Introduction
Anemiaceae is a monophyletic family (Labiak et al., 2015) that together with Lygodiaceae and Schizaeaceae constitute the Schizaeales, a different clade from the remaining leptosporangiate ferns (Smith et al., 2006; PPG I, 2016). This family has a single genus Anemia Sw., with 115 species that grow, in the Neotropics, Africa, India, and islands in the Indian Ocean (Smith et al., 2006; Mickel, 2016; Smith and Kessler, 2017).
The species of the genus Anemia are characterized by having sessile, subglobose or ovoid sporangia with an apical annulus, and trilete ornamented spores, tetrahedral to globose (De la Sota and Morbelli, 1987; Tryon and Lugardon, 1991). According to the spore ornamentation, Ramos Giacosa et al. (2012) proposed two morphological types for the species that grow in Argentina: Anemia australis-type, with ridges, and Anemia herzogii-type, with ridges and bacula. Irregular shapes were mentioned by Mickel (1962), De la Sota and Mickel (1968), and Ramos Giacosa (2014).
Regarding the gametophytic development, Nayar and Kaur (1971) classified the spore germination of Anemiaceae as Anemia-type and the gametophytic development as Ceratopteris-type. The development of the meristem was described by Pray (1971) in A. colimensis Mickel, and by Takahashi et al. (2012) in Anemia phyllitidis (L.) Sw.
Atkinson (1962) and Nayar and Kaur (1971) identified the Anemiaceae gametophytes as asymmetric cordate, raised on the substrate, bisexual, usually with simple trichomes, glandular or eglandular not secretory. Other studies carried out by Pray (1971), Nester and Schedlbauer (1981), Nester (1985), Grill (1988), Hernández-Solano (2004), and Escamilla-Aquino et al. (2008) pointed out similar features for the different species of Anemia. De la Sota and Morbelli (1987) described four types of adult laminar gametophytes for American species: filamentous, filamentous with an axial or pseudo-axial shape, tuberous axial, and cordate-thalloid type.
Experimental research carried out on gametophytes, of A. hirsuta (L.) Sw., A. phyllitidis, A. mexicana Klotzsch, shows that they produce antheridiogens that promote the development of antheridia in young gametophytes even in the darkness (Näf, 1969; Näf et al., 1975; Nester et al., 1987; Yamane, 1998). Nester and Coolbaugh (1986) recorded for A. mexicana and A. phyllitidis, pH 6 and eight light hours as optimal conditions to induce spore germination. This kind of information allows planning for Anemia spore culture to produce sporeling with ornamental aims or biological and conservation interest. For instance, Pinto et al. (2013) mention that A. tomentosa (Sav.) Sw. var. anthriscifolia (Schrad.) Mickel produces an essential oil with antibacterial activity apart from its potential value as ornamental.
Within the framework of biology and gametophytic diversity of ferns, we characterize living spores and gametophytic phase of three Anemia taxa, little studied or with fragmentary studies, A. herzogii Rosenst., A. tomentosa var. anthriscifolia and A. tomentosa (Sav.) Sw. var. tomentosa, in order to increase the knowledge on the gametophytic phase of this fern genus for future taxonomic studies.
Materials and Methods
The material for this study comes from samples collected in natural environments of northwestern Argentina. Reference samples were deposited in the MCNS Herbarium (Thiers, 2018 continuously updated), Universidad Nacional de Salta, Argentina.
Studied material
Anemia herzogii: ARGENTINA. Salta, Orán, Río Blanquito, 900 m, 23°06.8'S, 64°29.3'W, 6.XII.2011, C. J. Chambi 441 (MCNS).
Anemia tomentosa var. anthriscifolia: ARGENTINA. Salta, La Caldera, 1500 m, 25°30'S, 65°20.6'W, 2.II.2011, O. G. Martínez 2102 (MCNS).
Anemia tomentosa var. tomentosa: ARGENTINA. Salta, Rosario de la Frontera, 850 m, 25°50.1'S, 64°55.5'W, 27.VI.2011, O. G. Martínez 2146 (MCNS).
Fertile pinnae of each individual were kept in individually labeled paper bags. They were dried for seven days at room temperature to favor sporangia opening and subsequent spore liberation. For the spore analyses, the specimens were studied without chemical treatment, using light (LM) (Zeiss Standart Model 16, Carl Zeiss, Göttingen, Germany) and scanning electron microscopy (SEM) (JEOL JSM 6480 LV, JEOL, Tokyo, Japan) at the Facultad de Ciencias Naturales, Universidad Nacional de Salta, Argentina and a SEM at the Laboratorio de Microscopia Electrónica de Barrido y Microanálisis (LASEM), Universidad Nacional de Salta, Argentina. A total of 30 spores were measured for each taxon under a LM, the size of the equatorial and the polar diameter is indicated on average, and the lowest and highest values in parentheses (Table 1). For SEM studies the spores were mounted directly on a double-sided tape and coated with gold.
Table 1: Comparative analysis of spores of Anemia herzogii Rosenst., A. tomentosa (Sav.) Sw. var. anthriscifolia (Schrad.) Mickel and A. tomentosa (Sav.) Sw. var. tomentosa.
| Taxa | Ornamentation | Equatorial diameter (μm) |
Polar diameter (μm) |
|---|---|---|---|
|
Anemia
herzogii Rosenst. |
narrow parallel ridges
with bacula separated by ample depressions |
(53)66(78) | (42)57(69) |
|
A.
tomentosa (Sav.) Sw. var.
anthriscifolia (Schrad.)
