Introduction
In the moss family Hedwigiaceae, there is only one species with a combination of immersed capsules, green leaf tips, low leaf cell papillae, and broad undulate perichaetial leaves (Fig. 1). This unique blend of features has been conventionally regarded to justify the generic rank for this taxon as Hedwigidium Bruch & Schimp. As exposed by Allen (2010), the correct identity and nomenclature for the only species in the genus must be Hedwigidium imberbe (Sm.) Bruch & Schimp. Allen (2010) examined morphological differences and pointed out that the northeastern North American Hedwigia integrifolia P. Beauv. is not a synonym of Hedwigidium imberbe, originally described from Ireland. Therefore, Allen (2010) aptly discovered that the well-known name Hedwigidium integrifolium (P. Beauv.) Dixon was widely misapplied in the literature and herbarium specimens. Later, Dalton et al. (2012) also acknowledged that Hedwigia integrifolia differs from Hedwigidium imberbe in several important characters, supporting their taxonomic separation from Hedwigia P. Beauv. They examined the type for Hedwigia integrifolia and corroborated that it belongs in Hedwigia. The type specimen has leaves with a hyaline acumen, the leaf cells have branched papillae, and the perichaetial leaves are ciliate, as in Hedwigia ciliata (Hedw.) P. Beauv. In this case, Dalton et al. (2012) resolved prudently: “further study is needed to determine the exact status of this taxon, so it is provisionally left as a species of Hedwigia”. Notably, Dalton et al. (2012) recognized Hedwigia and Hedwigidium as different genera, despite both taxa having identical seta lengths (<1 mm) and similar capsule shapes (globose, urceolate).

Figure 1: Hedwigidium imberbe (Sm.) Bruch and Schimp. A plant showing several branches with sporophytes covered by long perichaetial leaves. One cluster of perichaetial leaves has been dissected to show the immersed urn with the operculum covered by the small calyptra still in place (from specimen De Luna 2717 (XAL), growing on rock; near Villareal, Tlaxcala, Mexico).
In contrast, in the same paper, Dalton et al. (2012) moved Hedwigidium imberbe (Fig. 1) to Braunia Bruch & Schimp. The genus Braunia is characterized by thick-walled, sinuose leaf cells with pluri-papillose longitudinal walls, exserted capsules, usually cylindrical urns, and a cucullate calyptra (Bruch and Schimper, 1846; Brotherus, 1909; Biasuso, 1992; De Luna, 1992, 2016). Dalton et al. (2012) made the new combination Braunia imberbis (Sm.) N. Dalton & D.G. Long, consequently synonymizing Hedwigidium with Braunia. As justification for this hypothesis, Dalton et al. (2012) asserted “… some Braunia species have a seta as short as that of Hedwigidium - in B. reflexifolia (Müll. Hal.) E.B. Bartram seta length is 2 mm or less”. They argued: “Seta length cannot therefore be used as a clear-cut generic character”. Dalton et al. (2012) concluded: “For these reasons, the new combination Braunia imberbis is made below, and thus Hedwigidium is synonymized with Braunia”. However, their taxonomic inference was based on untested premises about seta length variation.
The assertion by Dalton et al. (2012) “… some Braunia species have a seta as short as that of Hedwigidium” is a type of empirical statement that can be scrutinized in the context of the current standards of the science of biological systematics. Anyone applying the relevant technique can test empirical statements (Popper, 1992: 99) under refutationist and verificationist philosophies (Wiley, 1975; De Luna, 1995a; Kluge, 1997). The taxonomic hypothesis of Hedwigidium being identical to Braunia was presented without evidence and without any formal analysis (statistical, phenetic or phylogenetic) of actual measurement data. Therefore, this paper is intended as a simple exercise in Popperian refutation of the two competing hypotheses: Hedwigidium = Braunia and the alternative Hedwigidium ≠ Braunia. The goal of this research was to provide measurement data and statistical analyses required to appropriately test the taxonomic utility of seta length in the Hedwigiaceae. Seta length measurements were sampled to test the Dalton et al. (2012) assertion that some Braunia species have a seta as short as that of Hedwigidium. Measurements of seta length in numerous specimens worldwide were subjected to analysis of variance to compare variation in Hedwigidium imberbe with 17 species of Braunia, Pseudobraunia californica (Lesq.) Broth. and Hedwigia ciliata. This study appropriately tests the taxonomic utility of seta length in the Hedwigiaceae and refutes the hypothesis that Hedwigidium is identical to Braunia. Therefore, the alternative hypothesis which traditionally considers Hedwigidium as a monotypic genus cannot be rejected for now.
Material and Methods
Specimens
The specimens studied of Hedwigia, Hedwigidium, Braunia and Pseudobraunia (Lesq. & James) Broth. (Hedwigiaceae) belong to several herbaria (AAU, B, BM, BR, DUKE, F, JE, MEXU, MICH, MO, NSW, NY, QCA, S, US, and XAL). A list of the 20 species of Hedwigiaceae sampled and the number of specimens for each species examined are provided in Table 1.
Table 1: List of twenty species studied and summary statistics for seta length variation in Hedwigidium Bruch & Schimp., Braunia Bruch & Schimp., Pseudobraunia (Lesq. & James) Broth., and Hedwigia P. Beauv. (Hedwigiaceae). Species names are followed by the number of specimens sampled (n), sample mean, and standard deviation (sd) values of seta length, and the observed range of measurements (min, max). The eight species marked with an asterisk are the species included in the second analysis of variance and multiple comparison tests, as explained in the Material and Methods section and illustrated in Figure 2.
| Species | n | mean | sd | min | max | |
|---|---|---|---|---|---|---|
| 1 | Hedwigidium imberbe (Sm.) Bruch and Schimp.* | 40 | 0.7 | 0.21 | 0.2 | 1 |
| 2 | B. reflexifolia (Müll. Hal.) E.B. Bartram * | 29 | 2.0 | 0.31 | 1.3 | 2.6 |
| 3 | B. exserta Müll. Hal. * | 61 | 2.1 | 0.44 | 1.5 | 4 |
| 4 | B. tucumanensis Biasuso * | 28 | 2.3 | 0.32 | 1.5 | 3.1 |
| 5 | B. squarrulosa (Hampe) Müll. Hal. * | 32 | 4.1 | 1.34 | 3 | 10 |
| 6 | B. subincana Broth. * | 46 | 6.8 | 1.62 | 3 | 10 |
| 7 | B. incana Müll. Hal. | 2 | 6.5 | 0.70 | 6 | 7 |
| 8 | B. alopecura (Brid.) Limpr. * | 23 | 7.4 | 2.27 | 4 | 12 |
| 9 | B. subplicata E. Britton * | 19 | 7.8 | 2.54 | 4 | 11 |
| 10 | B. nephelogenes De Luna & W.R. Buck | 24 | 8.4 | 2.16 | 5 | 13 |
| 11 | B. cirrhifolia (Mitt.) A. Jaeger | 58 | 8.5 | 2.61 | 4 | 14 |
| 12 | B. rupestris (Mitt.) A. Jaeger | 22 | 8.8 | 1.59 | 5 | 11 |
| 13 | B. argentinica Müll. Hal. | 20 | 9.3 | 1.17 | 7 | 12 |
| 14 | B. canescens Schimp. ex E. Britton | 22 | 9.8 | 1.69 | 7 | 12 |
| 15 | B. andrieuxii Lorentz | 69 | 10.1 | 2.12 | 6 | 15 |
| 16 | B. plicata (Mitt.) A. Jaeger | 64 | 10.1 | 2.89 | 5 | 21 |
| 17 | B. attenuata (Mitt.) A. Jaeger | 24 | 11.0 | 2.71 | 7 | 16 |
| 18 | B. secunda (Hook.) Bruch & Schimp. | 71 | 12.0 | 3.36 | 7 | 22 |
| Braunia (17 spp.) | 614 | 7.8 | 3.94 | 1.3 | 22 | |
| 19 | Pseudobraunia californica (Lesquereux) Brotherus | 18 | 6.8 | 2.33 | 4 | 12 |
| 20 | Hedwigia ciliata (Hedw.) P. Beauv. | 10 | 0.5 | 0.29 | 0.2 | 1 |
Distribution map
The geographic distribution of Hedwigidium was reviewed and verified with herbarium specimens from each country previously recorded in the literature (except Europe where the genus is already well-documented). The list of representative specimens for this review of the distribution of Hedwigidium is presented below in the section “Specimens examined”.
