Introduction
The genus Mappia Jacq. (Icacinaceae) was described in 1797 by Nikolaus Joseph Jacquin and was forgotten until 1852, when John Miers assigned many new species to this genus (Howard, 1942). Baehni (1936) conducted a taxonomic review of Mappia and segregated the Asiatic species of Mappia into a new genus, Neoleretia Baehni (=Nothapodytes Blume). To date, Mappia consists of four species found in Mexico, Central America, and the Greater Antilles (Fig. 1). The species are trees or shrubs characterized by leaves without stipules, domatia on the abaxial surface of the leaves, malphigiaceous hairs on vegetative and floral structures, axillary inflorescences, bracts and bracteoles absent, pentamerous flowers, petals bearded on their inner surface and ovary surrounded by a disc. The group is particularly interesting because all the species, and especially Mappia mexicana B.L. Rob. & Greenm. and M. longipes Lundell, are notably rare (small distribution range and few individuals), hence, a better understanding of the Mappia circumscription might provide important insights for its conservation.
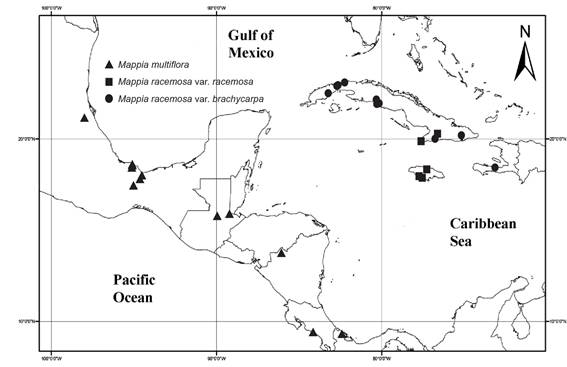
Figure 1: Geographic distribution of Mappia multiflora Lundell, Mappia racemosa Jacq. var. brachycarpa Griseb., and Mappia racemosa Jacq. var. racemosa.
On the one hand, Mappia mexicana and M. longipes are well-distinguished species based on their morphology and distribution. Mappia mexicana has small obovate leaves with a rounded apex (vs. acute to acuminate leaves in all other species) and a completely glabrous flower (vs. pubescent flowers in all other species) (Robinson and Greenman, 1895). It grows in thorny scrub and thorny forest in the border region between the states of Tamaulipas and San Luis Potosí in northeastern Mexico. Mappia longipes has a long floral peduncle (9 cm long) vs. ca. 3 cm long in all the other species (Lundell, 1942). It grows at 1300 m elevation in the cloud forest of Chiapas (southeastern Mexico) (Lundell, 1942).
On the other hand, Mappia racemosa Jacq. has elliptic or obovate leaves, a short peduncle (ca. 3 cm long) and a completely pubescent flower. Moreover, it has a larger distribution area, occurring in dry forest in Cuba, Jamaica, and Puerto Rico (Duno de Stefano and Angulo, 2010). Mappia racemosa has two infraspecific taxa; M. racemosa var. brachycarpa Griseb., and M. racemosa var. racemosa with few morphological differences; number of secondary veins (7-13 vs. 7-9), apex of the leaves (acute vs. acuminate), fruit size (14-22 × 1-16 × 1-13 vs. 12-17 × 1-11 × 08 mm) (Angulo et al., 2013). The most important morphological difference is the fruit. The mesocarp can be fleshy or not (Howard, 1942). However, this trait is highly variable within Mappia species (Angulo, 2006). Finally, Mappia multiflora Lundell occurs in the dry forest of Mexico to Costa Rica and has larger leaves than M. racemosa. Both Mappia racemosa and M. multiflora are morphologically similar, and have been confused throughout their distribution area. Mappia multiflora has longer leaves, 7 to 29.5 cm long (vs. 4.5 to 18 cm), secondary veins conspicuous on the abaxial surface of the leaves (vs. secondary veins inconspicuous), and leaves of the herbarium specimens dark green (vs. green-brown) (Angulo et al., 2013).
