. Therefore, the species information presented here represents a contribution to the knowledge of macroalgal diversity, for the conservation and management of the biodiversity present in the Biophere Reserve Seaflower.
Conclusions
With the results of the present study, the current algal flora of the Seaflower International Biosphere Reserve includes 153 species of Rhodophyta, belonging to 12 orders, 27 families and 73 genera. The Ceramiales is the most representative order with 91 species. The family Rhodomelaceae, with 33 species, is the most diverse family in the Archipelago, followed by Ceramiaceae with 21. The species information presented here represents a contribution to the knowledge of macroalgal diversity, for the conservation and management of the biodiversity present in the Biophere Reserve Seaflower.











 nueva página del texto (beta)
nueva página del texto (beta)