Digital images and seta length data
Prepared semi-permanent slides were used for photographs with a digital camera (D-SLR Canon 60D) attached to the C tube of a stereoscopic microscope (Zeiss, Stemi SV11, Jena, Germany). Digital images of complete sporophytes were obtained from specimens of the four genera in the Hedwigiaceae (Table 1). Images were used to record quantitative data for statistical analyses of seta length variation. Measurements of seta length were obtained with software ImageJ v. 1.53a (Schneider et al., 2012) and scaled in millimeters.
Morphometric analyses
This paper reports measurement data and an application of analyses of variance, a standard and well-known test procedure that uses sample measurements to decide between competing hypotheses for the equality of sample means (Zar, 1996). Statistical analyses of seta length variation were implemented with PAST v. 3.20 (Hammer et al., 2001) for MacOS (v. 10.13.6). Analyses of variance were designed at two grouping levels (PAST menu “Univariate>ANOVA etc. (several samples) >several sample tests”). First, all observations (n=682) were classified into four a priori groups to compare variation among Hedwigia (n=10), Hedwigidium (n=40), Braunia (n=614), and Pseudobraunia (n=18). Second, a subsample of only seven Braunia species with seta length average values lower than 8 mm were included to compare with Hedwigidium, so that the number of multiple comparisons remained within reasonable limits. In this case, measurements of seta length (n=278) were classified by species into eight a priori groups for analysis of variance and pairwise post-hoc comparison tests. In this analysis, the Mann-Whitney pairwise test available in PAST software was employed with the Bonferroni corrected p values. Besides Hedwigidium imberbe (n=40), the seven Braunia species included were: Braunia exserta Müll. Hal. (n=61), B. tucumanensis Biasuso (n=28), B. reflexifolia (n=29), B. squarrulosa (Hampe) Müll. Hal. (n=32), B. alopecura (Brid.) Limpr. (n=23), B. subplicata E. Britton (n=19), and B. subincana Broth. (n=46). The geographical amplitude of this subsample of seven species of Braunia with short setae covers South America, Mexico, Central America, and Europe. All other species of Braunia present in Africa and India have seta length averages well above 8 mm (Table 1).
Results
Summary statistics of seta length variation in the Hedwigiaceae are presented in Table 1. The first analysis of variance comparing four genera revealed that seta length is very variable in the 17 species of Braunia sampled (Table 1). Unquestionably the sample mean in Braunia (7.8 mm, sd=3.9) differs from the value recorded for Hedwigidium (0.7 mm, sd=0.21). However, as expected, the average seta length in Hedwigidum is not different from the sample mean in Hedwigia (0.5 mm, sd=0.29).
The second analysis of variance shows there is no overlap in measurements between Hedwigidium and the subsample of seven species of Braunia with short seta lengths (Fig. 2). The average seta length in B. exserta, B. tucumanensis, and B. reflexifolia is in the range of 2.0-2.3 mm. These values might seem very close to the values in Hedwigidium, but the Mann-Whitney pairwise test assessed the difference, and it is significant between sample means. This a posteriori test reveals that measurements of the seta in Hedwigidium are different from the seven Braunia species in all pairwise comparisons. The average seta length in B. squarrulosa is 4.1 mm (sd=1.34), in B. subincana it is 6.8 mm (sd=1.62), in B. alopecura it is 7.4 mm (sd=2.27) and in B. subplicata it is 7.8 mm (sd=2.54). All other species of Braunia not included in the second analysis have seta lengths longer than 8 mm (Table 1).
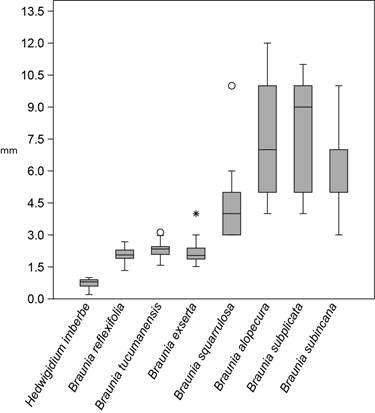
Figure 2: Variation in seta length in Hedwigidium Bruch & Schimp. and Braunia Bruch & Schimp. Comparing distributions of measurements from 278 specimens sampled for Hedwigidium imberbe (Sm.) Bruch and Schimp. and seven species of Braunia Bruch & Schimp. with short setae. Box and whisker plots for each species illustrate estimated median value, quartiles, and the range of seta length measurements. Outliers are identified with symbols.
Discussion
This numerical exercise corroborates the intuitively perceived differences in seta length between Hedwigidium and Braunia. The two analyses of variance presented here reject the hypothesis of equal sample means. None of the Braunia species has a seta as short as that of Hedwigidium, as Dalton et al. (2012) asserted. Their declaration: “some Braunia species have a seta as short as that of Hedwigidium - in B. reflexifolia (Mull. Hall) E.B. Bartram seta length is 2 mm or less” does not hold as true when the assessment is based on actual data and on formal statistical analyses. The data and results of analyses presented here refute the taxonomic conclusion by Dalton et al. (2012) on the synonymy of Hedwigidium with Braunia.
This quantitative example illustrates a simple exercise in Popperian refutation to test a very specific hypothesis on the nature of seta length variation. If as a result of the test, the hypothesis of Hedwigidium as a separate genus survives, this does not imply the use seta length variation as a classical ‘key character’, indicating generic differences. Neither this analysis advocates a proposal of a new taxonomic group nor a change in phylogenetic structure within the family. It must be clear that the central result is the refutation of the hypothesis Hedwigidium = Braunia and the failure to refute the hypothesis Hedwigidium ≠ Braunia. The surviving hypothesis of Hedwigidium as a different genus from Braunia, as originally proposed by Bruch and Schimper (1846), cannot be rejected on the basis of current data. Hedwigidium can still be used as an alternative to the taxonomic change proposed by Dalton et al. (2012).
Notwithstanding, the taxonomic hypothesis Hedwigidium = Braunia (Dalton et al., 2012) has been accepted by some. Buchbender et al. (2014) and Ignatova et al. (2016) used this generic taxonomy by labelling three terminals as B. imberbis in their phylogenetic analyses of several species of Hedwigia. However, Fife (2014), De Luna (2016) and Wigginton et al. (2020) did not accept such taxonomic hypothesis and the nomenclatural implications. Synapomorphic characters justifying Hedwigidium had already been uncovered in a preliminary phylogenetic morphological analysis of the Hedwigiaceae by De Luna (1995b), namely, broad perichaetial leaves with undulate margins, a short seta, ribbed capsules, a conical operculum, and a small calyptra with a subtubulose base. Therefore, De Luna (2016) excluded B. imberbis from his key and list of 23 species of Braunia worldwide, highlighting the need for combined morphological and molecular phylogenetic analyses for the grouping and generic classification in the Hedwigiaceae.