Previous phylogenetic analyses with a molecular plastid marker (ndhF) and morphological data have been realized within Mappia and related groups (Angulo et al., 2013). The results suggest that within Mappia, there is morphological support for a continental clade (M. longipes, M. mexicana and M. multiflora) sister to the Antillean species M. racemosa. However, these relationships were not resolved in the molecular analyses or in the combined molecular-morphological analyses (Angulo et al., 2013). Two fundamental reasons are responsible for these results: insufficient data and low phylogenetic signal of the marker used (Wortley et al., 2005).
In addition to traditional morphological and/or molecular differences (Wiens and Penkrot, 2002; Sites and Marshal, 2003, 2004), the inclusion of geographic (Barraclough et al., 1998; Schneider and Moritz, 1998), and ecological data (Anderson et al., 2002a, b; Johnson and Cicero, 2002; Martínez-Gordillo et al., 2010) has given an important new impulse to assessing the status and distribution of poorly know taxa and species conservation (Gaubert et al., 2006).
In recent years there has been a substantial increase in the digitization of natural history collections, which has contributed to the integration of this information in global repositories (e.g. REMIB, 2019; GBIF, 2019). Parallel to this there has been a considerable development of global environmental data at fine scale resolutions (e.g. WorldClim, (Hijmans et al., 2005); SoilGrids (Hengl et al., 2017)). The integration of these data, although with potential caveats, has contributed to important insights in the biodiversity distribution around the globe, as well to understanding the role of ecological divergence on speciation.
Ecological divergence is one of the most widely reported processes that promotes diversification in natural populations (e.g., Jansson and Dynesius, 2002; Barnosky, 2005; Mittelbach et al., 2007) and has recently received much attention (Hua and Wiens, 2013).
Environmental niche modeling is a method that uses occurrence data in conjunction with environmental data to make a correlative model of the environmental conditions that meet a species’ ecological requirements and predict the relative suitability of habitat (Warren and Seifert, 2011). These methods have now been applied to evaluate ecological divergence (Rissler and Apodaca, 2007; Murienne et al., 2009) and are widely used in diverse biodiversity studies (e.g. predicting species’ geographic potential (Peterson, 2003)); and species’ potential distributions under different climatic conditions (Martínez-Meyer et al., 2004).
Niche conservatism can be defined as the tendency for many ecological traits to remain similar over time. Outside the niche, individuals are not expected to leave descendants, nor populations to persist, nor clades to endure and proliferate (Wiens et al., 2010). However, a niche shift is possible, and a new lineage can utilize a new diet, host, habitat, and climatic regime. This adaptative divergence ensures not only the occupancy of two or more niches, but also reproductive isolation (revised in Wiens and Graham, 2005). Adaptative divergence in most species is more likely to occur at the level of local population as has been demonstrated in a fen orchid (Vanden Broeck et al., 2014). At local level, ecological divergence could be related with shifts in the pollinator’s assembly that have also contributed to maintaining genetic isolation as has been observed in other species (Angulo et al., 2014a, b). Additionally, different climate habitats could act as a barrier to gene flow influencing reproductive isolation, and minimizing connectivity among populations (e.g., Kozak and Wiens, 2007; Sobel et al., 2010). A divergent niche among close relatives has been detected in other plants as a response to different factors such as drought stress (Mimulus L.; Peterson et al., 2013), salt tolerance (Mimulus guttatus DC.; Lowry et al., 2008), and host plant adaptation with the beetle Neochlamisus Karren (Funk et al., 2011).
Here, we used ecological niche analysis (environmental niche modeling and niche divergence/conservatism tests) and multivariate analysis of variance (MANOVA) to evaluate whether ecological factors support previous taxonomic conclusions based on morphology (Angulo et al., 2013), which suggests that Mappia multiflora (continental), and Mappia racemosa (Antillean) are morphologically different species with different distribution areas. In the case of the Antilles, both intraspecific taxa proposed by Howard (1942) are morphologically identical with a similar distribution area.