In the context of available phylogenetic information, variation in seta length is congruent with other morphological and molecular data in the Hedwigiaceae. In separate morphological and molecular phylogenetic analyses, one clade includes Hedwigia and Pseudobraunia as sister taxa (De Luna, 1995b; Cox et al., 2010), and this clade is sister to another clade that includes Braunia and Hedwigidium (De Luna, 1995b; Cox et al., 2010). The same basic topology ((Braunia, Hedwigidium) (Pseudobraunia, Hedwigia)) was suggested in recent phylogenetic analyses of molecular sequence data (Ignatova et al., 2016:Fig. 1; Liu et al., 2019: Fig. 1). Variation in seta length is congruent with this molecular sequence phylogenies. The seta is uniformly very long in the Rhacocarpaceae (Frahm, 1996), the sister group of the Hedwigiaceae (Cox et al., 2010). Sporophytes with long setae and exserted capsules are also present in Braunia and Pseudobraunia, whereas sporophytes with very short setae and immersed capsules are known in Hedwigia and Hedwigidium. Therefore, character optimization over the backbone tree topology within the Hedwigiaceae suggests two independent changes from long to very short seta in Pseudobraunia to Hedwigia and Braunia to Hedwigidium.
Obviously, seta length as a single morphological character can hardly be used as an argument for a certain taxonomic ranking. As Allen (2010) had already discussed, “if setae length is disregarded one might consider merging all four genera”. Merging two pairs of genera would also be an option for a ranking compatible with the backbone phylogeny, in which Hedwigia includes Pseudobraunia, and Hedwigidium is merged with Braunia. Still one more ranking option consists of just merging one sister pair, Pseudobraunia with Hedwigia, and leaving the other two genera separate. However, we still do not know how many species there are in Hedwigidium and if all species in Braunia belong to a monophyletic or a paraphyletic group. A combined morphological and molecular phylogenetic analysis of the relationships of species and genera within the Hedwigiaceae is still missing. Meanwhile, I prefer a ranking system of four genera.
In summary, it was premature to consider Hedwigidium as synonym of Braunia given the paucity of data and lack of detailed taxonomic and phylogenetic analyses. The proposition that Dalton et al. (2012) presented for the generic classification and for the name of this species, as Braunia imberbis, was unsupported and flawed. Their taxonomic inferences were undermined, because they did not report data and formal analysis of seta length to test the generic status of this species in Hedwigidium or as a species in Braunia. When assessing Hedwigidium and Braunia, they disregarded differences in seta length. The data and analyses presented here overturn the proposed synonymy of Hedwigidium and Braunia in favor of conserving the original hypothesis of Hedwigidium as a separate genus.
Generic taxonomic characters
There are a number of morphological features that distinguish Hedwigidium from the other three genera in Hedwigiaceae. Originally, Bruch and Schimper (1846) distinguished Hedwigidium by the less-branched stems, the frequent stoloniform branches, the plicate leaves, the conical operculum, and the cucullate calyptra, as compared to other genera in the Hedwigiaceae. The detailed description by Müller (1851, as Neckera imberbis (Sm.) Müll. Hal.), provided additional important character states which circumscribed this taxon: “densifolia ferruginea, apicibus viridibus obtusis; folia caulina ... anguste ovato-acuminata, margine lato revoluta, ... perich. longius vaginanti-lanceolato-acuminata, plicata inferne anguste elongate, basi laxe, apice incrassate quadrate reticulata; ... operc. brevi conico oblique, calyptra parva cucullata, saepius fissa”. These morphological features were illustrated in the early literature of the genus (Figs. 3A-K).

Figure 3: Historical illustrations of the morphological characters in the diagnoses of genus Hedwigidium Bruch & Schimp. A-C. leaf, perichaetial leaf, and capsule (redrawn from Smith (1811), as Gymnostomum imberbe Sm.); D-F. leaf, perichaetial leaf, capsule, and operculum (redrawn from Hooker and Taylor (1827), as Anictangium imberbe (Sm.) Hook. and Taylor); G-K. stem, leaf, perichaetial leaf, capsule, operculum, and calyptra (redrawn from Bruch and Schimper (1846), as Hedwigidium imberbe (Sm.) Bruch and Schimp.).
Among the important characters that distinguish Hedwigidium from the other three genera in the family are the very broad perichaetial leaves with undulate margins. The size of the perichaetial leaves is variable from twice or more the length of the vegetative leaves (Figs. 4C-D, H). There are some species in Braunia with large perichaetial leaves, but these are narrowly lanceolate and never undulate at the margins. Besides the broad perichaetial leaves, other features are found in the immersed sporophytes. Synapomorphic characters justifying Hedwigidium are a short seta, ribbed capsules, a conical operculum, and a small calyptra with a subtubulose base (De Luna, 1995b). The very short seta, (0.5-)0.7-0.9(-1.1) mm, distinguishes Hedwigidium from Pseudobraunia and all species of Braunia. There are some species in Braunia with short setae, such as B. exserta (2-3 mm), B. reflexifolia, (2-3 mm), and B. tucumanensis (2.-3.5 mm); however, the capsules in these Braunia species are always fully exserted and the operculum is conic-rostrate (Biasuso, 1992). Moreover, the urn in Hedwigidum is broadly urceolate or cyathiform and deeply furrowed or ribbed (Fig. 5B). In some species of Braunia the urn is broad, and sulcate, such as in B. nephelogenes DeLuna & W.R. Buck and in B. subincana (Figs. 5C-D). However, most species in Braunia have sporophytes with a cylindrical or ellipsoidal urn with a narrow capsule mouth (Figs. 5E-I). The low conical, shortly rostrate operculum, and a small cucullate calyptra, with a subtubulose base also distinguish Hedwigidium (Figs. 6D-G). The calyptra in all species of Braunia is large and cucullate (Figs. 6H-K), and the operculum in all species of Braunia is conical with a high rostrum (Figs. 6L-O).

Figure 4: Morphological features of Hedwigidium Bruch & Schimp. A-B. leaves; C-D. perichaetial leaves; E. sporophyte (A-E, from Sharp 5449 (DUKE), Guatemala); F-G. leaves; H. perichaetial leaf (F-H, from Adams s.n. (BM), Cameroon).

Figure 5: Capsule shape in Hedwigidium Bruch & Schimp. compared with variation in other genera of the Hedwigiaceae. A. Hedwigia ciliata (Hedw.) P. Beauv.; B. Hedwigidium imberbe (Sm.) Bruch and Schimp.; C. Braunia nephelogenes De Luna & W.R. Buck; D. Braunia subincana Broth.; E. Braunia exserta Müll. Hal.; F. Braunia schimperi Bruch & Schimp.; G. Braunia plicata (Mitt.) A. Jaeger; H. Braunia alopecura (Brid.) Limpr.; I. Braunia secunda (Hook.) Bruch & Schimp.

Figure 6: Calyptrae and operculum in Hedwigidium Bruch & Schimp. compared with variation in other genera of the Hedwigiaceae. A-B. Hedwigia cilata (Hedw.) P. Beauv.; C. Pseudobraunia californica (Lesquereux) Brotherus.; D-G. Hedwigidium imberbe (Sm.) Bruch and Schimp.; H. Braunia argentinica Müll. Hal. I. Braunia rupestris (Mitt.) A. Jaeger; J. Braunia exserta Müll. Hal.; K. Braunia cirrhifolia (Mitt.) A. Jaeger; L. Braunia alopecura (Brid.) Limpr.; M. Braunia schimperi Bruch & Schimp.; N. Braunia secunda (Hook.) Bruch & Schimp.; O. Braunia squarrulosa (Hampe) Müll. Hal.
Hedwigidium is further distinguished from Hedwigia and Pseudobraunia in the flagelliform-stoloniferous branches emerging from the stems. Moreover, the papillose pseudoparaphyllia in Hedwigidium are broad and short, similar to those in Braunia, while the pseudoparaphyllia are filamentous in Pseudobraunia and Hedwigia. The leaves in Hedwigidium are ovate-lanceolate, plicate, with strongly recurved margins, and have concolorous short-acuminate apices; whereas in Hedwigia and Pseudobraunia the leaves are narrowly lanceolate and have hyaline apices (Figs. 7A-P). The upper leaf cell papillae in Hedwigidium are low, unbranched, and scattered over the cell lumina, rather than tall and centered over the cell lumina as in Pseudobraunia, or tall and branched as in Hedwigia. The upper leaf cells in Hedwigidium and some species in Braunia are similarly rectangular, sinuose, and pluripapillose. There are very nice SEM pictures in the paper by Dalton et al. (2012, Figs. 1A, C), showing the similar leaf cell papillae in Hedwigidium imberbe and Braunia attenuata (Mitt.) A. Jaeger. Previously, Sharp et al. (1978) had shown also the similar leaf cell papilla in B. secunda (Hook.) Bruch & Schimp., B. squarrulosa and Hedwigidium imberbe.

Figure 7: Leaf shape in Hedwigidium Bruch & Schimp. compared with variation in other genera of the Hedwigiaceae. A. Braunia diaphana (Müll. Hal.) A. Jaeger; B. B. subincana Broth.; C. Braunia alopecura (Brid.) Limpr.; D. Braunia camptoclada P. de la Varde & Thér.; E. Braunia exserta Müll. Hal.; F. Braunia schimperi Bruch & Schimp.; G. Braunia squarrulosa (Hampe) Müll. Hal.; H. Braunia secunda (Hook.) Bruch & Schimp.; I-J. Hedwigidium imberbe (Sm.) Bruch and Schimp.; K. Braunia canescens Schimp. ex E. Britton; L. Braunia arbuscula (Welw. & Duby) A. Gepp.; M. Hedwigia ciliata (Hedw.) P. Beauv.; N. Braunia attenuata (Mitt.) A. Jaeger; O. B. cirrhifolia (Mitt.) A. Jaeger; P. Braunia nephelogenes De Luna & W.R. Buck.
In the absence of sporophytes, Hedwigidium is indeed hard to distinguish from some species of Braunia, but this happens only in some regions. In Mexico, Hedwigidium is difficult to distinguish from Braunia secunda because of the similar ovate-lanceolate leaves, with recurved leaf margins and concolorous leaf apices. In South America the same similarities in the lanceolate leaves mask the identity of B. subplicata and Hedwigidium. Likewise, in Africa it is difficult to separate Hedwigidium from B. entodonticarpa Müll. Hal. and B. rupestris (Mitt.) A. Jaeger, because of the ovate-lanceolate leaves with recurved margins in these species. The leaves in all other species of Braunia in Mexico, South America and Africa are different from Hedwigidium (Figs. 7A-P). For example, only species of Braunia will have hyaline leaf apices. This feature will distinguish B. plicata (Mitt.) A. Jaeger in Mexico. In South America the hyaline apices separate B. canescens Schimp. ex E. Britton (Fig. 7K) and B. incana Müll. Hal. Similarly, in Africa the green leaf apices differentiate Hedwigidium from B. diaphana (Müll. Hal.) A. Jaeger (Fig. 7A) and B. arbuscula (Welw. & Duby) A. Gepp. (Fig. 7L). In Africa there are three species of Braunia with concolorous leaf acumen: B. entodonticarpa, B. camptoclada P. de la Varde & Thér. (Fig. 7D), and B. schimperi Bruch & Schimp. (Fig. 7F). However, these species can be distinguished from Hedwigidium, even without sporophytes. The leaves in these species are widely ovate, oblong, and orbiculate, with a sharply differentiated mucronate or cuspidate leaf apex. In Hedwigidium the leaves are ovate-lanceolate with a gradually acuminate apex (Figs. 7I-J).
Taxonomic treatment
HedwigidiumBruch & Schimp., Bryol. Eur. 3: 155. 1846. TYPE: Hedwigidium imberbe (Sm.) Bruch & Schimp.
Hedwigia sect. Hedwigidium (Bruch & Schimp.) Mitt. J. Proc. Linn. Soc., Bot. 7: 160. 1863.
Harrisonia subg. Hedwigidium (Bruch & Schimp.) Hampe. Verh. Zool.-Bot. Ges. Wien 21: 386. 1871.
Hedwigia subg. Hedwigidium (Bruch & Schimp.) Lindb. Musci Scand. 40. 1879.
Braunia sect. Hedwigidium (Bruch & Schimp.) Müll. Hal. Linnaea 42: 378. 1879.
Plants medium or robust (2-3 cm), in loose or dense tufts or mats, dark green or red-brown; stems sympodially branched, plagiotropic, tips ascending; branches short, terete and blunt, some stoloniform; pseudoparaphyllia foliose, base much wider than long, lobed-dentate, cells papillose; leaves imbricate, spreading, concave, weakly plicate, short-ovate, ovate-lanceolate to narrowly lanceolate, apiculate, or gradually acuminate, ecostate; leaf apex concolorous, entire, erose, crenulate or serrulate; leaf margins revolute or narrowly recurved up to the base of the acumen; leaf cells thick-walled, sinuose, medial and upper cells with 3-4 low, rounded, unbranched papillae, marginal, bending over lumen; apical cells short- to long-elliptical; upper cells long-rectangular to subquadrate or oblate; basal median cells long-rectangular, yellow; basal marginal cells shortly rectangular, quadrate, or oblate, smooth, dark reddish brown; autoicous; perigonia terminal on short sympodia, alternating with sympodia terminated with perichaetia; perichaetial leaves erect, elongate, overtopping the capsules, margins entire, undulate; paraphyses short, or as long as the perichaetial leaves; setae short, neck very short ampullaceous, capsules immersed, erect, symmetric, broadly urceolate, subglobose, to cyathiform, furrowed when wet or dry, red brown, macrostomus, exothecial cells subquadrate, isodiametric to oblong, stomata few, superficial, only at neck, operculum base conic, umbonate, rounded or small rostellate; calyptra cucullate, small, or conic-mitrate, 1.5 mm long, 2-4-lobed, covering only the operculum; spores 20(24-27)-33 μm, vermiculate-papillose.
The number of species in Hedwigidium is unknown. Since Bruch and Schimper (1846) established Hedwigidium, a few more species were classified in the genus. Jaeger (1876), besides H. imberbe, listed H. emersum (Müll. Hal. & Hampe) A. Jaeger from New Zealand, and H. drummondi (Taylor) A. Jaeger from western Australia. A few years later he added H. rhabdocarpum (Hampe) A. Jaeger from Colombia and H. glyphocarpum (Hampe) A. Jaeger from Brazil (Jaeger, 1880). Two more species were included in the genus when Paris (1896) first listed H. teres (Müll. Hal.) Paris from central Africa (Mount Kilimanjaro); from South Africa he added H. erosum (Müll. Hal.) Paris. The last two species to be included in the genus were H. macrocalyx (Müll. Hal.) Paris and H. serrae (Müll. Hal.) Paris, both from southwestern Brazil (Paris, 1900). Brotherus (1925: 70) considered the genus to have two species; however, Magill and van Rooy (1998) placed the South African H. erosum as a synonym of H. imberbe. Eventually, all nine species were considered synonyms under H. imberbe. Taxonomic work in progress evaluating type specimens and worldwide morphological variation might reveal several species. Meanwhile the genus is here considered monotypic. Valid names are listed as synonyms of H. imberbe, some of which need lectotypification.