Materials and Methods
Sample collection
One hundred specimens of Mappia were studied from sixteen herbaria: BM, CICY, F, G, GH, GOET, K, LL, MA, MEXU, MO, NY, P, US, TEX and XAL (acronyms according to Thiers, 2020). The species delimitation of Mappia racemosa and M. multiflora used in this study was based on Angulo et al. (2013). In order to test the infraspecific taxa recognition of Mappia racemosa, we analyzed only the original label determined by Howard (1942) as M. racemosa var. brachycarpa and M. racemosa var. racemosa.
Rare species, those with either a small range or a low abundance (Rabinowitz, 1981), represent the vast majority of species (Longino et al., 2002) and are consequently represented by few samples in natural history collections, the primary source of distributional data (van Proosdij et al., 2016). This is the case of the genus Mappia, as the analysis was originally intended for the whole genus. However, two species are poorly known: Mappia longipes and M. mexicana are only recorded from one and four collections, respectively, and were excluded from the analyses. Of the reviewed herbarium specimens, we only used those with georeferences of Mappia multiflora (33 specimens) and M. racemosa (36 specimens; 12 for M. racemosa var. racemosa and 24 for M. racemosa var. brachycarpa).
Environmental niche modeling
Environmental niche modeling (ENM) was used in order to visualize whether the climatic niches of different taxonomic entities are inter-predictable; we estimated environmental niche models for each entity. We employed the MaxEnt software (Phillips et al., 2006) since it offers many advantages, and few drawbacks as compared with other modeling methods: (1) it requires only presence data, together with environmental information, (2) it can utilize both continuous and categorical data, including interactions between different variables, (3) it possesses efficient deterministic algorithms that guarantee to converge to the maximum entropy probability distribution, (4) the MaxEnt probability distribution has a concise mathematical definition, and is therefore amenable to analysis (Phillips et al., 2006). Moreover, MaxEnt has shown good performance with small or incomplete data sets and still produces near maximum accuracy levels (Hernandez et al., 2006).
ENMs were performed using standard bioclimatic variables obtained from the WorldClim 1.4 database (Hijmans et al., 2005) with ~1 km2 resolution. We performed a correlation analysis using the R statistical software (R Core Team, 2017) and selected nine not correlated variables (pairwise r<0.7 based on all sample locations) (Peterson, 2007; Nakazato et al., 2010). These variables were derived from temperature and precipitation (Table 1). Parameters for all the MaxEnt analyses were as following: convergence threshold=10−5, maximum iterations=1000, regularization multiplier=1, in addition to other default modeling parameters. Binary maps (predicted presence or absence) were created from the MaxEnt-generated niche distribution models using a threshold value of >0.5. Model performance was evaluated by the area under the receiver-operating characteristic curve (AUC) and the lowest presence threshold value (LPT, Peterson et al., 2007; Lobo et al., 2008). AUC scores were calculated using training data (Fielding and Bell, 1997).
Table 1: Bioclimatic parameters, sets used for ecological niche model (ENM) generation and climatic PCA. Mappia multiflora Lundell (MM), M. racemosa Jacq. var. racemosa (MRR), and M. racemosa Jacq. var. brachycarpa Griseb. (MRB). Temperature in °C and precipitation in mm.