Hedwigidium imberbe (Sm.) Bruch & Schimp. Bryol. Eur. 3 (fasc. 29-30): 157. 1846.
Gymnostomum imberbe Sm., Engl. Bot. 32: 2237. 1811. Anictangium imberbe (Sm.) Hook. & Taylor, Muscol. Brit. 14. 1818. Schistidium imberbe (Sm.) Nees & Hornsch., Bryol. Germ. 1: 99. 1823. Anictangium ciliatum var. rufescens Arnott, Mém. Soc. Linn. Paris 5: 226. 1827. Schistidium ciliatum var. imberbe (Sm.) Huebener, Muscologia Germanica 30. 1833. Hedwigia imberbis (Sm.) Spruce, Musci Pyren. 263. 1847. Neckera imberbis (Sm.) Müll. Hal., Syn. Musc. Frond. 2: 105. 1851. Braunia imberbis (Sm.) N. Dalton & D.G. Long, J. Bryol. 34(1): 60. 2012. Type citation: “Discovered on dry rocks upon mountains in the west of Ireland by Miss Hutchins, who in 1809 sent specimens to Mr. Turner, which he has kindly communicated to us”. TYPE: UNITED KINGDOM. Ireland, Glengariff, 1810, Miss (Ellen) Hutchins. Mr. Turner s.n. (holotype: LINN, isotype: BM).
= Schistidium drummondii Taylor, London J. Bot. 5: 57. 1846. (Schistidium australe Wilson. London Journal of Botany 5: 58. 1846, invalid). º Neckera drummondii (Taylor) Müll. Hal., Syn. Musc. Frond. 2: 106. 1851. º Hedwigia imberbis var. drummondii (Taylor) Wilson, Flora Tasmaniae 2: 179. 1859. º Hedwigidium drummondii (Taylor) A. Jaeger. Ber. Thätigkeit St. Gall. Naturwiss. Gesell. 1874-1875: 173 (Gen. Sp. Musc. 2: 89). 1876. º Hedwigia drummondii (Taylor) Kindb., Enumeratio Bryinearum Exoticarum 15. 1888. Type citation: “S. australe, Wils. Mss. et Herb. Hook. n. 3658 (fide G. J. Lyon). -Swan River, Mr. James Drummond”. TYPE: PATRIA NOVA-HOLLANDIA, ad Swan-river, J. Drummond 3658 (Herb. Lyon-BM000986156, Herb. Lyon-BM000986155, Herb. Wilson-BM000986150, Herb. Wilson-BM000986151, Herb. Wilson-BM000986152, Herb. Wilson-BM000986154, Herb. Wilson-BM000986149, Herb. Wilson-BM000986148).
= Neckera emersa Müll. Hal. & Hampe, Linnaea 26: 502. 1855. º Hedwigidium emersum (Müll. Hal. & Hampe) A. Jaeger, Ber. Thätigkeit St. Gall. Naturwiss. Gesell. 1874-75: 173. 1876. Type citation: “Nova-Hollandia austr., sine loco indicato”. TYPE: F. J. H. von Müller s.n. (Herb. Hampe-BM000986141, Herb. Hampe-BM000986142, Herb. Hampe-BM000986143, BM000986165).
= Harrisonia rhabdocarpa Hampe, Linnaea 32: 148. 1863. º Braunia rhabdocarpa (Hampe) Müll. Hal, Linnaea 42: 378. 1879. º Hedwigidium rhabdocarpum (Hampe) A. Jaeger, Ber. Thätigkeit St. Gall. Naturwiss. Gesell. 1877-78: 508 (Gen. Sp. Musc. 2: 772). 1880. º Hedwigia rhabdocarpa (Hampe) Kindb., Enumeratio Bryinearum Exoticarum 15. 1888. Type citation: “(Lindig) 2005. Hab. Bogota Chapinero ad saxa riparia, 2700 metr., October”. TYPE: NOVA GRANADA. Bogota Chapinero, alt. 2700 metr, ad saxa riparia, X.1859, Lindig 2005 (Herb. Hampe-BM000960783).
= Harrisonia glyphocarpa Hampe, Vidensk. Meddel. Dansk Naturhist. For. Kjøbenhavn, ser. 3, 10: 263. 1878. º Hedwigidium glyphocarpum (Hampe) A. Jaeger, Ber. Thätigkeit St. Gall. Naturwiss. Gesell. 1877-1878: 508 (Gen. Sp. Musc. 2: 772). 1880. º Hedwigia glyphocarpa (Hampe) Kindb., Enumeratio Bryinearum Exoticarum 15. 1888. Type citation: (Brazil), “Rio Preto; (Glaziou) nr. 9070”. TYPE: BRASIL. Rio de Janeiro, Glaziou 9070 (Herb. Hampe-BM000986128, Herb. Bescherelle-BM000986129, BM000986132, NY).
= Braunia teres Müll. Hal., Flora 71: 415. 1888. º Hedwigidium teres (Müll. Hal.) Paris, Index Bryol. 555. 1896. Type citation: “(H. Meyer), Monte Kilimandscharo, ad finem sylvestrem superiorem inter 3000-4000 m.” TYPE: Monte Kilimandscharo, Dr. Hans Meyer (n.v.).
= Braunia novae-seelandiae Müll. Hal. in Beckett, Trans. Proc. New Zealand Inst. 26: 275. 1894. Type citation: “Selwyn Gorge, Canterbury, September 1892; No. 417; T. W. N. B. (named by Dr K. Müller)”. TYPE: NEW ZEALAND. Canterbury, Selwyn Gorge, on dry rock, IX.1892, T.W.N. Beckett 417 (NSW).
= Braunia macrocalyx Müll. Hal., Bull, Herb. Boissier 6: 110. 1898. º Hedwigidium macrocalyx (Müll. Hal.) Paris, Index Bryol. Suppl. 1: 179. 1900. º Hedwigidum imberbe var. macrocalyx (Müll. Hal.) Herzog, Biblioth, Bot. 87: 104. 1916. Type citation: (Brazil), “Brasilia, Serra Itatiaia, in rupibus alt. 2100 et 220 m., Febr. 1894: E. Ule, Coll No. 1848, 1849; Agulhas Negras, in rupibus, 2300 m.: idem No. 1850, 1854”. TYPE: (n.v.).
= Braunia serrae Müll. Hal., Bull. Herb. Boissier 6: 111. 1898. º Hedwigidium serrae (Müll. Hal.) Paris, Index Bryol. Suppl. 1 180. 1900. Type citation: “(Brazil), Brasilia, Sa Catharina, Serra do Oratorio, ad cataractam rivuli Pelotas, Aprili 1889: E. Ule, Coll. No. 536; Serra Geral, ad rupes, Januario 1890: idem, Coll”. TYPE: BRAZIL. Prov. Santa Catharina, Serra Geral, I.1890, Ule 536, Ule 79 (JE-04008589, PC, US).
= Braunia macowaniana Müll. Hal., Hedwigia 38(2): 123. 1899. Type citation: “Prom, bonae spei, Mte. Boschberg prope Somerset East, in fissuris irrigates rupium basalticarum, 1877: Prof. Mac Owan, misit 1878 sterilem; Natal, Jammerlappen: J. Dittrich (1898), lg. Hb. Arboreti Zoeschen-Diek”. TYPE: SOUTH AFRICA. Somerset East, monte Boschberg, in rupibus basalticus, 1877, Mac Owan 41 (Hb. C. Müller, Vereinigte Hb, JE); Mac Owan s.n. (JE_04008596); SOUTH AFRICA. Jammerlapen, Natal, 1898, J. Ditrich s.n. (JE_04008593).
= Braunia erosa Müll. Hal., Hedwigia 38: 124. 1899. º Hedwigidium erosum (Müll. Hal.) Paris, Index Bryol. Suppl. 1 179. 1900. Type citation: (South Africa), “Prom. bonae spei, Capetown, ad rupes prope Rondebosch, 1875: Dr. A. Rehmann”. TYPE: 1875, Rehmann s.n. (n.v.).
= Hedwigidium imberbe var. andesiticum M. Fleisch., Hedwigia 44: 315. 1905. Type citation: “Exsiccata: M. Fleischer, Musci Archip. Ind. No. 314 (1904). An Andesitfelsblöcken. Ost_Java: am Ardjoeno im Hochgebirge auf waldfreien bei Lalidjiwa 2500-2800 m im Mai 1901 vom Verfasser entdeckt”. TYPE: JAVA. Ost-Java, Am Ardjoenogebirge bei lali Djiwa an Andesitfelsen, 2500-2700 m, 10.V.1901, Fleischer 314 (BM000986130, JE, NY).
Not: Hedwigidium integrifolium (P. Beauv.) C.E.O. Jensen, Skand. Bladmossfl., 369. 1939. (= Hedwigia integrifolia P. Beauv., Prodr. Aethéogam, 60. 1805).
Illustrations
Bartram (1949: fig. 105 H-J); Beckett (1894: plate xxvi, 1-6 as Braunia novae-seelandiae Müll. Hal.); Bruch and Schimper (1846); Catcheside (1980: fig. 178, as Hedwigia integrifolia); Churchill and Linares (1995: fig. 101 a-, as Hedwigidium integrifolium); Crum (1994: fig. 496); Dixon and Jameson (1896: plate XXIV, J, as Hedwigia imberbis); Fleischer (1906: fig. 135, as Hedwigidium imberbe var. andesiticum); Fife (2014: plate 1, I-M, as Hedwigidium integrifolium); Husnot (1890: plate XLI, as Hedwigia imberbis); Meagher and Fuhrer (2003: plate 61, as Hedwigia integrifolia); Scott and Stone (1976: plate 68, as Hedwigia integrifolia); Seppelt et al. (2013: plate 10).
Distribution
Europe: United Kingdom, Norway, France, Italy, and Spain.
Africa: Cameroon, DR Congo, Malawi, Tanzania, Uganda, Zimbabwe, Reunion Island, Kenya, South Africa. Asia: India, Sri Lanka, Indonesia. Australia, New Zealand. Central America: Mexico, Guatemala, El Salvador, Honduras, Costa Rica, Dominican Republic. South America: Venezuela, Colombia, Ecuador, Peru, Bolivia, Chile, Argentina, Brazil.
Specimens examined. The records for most countries are documented with herbarium specimens. Countries are grouped by continent. Literature references are also included after the specimens.
Europe
UNITED KINGDOM. Ireland, Glengarriff, 1810, Hutchins (Mr Turner) s.n. (BM). Bruch and Schimper (1846), Wilson (1841), Smith (1978, as Hedwigia integrifolia). NORWAY. Bruch and Schimper (1846), Nyholm (1960, as Hedwigia integrifolia). FRANCE. Müller (1851), Ros et al. (2013), Hugonnot (2015); Le Bail (2015, as Braunia imberbis). SPAIN. Ros et al. (2013, as Braunia imberbis). ITALY. Ros et al. (2013, as Braunia imberbis); Frahm (1976, as Hedwigidium integrifolium).
Africa
CAMEROON. West Africa, III.1898, Bornmüller s.n. (B), as Hedwigidium montis-deorum; Cameroon Mnt, 2.IV.1952, No collector given, M45 (BM) as B. rupestris; Mt. Cameroon, 2.IV.1952, Adams s.n. (BM). O´Shea (2006). DEMOCRATIC REPUBLIC OF CONGO (formerly Zaire). Virunga, flanc nord du Kirisimbi, mixed with Braunia arbuscula, De Sloover 13090 b (DUKE); Virunga, De Sloover 13092 (DUKE); Ruwenzori, Bequaert 4579 (BR). O´Shea (2006). KENYA. Bizot et al. (1978), Kis (1985), O´Shea (2006). MALAWI. Mt. Mulanje, rio Muloza, Hodgetts 7227 (BM); Mt. Mulanje, Sapitwa, Hodgetts 7735 (BM); Mt. Mulanje, Chisongoli, Porley 16 (BM); Mt. Mulanje, Chinzama Hut, Wigginton M1302 (BM). Wigginton et al., (2020, as H. integrifolium). REUNION ISLAND. Crosby 8421 (DUKE), 8429 (DUKE). Ah-Peng and J. Bardat (2005). O´Shea (2006). RWANDA. O´Shea (2006). SOUTH AFRICA. Natal, Jammerlapen, 1898, Ditrich s.n. (JE); Somerset East monte Boschberg, MacOwan 41 (JE); Mt. Boschberg, 1887, MacOwan s.n. (JE); Natal, Royal National Park, Magill 6730 (MO), as Braunia secunda; van Rooy 1203 (MO). Magill and van Rooy (1998), O´Shea (2006). TANZANIA. Kilimanjaro Mt., De Sloover B-478 (BR); Kilimanjaro Mt., Pócs 6994/I (F); Kilimanjaro Mt. Philipia, Pócs 6720/M (NY). Bizot et al. (1978), Kis (1985), O´Shea (2006). UGANDA. Mt. Kineti Imatong Mts, Thomas 1846 (BM), as Braunia schimperiana; Kigezi, Hedberg 2069 (DUKE), 2093 (DUKE). Kis (1985), O´Shea (2006). ZIMBABWE. Magill 10213 (MO). O´Shea (2006).
Asia
INDIA. Chopra (1975) , Vashistha (1998), Daniels (2010). All as Hedwigidium integrifolium. SRI LANKA (formerly Ceylon). Au deu Sipfelfshen des Kirigalpota, II.1906, Herzog s.n. (JE), 2320 (DUKE). INDONESIA. 10.V.1901, Fleischer s.n. (JE, NY); Ost-Java, Am Ardjoenogebirge bei lali Djiwa an Andesitfelsen, 10.V.1901, Fleischer s.n. (BM, JE, NY), 314 (BM, JE, NY), as Hedwigidium andesiticum. AUSTRALIA. Western Australia, Swan River, Drummond 3658 (BM); Müller s.n. (BM); Streimann 35662 (DUKE); Tasmania, Watts s.n. (NSW), as H. campbellii. Sainsbury (1955), Scott and Stone (1976), Catcheside (1980) as Hedwigia integrifolia. NEW ZEALAND. Selwyn Gorge, Canterbury, Beckett 417 (NSW), as B. novae-sealandiae; Selwyn Gorge, Becket s.n., (BM). Fife (2014).