| Climate variable | MM | MRR | MRB |
|---|---|---|---|
| Isothermality (Bio3) | 6.2 | 6.84 | 6.4 |
| Max Temperature of Warmest Month (Bio5) | 32.2 | 30.1 | 31.1 |
| Min Temperature of Coldest Month (Bio6) | 17.1 | 17.7 | 15.9 |
| Temperature Annual Range (Bio7) | 15.1 | 12.4 | 15.2 |
| Mean Temperature of Wettest Quarter (Bio8) | 25.6 | 25.1 | 25.6 |
| Precipitation of Driest Month (Bio14) | 5.8 | 4.4 | 3.4 |
| Precipitation Seasonality (Bio15) | 6.2 | 5.2 | 5.5 |
| Precipitation of Wettest Quarter (Bio16) | 116.1 | 60.7 | 56.5 |
| Precipitation of Warmest Quarter (Bio18) | 63.7 | 45.5 | 52.8 |
Ecological differentiation between species
Ecological differentiation was evaluated with niche divergence/conservatism tests using environmental data and taxa occurrence points. These tests were run following a multivariate analysis-based methodology developed by McCormack et al. (2010), and employing the same environmental variables used in environmental niche modeling (see above). For each entity, bioclimatic data for the actual occurrence points were extracted, and 1000 points were taken from the background environmental area (based on polygons drawn around the occurrence points). A principal component analysis (PCA) was run using PAST 1.99 (Hammer et al., 2001), and the first six axes, which explained over 95% of the total variance, were used to test for niche divergence/conservatism. Each axis’s niche was tested against a null model of background divergence by comparing the observed difference in mean niche values on a given axis to the difference in mean background values between paired comparisons. Significance was assessed with 1000 jackknife replicates of the mean background values. A 95% significance level was applied for null model rejection. All these analyses were performed in STATA v. 11.0 (StataCorp., 2009).
Finally, a conventional multivariate analysis of variance (MANOVA) was performed in SPSS v. 19.0 (IBM, 2010), using environmental variables for each species pair to determine whether the observed environmental conditions differed significantly. The F-statistic was reported, and a test of between-subject effects was run to determine which PCs accounted for significance in the overall test (Graham et al., 2004).
Results
Environmental niche modeling
The environmental niche modeling (ENM) results indicated good model performance (AUC>0.90). Isothermality and annual temperature range were the most contributive variables. In the Antillean entities, precipitation in the warmest quarter contributed most to the MaxEnt model in Mappia racemosa var. brachycarpa, while for M. racemosa var. racemosa annual temperature range was the variable with the greatest contribution to the model.
In the continental entities, precipitation in the wettest quarter showed a greater contribution to the distribution predicted in the MaxEnt model. Potential occurrence areas for M. racemosa var. racemosa and M. racemosa var. brachycarpa were identified in Florida, the Antilles, some areas in Mesoamerica and South America. For M. multiflora, areas of potential occurrence were identified mainly in Mesoamerica (Fig. 2).
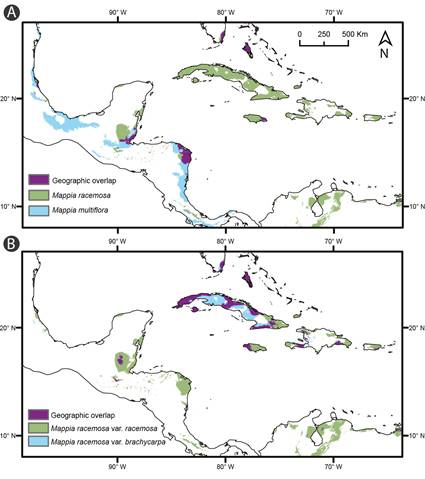
Figure 2: Ecological niche models of Mappia Jacq. entities. A. predictions of potential habitats for M. racemosa Jacq., M. multiflora Lundell, and geographic overlap. B. predictions of potential habitats for M. racemosa Jacq. var. brachycarpa Griseb., and M. racemosa Jacq. var. racemosa, and geographic overlap.
The environmental niche models predicted well the documented geographic distribution of the different Mappia entities, as known by information of herbarium specimens and taxonomic revisions of the genus (Angulo et al., 2013). The climatic niches of the Antillean and continental species might not be ecologically interchangeable since they do not inter-predict each other (low niche overlap). On the other hand, a strong niche overlap between the Antillean entities was observed on the potential distribution, suggesting the entities might be ecologically interchangeable (Fig. 2).