Central America and the caribean
MEXICO. Ciudad de México, Cárdenas 3741 (MEXU, XAL); Cuajimalpa, Vivas 337 (MEXU, XAL). Chiapas, Motozintla, Delgadillo 4780 (DUKE). Durango, near Buenos Aires, Breedlove 69168 (DUKE). Estado de México, Nevado de Toluca, De Luna 1754 (DUKE, MEXU), 1755 (DUKE, MEXU), 1759 (DUKE, MEXU). Hidalgo, Cárdenas 3429 (DUKE, MEXU); Parque Nacional El Chico, De Luna 1798 (XAL). Jalisco, Nevado de Colima, De Luna 45 (DUKE, MEXU), 548 (DUKE, MEXU). Michoacán, volcán Paricutín, cerca de San Lorenzo, De Luna 1778 (MEXU, XAL). Oaxaca, cerro Corral de Piedra, Delgadillo 4844 (DUKE). Puebla, Hermann 26448 (DUKE); Pico de Orizaba, entre Tezmola y San Miguel, De Luna 1443 (MEXU, XAL). Tlaxcala, al norte de Villareal, De Luna 2647 (XAL), 2717 (XAL); volcán La Malinche, Marin 239 (XAL). Veracruz, Cofre de Perote, De Luna 2287 (XAL), 2711 (XAL), 2713 (XAL). Nuevo León, Peña Nevada, Nelson and Wells 6 (DUKE). Crum (1994). EL SALVADOR. Allen (2010). GUATEMALA. San Marcos, Sharp 5449 (DUKE). Bartram (1949). COSTA RICA. Holz et al. 99-0913 (DUKE); San José, Crosby 3888 (DUKE, MO). Allen (2010). HONDURAS. Allen 12255 (MO). DOMINICAN REPUBLIC. La Vega, Norris et al. 5680 (DUKE); La Vega, Steere 23037 (DUKE), 23104 (DUKE). Sastre-De Jesús et al. (2010).
South America
VENEZUELA. Mérida, Griffin III PV-510 (DUKE, MO). Delgado and León-Vargas (2017). COLOMBIA. Sumapaz, Cleef 149 (QCA); Belén, Cleef 2127 (QCA); Bogotá, Chapinero, Lindig 2005 (BM); Antioquia, Churchill et al. 13342 (DUKE); Cundinamarca, Schultes 12262 (DUKE). Churchill and Linares (1995). ECUADOR. Azuay, Cerro Urucaca, south of Cuenca, Lewis 78-2202 (F, NY), as Braunia cirrhifolia; Cañar, Lewis 78-2379 (QCA); Cajas, Lewis 78-2278 (F, QCA), 78-3236 (F, QCA); Cajas, Steere 27716 (NY, QCA); Cajas, Sastre-De Jesús 697 (QCA); entre Sigsig y Ludo, al sureste de Cuenca, De Luna 2010 (DUKE, XAL), Sierra de Cajas, camino de Cuenca a Molleturo, De Luna 1991 (DUKE, XAL); Chimborazo, alrededor de Achupallas, De Luna 2018 (DUKE, XAL); Cotopaxi, Quevedo - Latacunga road, Holm-Nielsen et al. 3381 (AAU, S, US), as B. cirrhifolia; cerca de Mulalo, De Luna 1902 (DUKE, XAL); Volcán Cotopaxi, De Luna 1946 (DUKE, XAL), 1953 (DUKE, XAL),1958 (DUKE, XAL); Cotopaxi, Buck 10298 (NY, QCA); Rio Cutuchi, Gradstein et al. 124 (QCA), Bryophyta Neotropica Exiccatae 113 (QCA); Pichincha, camino al volcán Pululahua, cerca de San Antonio, De Luna 1879 (DUKE). Steere (1948). PERU. Cuzco, Herrera 2386 (F), 2391 (F), as B. secunda; Lima, Hampe s.n. (slide 3817, BM), as Braunia secunda; Mathews s.n. (QCA); Lima, Lomas de Granados, Vargas 9554 (MICH). Menzel (1986). BOLIVIA. Sorata, Balslev 1097 (MO, NY, QCA); Cochabamba, Hermann 25155 (MO); Herzog 3254 (JE), as Braunia macrocalyx; Cochabamba, I.1908, Herzog s.n., (JE); I.1908, Herzog s.n. (JE), as B. subplicata; Chuquisaca, al sur de Tarabuco, Lewis 1628 (MO); al noroeste de Santa Elena, Lewis 1948 (MO); Cochabamba, Ayopaya, De Luna 2122 (DUKE), 2124 (DUKE), Chaparé, De Luna 2111 (DUKE); Tunari, Monte Tunari, De Luna 2128 (DUKE), 2134 (DUKE); La Paz, Larecaja, De Luna 2047 (DUKE), 2060 (DUKE), 2063 (DUKE, MO); Larecaja, alrededores de Sorata, De Luna 2065b (DUKE). Hermann (1976). CHILE. Cordillera de la Costa, 4.XI.1897, Dusén 344 (JE, NY). Mahú (1979), He (1998), Müller (2009, as H. integrifolium). ARGENTINA. Argentina subtropica, Siambon regione Aliso, Lorentz s.n. (JE), as Braunia rhabdocarpa Hampe; Argentina Patagonica, Sierra Ventana, I.III.1881, Lorentz s.n. (JE), as B. patagonica; Buenos Aires, Cerro de la Ventana, Roivainen 460 (MICH), as B. patagonica; Cordoba, cerro Uritoco, Hosseus 360 (BM, BR, NY); Cordoba, von Hübschmann 5 (NY); Tandil, IX.1907, Hicken 18 (JE); Churchill and Schiavone 19993e (MO). Blockeel et al. (2003, as H. integrifolium). BRAZIL. Rio de Janeiro, Glaziou 9070 (NY); Sierra do Itatiaia, 17.VI.1902, Dusén s.n. (B, JE), as Braunia macrocalyx; Jaquirana, Wasum et al. 6041 (DUKE); Vital 7111 (MO); Serra Geral, Ulé 79 (JE, US), as Hedwigidium serrae. Costa and Lima (2005), Yano (2004, 2011, as H. integrifolium).
Verified specimens, worldwide distribution and habitat range
Since its establishment, Hedwigidium was first known from Europe, and later found throughout the mountains in Mexico, South America, Africa, New Zealand and Australia. For the first time, a list of specimens is provided, and the worldwide geographical distribution of the genus is documented and mapped. The specimens listed above were used for mapping the distribution of genus Hedwigidium. The map also includes literature country records (Fig. 8).

Figure 8: Map of the worldwide distribution of Hedwigidium Bruch & Schimp. A little square on the map indicates a literature citation. Each point on the distribution map indicates a particular specimen, of which I have corroborated the identity (see “Specimens examined” section). A question mark indicates a literature record not verified yet.
Hedwigidium imberbe was originally described from a specimen growing on rocks in Glengarriff, southwestern Ireland. Afterwards, a second specimen was collected in a locality in Lofoten, northwestern Norway (Bruch and Schimper, 1946). Shortly after, Müller (1851) cited these two specimens (“Ad rupes prope Glengariff Hiberniae: Miss Hutchins, Wilson; in insula Osteröe Finmarkiae: Blytt”) and added the following three specimens from southern France: “in Pyrenaeeis ad Bagnëres de Bigorre in rupib. porphyraceis: W.P. Sch., ad saxa granitica pr. Laruns: Spruce et in rupib. schistosis pr Pouzac et Gazos: Philippe”. Currently, H. imberbe is well-documented in Europe from northern, western and southwestern regions, where it is listed as rare and vulnerable (Hodgetts, 2015). European specimens have short perichaetial leaves, less than twice the length of vegetative leaves. Hedwigidium imberbe is present at low to moderate elevations in England. Wilson (1841) recorded “It is found rather plentifully near Llanberis, and near Beddgelert in N. Wales.” In modern times, Smith (1978: 493) documented this taxon from North Wales, West Scotland, north to Skye and West Ross. He used the name Hedwigia integrifolia, but the description certainly corresponds to H. imberbe. In Norway, Nyholm (1960) treated this taxon as Hedwigia integrifolia, but the description of elongate and non-ciliate perichaetial leaves undoubtedly corresponds to Hedwigidium imberbe. This species was reported erroneously in Germany, according to Düll (1992). In France this species is rare and it has recently been rediscovered (Hugonnot, 2015; Le Bail, 2015, as Braunia imberbis). It is listed for Italy with a report of Braunia imberbis based on collections published before 1962 (Ros et al., 2013). It was rediscovered in Italy in the area east of Lake Como, according to Frahm (1976, as Hedwigidium integrifolium). It has also been confirmed for Spain (Ros et al., 2013).