Ecological differentiation
Evidence of niche divergence was detected for all taxa in the niche divergence/conservatism tests. The first three components explained over 80% of the observed divergence. High loadings were observed mainly for temperature related variables rather than precipitation, suggesting the former might be an important factor associated with the distribution of species. The first three axes that represent the largest explained variation show that Mappia multiflora and M. racemosa var. racemosa diverged in all niche axes, M. multiflora and M. racemosa var. brachycarpa diverged in two of the three, while M. racemosa var. racemosa and M. racemosa var. brachycarpa diverged in one of the three (Table 2).
Table 2: Divergence in niche axes between Mappia multiflora Lundell (MM), M. racemosa Jacq. var. racemosa (MRR), and M. racemosa Jacq. var. brachycarpa Griseb. (MRB). Bold values indicate significant niche divergence (D) or conservatism (C) compared to null distribution (in parentheses) based on background divergence between their respective geographic ranges. 1 See Table 1 for variable descriptions. Values in italics indicate opposite sign.
| Pairwise comparison | Niche axes | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PC 1 | PC 2 | PC 3 | PC 4 | PC 5 | PC 6 | |||||||
| 1 vs. 2 | 2 vs. 1 | 1 vs. 2 | 2 vs. 1 | 1 vs. 2 | 2 vs. 1 | 1 vs. 2 | 2 vs. 1 | 1 vs. 2 | 2 vs. 1 | 1 vs. 2 | 2 vs. 1 | |
| MM vs. MRR | 0.70 D | 0.70 D | 1.62 D | 1.62 D | 0.81 D | 0.81 D | 0.10 C | 0.10 C | 0.32 C | 0.32 C | 1.26 D | 1.26 D |
| (0.20,0.53) | (0.11,0.51) | (0.63,0.73) | (1.03,1.56) | (0.01,0.26) | (0.44,0.82) | (0.17,0.34) | (0.18,0.68) | (0.45,0.68) | (0.92,1.38) | (0.23,0.52) | (0.44,1.16) | |
| MM vs. MRB | 0.26 D | 0.26 C | 1.47 D | 1.47 D | 0.35 | 0.35 C | 0.66 D | 0.66 D | 0.89 D | 0.89 D | 1.30 D | 1.30 D |
| (0.10,0.27) | (0.16,0.56) | (0.79,0.94) | (0.26,0.79) | (0.17,0.48) | (0.24,0.62) | (0.01,0.23) | (4.3E- 05,0.42) | (0.01,0.20) | (5.15E- 05,0.32) | (0.11,0.32) | (0.03,0.45) | |
| MRR vs. MRB | 0.96 D | 0.96 D | 0.15 C | 0.15 C | 0.46 | 0.46 | 0.55 D | 0.55 D | 0.57 D | 0.57 D | 0.03 C | 0.03 C |
| (0.002,0.27) | (0.42,0.60) | (0.74,0.84) | (0.67,0.83) | (0.34,0.62) | (0.32,0.64) | (0.31,0.48) | (1.59E- 05,0.12) | (0.21,0.44) | (0.11,0.34) | (0.78,1.06) | (0.94,1.15) | |
| % variance explained | 37.4 | 25.9 | 18.9 | 9.1 | 4.5 | 2.6 | ||||||
| Eigenvalues | 3.368 | 2.334 | 1.704 | 0.823 | 0.411 | 0.24 | ||||||
| Variable loadings1 | Bio 7 | Bio 6 | Bio 16 | Bio 3 | Bio 18 | Bio 3 | ||||||
| Bio 15 | Bio 8 | Bio 3 | Bio 14 | Bio 14 | Bio 7 | |||||||
| Biological interpretatio | Temp/Precip | Temperature | Precip/ Temp | Temp/ Precip | Precipitation | Temperature | ||||||
The overall MANOVA showed statistically significant differences between the continental and Antillean entities (Mappia multiflora/M. racemosa var. racemosa, F=4.41, P=0.02; Mappia multiflora/M. racemosa var. brachycarpa, F=6.34, P<0.01), but no differences among the Antillean entities (M. racemosa var. racemosa/M. racemosa var. brachycarpa, F=3.19, P=0.06).
Discussion
In general, our results in both ecological niche analysis (environmental niche modeling and niche divergence/conservatism tests) and multivariate analysis of variance (MANOVA) support previous phylogenetic study, where a continental (Mappia multiflora) and an Antillean clade (Mappia racemosa) were found (Angulo et al., 2013). Furthermore, at the infraspecific level our results reject Howard´s hypothesis where two distinct entities are recognized (Howard, 1942). We discuss these results in detail below.
Environmental niche modeling and ecological differentiation
Levin (2000, 2003) stated that changes in the ecological attributes of populations are an important component of speciation in many flowering plant lineages, and such changes alone may result in the origin of species. In addition, Rissler and Apodaca (2007) stated that the extent of cross-lineage divergence varies in response to isolation, this being either geographic or environmental. In the environmental niche modeling analyses, the potential distribution of Mappia multiflora did not show any distribution in the Antillean region. However, M. racemosa distribution did predict few areas in the continent. Mappia multiflora and M. racemosa are found in areas with different climatic regimes, mainly related to temperature.
These potential distribution lead to the conclusion that niche divergence exists between both species supporting our previous results (Angulo et al., 2013). This trend toward a specialized niche is also observed in the multivariate niche evolution method that suggests niche divergence, in most PC axes. This multivariate method provides detailed information on niche divergence, as it is in closer agreement with the Hutchinsonian idea of the niche as a multidimensional hypervolume (Hutchinson, 1957), in which some axes will remain conserved while others diverge. Both environmental niche modeling and PC analysis suggested trends toward an adaptative divergence to local specialized niche between Mappia multiflora and Mappia racemosa. That is, ecological discontinuity between Mappia species is strongly influenced by environmental factors (most notably temperature), suggesting different climate selection pressures in each habitat. Hence, the results show that the continental and Caribbean populations have different ecological niches, despite sharing similar preferences: latitudes and vegetation, dry forest mainly on calcareous soils (Angulo, 2006). Although the other two species of the genus were not included in the analysis because of the low number of specimens, both occupy different ecological niches; M. longipes occurs at 1300 m, while M. mexicana grows in lowlands near the limit of the tropical region. All cases suggest allopatric and adaptive ecological divergence.
The evolutionary divergence of the genus Mappia implies primarily an allopatric distribution, a body of salt water between the continental and the island species that interrupts the gene flow between both species. Secondly, an ecological divergence (climatic preference) where sympatric species exploit alternative ecological niches through cumulative morphological changes (Chase and Leibold, 2003).
The most important difference among infraspecific taxa of Mappia racemosa, the fruit with a fleshy mesocarp or not (Howard, 1942), is highly variable within Mappia species. For example, this variation in mesocarp consistency also occurs within individuals of Mappia multiflora growing in the Los Tuxtlas Biological Station in Veracruz (Angulo, 2006). Furthermore, the sympatric spatial distribution of the infraspecific taxa of Mappia racemosa´s island population (Antilles) greatly raises the possibility of gene flow among their populations, which could have reduced the evolutionary divergence.
This study gives an important signal about the ecological divergence between closely related species (Mappia multiflora and M. racemosa). Other studies incorporating physiological, ecological, and molecular markers with faster mutation rates, such as microsatellites or Single Nucleotide Polymorphisms (SNPs), are necessary to understand the complete evolutionary history of the genus.











 text new page (beta)
text new page (beta)