Hedwigidium is not known to occur in northern North America. However, in Mexico Hedwigidium imberbe is fairly common in high mountain areas, mostly above 3000 m. In Central America H. imberbe is also common on rocks at high elevations (“On rocks at high to very high altitudes” fide Bartram, 1949: 233; Allen, 2010). In the Dominican Republic the species has been collected in La Vega, a high elevation mountain (Sastre-De Jesús et al., 2010).
In northern, western and southern South America Hedwigidium has been documented in localities at high elevations along the Andes and also in southern Brazil. The illustrations of H. imberbe from Colombia correctly depict the morphology of this species (Churchill and Linares, 1995: fig. 101 a-f). In Ecuador, Hedwigidium grows mixed with Hedwigia, above 2400 m. I found infrequent populations on the Pululahua volcano, only growing on rocks covered under small bushes. In contrast, every rock had abundant Hedwigidium, but not Hedwigia or Braunia, in a locality nearby Mulaló, above 3000 m. Also, Hedwigidium is abundant in localities on the skirts of Cotopaxi volcano, between 2800 and 3200 m (see e.g., De Luna 1946 (DUKE), 1953 (DUKE)). In the area close to Achupallas, at 3000 m, Hedwigidium grows abundantly on rocks in open agricultural fields (see e.g., De Luna 2027 (DUKE), 2030 DUKE). Britton (1896) discussed early collections of Hedwigidium in Bolivia and compared these with those in Colombia and Peru. Later, Herzog (1916: 104) reported two taxa of Hedwigidum from Bolivia. One was H. imberbe var. macrocalyx (Müll. Hal.) Herzog, based on his collection nr. 3254. I have seen this Herzog specimen at JE; indeed, it has extremely long perichaetial leaves reaching six times the size of vegetative leaves. Others of his collections (nrs. 3583 and 4914) were reported as Hedwigidium imberbe. In Bolivia, sterile populations of Braunia growing on rocks can be easily confused as Hedwigidium. But close examination reveals some actually are Braunia subplicata, a species that also has ovate-lanceolate leaves and strongly revolute margins, but exserted capsules (see e.g., De Luna 2082 (DUKE), 2129 (DUKE)). In Chile, Mahú (1979) recorded the presence of the genus, and it has been documented along the western side of the Andes, from Santiago to Valdivia (He, 1998; Müller, 2009, as Hedwigidium integrifolium).
The genus is also recorded in Argentina. Recently Hedwigidium was found in northern Patagonia, the most southerly locality of the genus in the Americas (Blockeel et al., 2003). Blockeel et al. (2003) believed that the Lorentz specimen cited in the original report by Müller (1879), as Braunia rhabdocarpa was probably lost with Müller’s herbarium. However, I was able to locate a specimen in JE collected by P.G. Lorentz, labelled “Braunia rhabdocarpa Hampe, Argentina subtropica, Siambon regione Aliso”, an area that corresponds to northwestern Tucumán. This Lorentz specimen has the immersed capsules, entire perichaetial leaves, and other gametophytic features, which indicate it is clearly a genuine Hedwigidium from Argentina. In Brazil, Costa and Lima (2005) recorded H. imberbe (as H. integrifolium) in the montane and upper montane Atlantic rainforest of southeastern Brazil (1500-2700 m). Yano (2004, 2011) examined new specimens of this species (as Hedwigidium integrifolium) collected in six states in Brazil.
In Africa, H. imberbe is well-known from high altitude mountains in western, central, eastern and southern regions (O´Shea, 2006). Sim (1926) recorded localities at middle elevations (1500 m) with plants up to 5 cm long, but noted that at that time, sporophytes were not known in South Africa. More recently, Magill and van Rooy (1998) presented a detailed map of known localities of Hedwigidium in southern Africa. In Africa, the gametophytes of H. imberbe are very similar to those of Braunia rupestris. With some frequency, I have found specimens wrongly identified as Braunia rupestris, but on close examination, and having found sporophytes, undoubtedly these belong to Hedwigidium. The report of Hedwigidium from Ethiopia (Bizot et al., 1978: 274) is not correct, since the two specimens cited are from localities (21 and 33) not in this country. According to their map in p. 261, one locality is in Kenya, and the other site is on Tanzania. A few years later, Kis (1985: 88) listed several specimens of Hedwigidium for Tanzania, Uganda and Kenya, but none for Ethiopia. O´Shea (2006) listed H. imberbe (as H. integrifolium) from Cameroon, Cape Verde Island, Kenya, Lesotho, Réunion, Rwanda, South Africa, Swaziland, Tanzania, Uganda, Democratic Republic of Congo (former Zaire), and Zimbabwe. Recently, Hedwigidium was first reported from Malawi (Wigginton et al., 2020). I can confirm such record with four specimens found at BM: Hodgetts 7227, 7735, Porley M16a (cited in Wigginton et al., 2020), and Wigginton M1302a (cited in Wigginton et al., 2020). The latter specimen mostly consists of Hedwigidium, but it is mixed with some Hedwigia. Capsules have a very wide-open mouth, and the perichaetial leaves are strongly undulate.
In Asia, Hedwigidium imberbe has been recorded from Palni Hills, southern India (Chopra, 1975). Vashistha (1998) and Daniels (2010) also listed this species, as Hedwigidium integrifolium, from southern India. Additionally, I was able to confirm collections from Sri Lanka (formerly Ceylon, Herzog 2320 (DUKE); II.1906, Herzog s.n. (JE)) and Indonesia (10.V.1901, Fleischer s.n. (BM, JE, NY)).
In Australia and New Zealand, Hedwigidium imberbe is well-documented, although Sainsbury (1955) and Scott and Stone (1976) incorrectly named this taxon as Hedwigia integrifolia. In southern Australia, plants are more than 6 cm long, with frequent flagelliform shoots. The illustrations in Gilmore (2012) and Fife (2014) are good representations of the morphological features of this taxon. Catcheside (1980: 295) also misapplied the name Hedwigia integrifolia, but the description fits very well the taxonomic concept of Hedwigidium imberbe. There are six drawings of Braunia novae-seelandiae in Plate xxvi in Beckett (1894). One leaf is shown as shortly ovate (Beckett, 1894: fig. 2), with perichaetial leaves twice the size of vegetative leaves (Beckett, 1894: figs. 5, 6). Previously, Dixon (1927: 240) considered that B. novae-seelandiae was a synonym of Hedwigidium integrifolium, a taxonomy with which Fife (2014) also agreed. I am also interpreting these features as an indication that Braunia novae-seelandiae Beckett belongs in Hedwigidium.
The records of Hedwigidium (as Braunia obtusicuspes Broth.) from China, by He and De Luna (2004) might be Braunia alopecura as re-interpreted by Wang (2011) and Dalton et al. (2013). However, my recent re-examination of the type specimen, Handel-Mazzetti 788 (S), is not so conclusive. The specimen has no sporophytes and the flat leaf apex indeed resembles that of Hedwigidium, not the tubulose leaf apex of B. alopecura. Thus, I prefer to leave this identification as doubtful.











 nueva página del texto (beta)
nueva página del texto (beta)