Mickel |
ample parallel ridges separated by
narrow depressions |
(76)88(94) | (68)76(82) |
| A. tomentosa (Sav.) Sw. var. tomentosa | (75)100(113) | (69)80(95) |
For sowing spores with 10 days of storage were used. Before sowing the material was sieved with a metallic mesh (pores 120 µm diameter) to eliminate traces of leaves and sporangia. Spores were sterilized with 10% sodium hypochlorite for five minutes, rinsed with sterilized water, and sown in Petri dishes (5 cm diameter), with Dyer medium (Dyer, 1979) gelled with agar 10g/l. A total of 30 Petri dishes were sown, 10 replicas for each taxon. The cultures were kept in growth chambers (BS610, Bioamerican Science, Córdoba, Argentina) at 21±3 °C with periods of 12 hours of light. The observations were made periodically, the first week every day and from the second week, once every seven days for 10 weeks. In each record, 10 fields were evaluated. For evaluating the germination percentage, we considered as viable spores those with cellular content, and no viable spores those that were collapsed and without cellular content.
Gametophyte development was observed for two years. For gametophyte measurements 10 samples were considered for each taxon, the sizes were expressed as means of length and standard deviation. Spores and gametophytes extracted for observation were removed to avoid contamination.
The gametophytes were fixed in acetocarmine choral hydrate (Edwards and Miller, 1972) for the observations with LM, and with 2.5% glutaraldehyde in phosphate buffer and dehydrated in a graded ethanol series for SEM studies. The photographs of the spores and gametophytes were taken with an optical microscope and a SEM.
Results
Spores
Living spores from the three studied taxa are dark brown, trilete, tetrahedral-globose shaped, with prominent angles and ornamented with ridges, with or without bacula (Fig. 1). The measurements of equatorial and polar diameter corresponding to each taxon are shown in Table 1. In A. tomentosa var. tomentosa, ca. 30% of irregular spores with variable dimensions, atypical shapes of monoletes, and spores grouped in diades were recorded. The three taxa showed 2% of spores without cellular content.

Figure 1: Spores. A. spores of Anemia herzogii Rosenst., two spores in distal view (left and middle) and one in equatorial view (right); B. spores of Anemia tomentosa (Sav.) Sw. var. tomentosa, one spore in distal view (left), three in equatorial view (middle) and one in proximal view (right); C. spore of Anemia tomentosa (Sav.) Sw. var. anthriscifolia (Schrad.) Mickel in proximal view.
Germination
Spore germination in all taxa begins 4-5 days after sowing, as in A. herzogii (Fig. 2A). The first cell divides in two equal cells, one of which remains inactive, whereas the other, divides in two cells of different dimensions. The largest one will result in the gametophyte and the other one in the rhizoid.

Figure 2: A. spore germination of Anemia herzogii Rosenst., B-D. filamentous gametophytes: B. Anemia tomentosa (Sav.) Sw. var. anthriscifolia (Schrad.) Mickel (5 days); C. Anemia tomentosa (Sav.) Sw. var. tomentosa (10 days); D. Anemia herzogii Rosenst. (10 days); E-H. laminar gametophytes of spatulate shape; E. Anemia herzogii Rosenst. (14 days); F. Anemia tomentosa (Sav.) Sw. var. tomentosa (20 days); G. Anemia tomentosa (Sav.) Sw. var. anthriscifolia (Schrad.) Mickel (20 days); H. Anemia tomentosa (Sav.) Sw. var. tomentosa (30 days); I. young laminar gametophyte of Anemia tomentosa (Sav.) Sw. var. tomentosa (50 days). (m) meristem; (pr) prothallus; (r) rhizoid.
In the three taxa germination increases gradually during the first ten weeks. The highest germination percentage is recorded in A. tomentosa var. anthriscifolia of 98% germinated spores out of a total of 709 spores, in A. herzogii 78% out of a total of 1302 spores and the lowest percentage in A. tomentosa var. tomentosa with 72 % germinated spores out of a total of 1073 spores.
Filamentous gametophytes
The filamentous gametophytes have variable length, from 4-20 cells, germinated between 5-10 days after sowing (Fig. 2 B). In Anemia tomentosa var. tomentosa, they are short, with 4-10 cells with an average length of 0.59±0.26 mm (Fig. 2C). In A. herzogii and A. tomentosa var. anthriscifolia, they are long, with more than seven cells. In Anemia herzogii they consist of 12-18 cells with an average length of 1.34±0.20 mm (Fig. 2D) while in A. tomentosa var. anthriscifolia they consist of 7-20 cells with an average length 1.28±0.23 mm. One to three rhizoids are found in cells near the spore. The cells of filamentous gametophytes are anisodiametric of 32±7 μm and 132±34 μm length, respectively.
Laminar gametophytes
The laminar phase began between 10-15 days after the spores’ germination with the multiplication of the filamentous gametophytes distal cells. In the first stages of the laminar phase, the gametophytes are largely spatulate with marginal meristem (Figs. 2E-I). Twenty days after sowing, the length of gametophytes of A. herzogii have an average size of 1.95±0.23 mm (Fig. 2E), while in A. tomentosa var. tomentosa they measure 1.36±0.43 mm (Fig. 2F) and in A. tomentosa var. anthriscifolia 2.45±0.57 mm (Fig. 2G). After the meristematic region becomes apical, cordate or spatulated adult gametophytes are formed, 40-60 days after germination.
In the three taxa, the laminar gametophytes have few clavate trichomes, 1-2-cellular, 20-30 µm long, on both margins and surface (Figs. 3A, B). Generally, marginal trichomes are curved, directed to the notch. The trichomes are formed 20 days after the sowing of the spores, when the notch starts being noticed. Also, in A. herzogii, branched trichomes 50-60 µm long are formed on the midrib between the gametangia, consisting of one basal short cell and two different distal cells, a short papillate cell and an eglandular elongate cell (Fig. 3C).
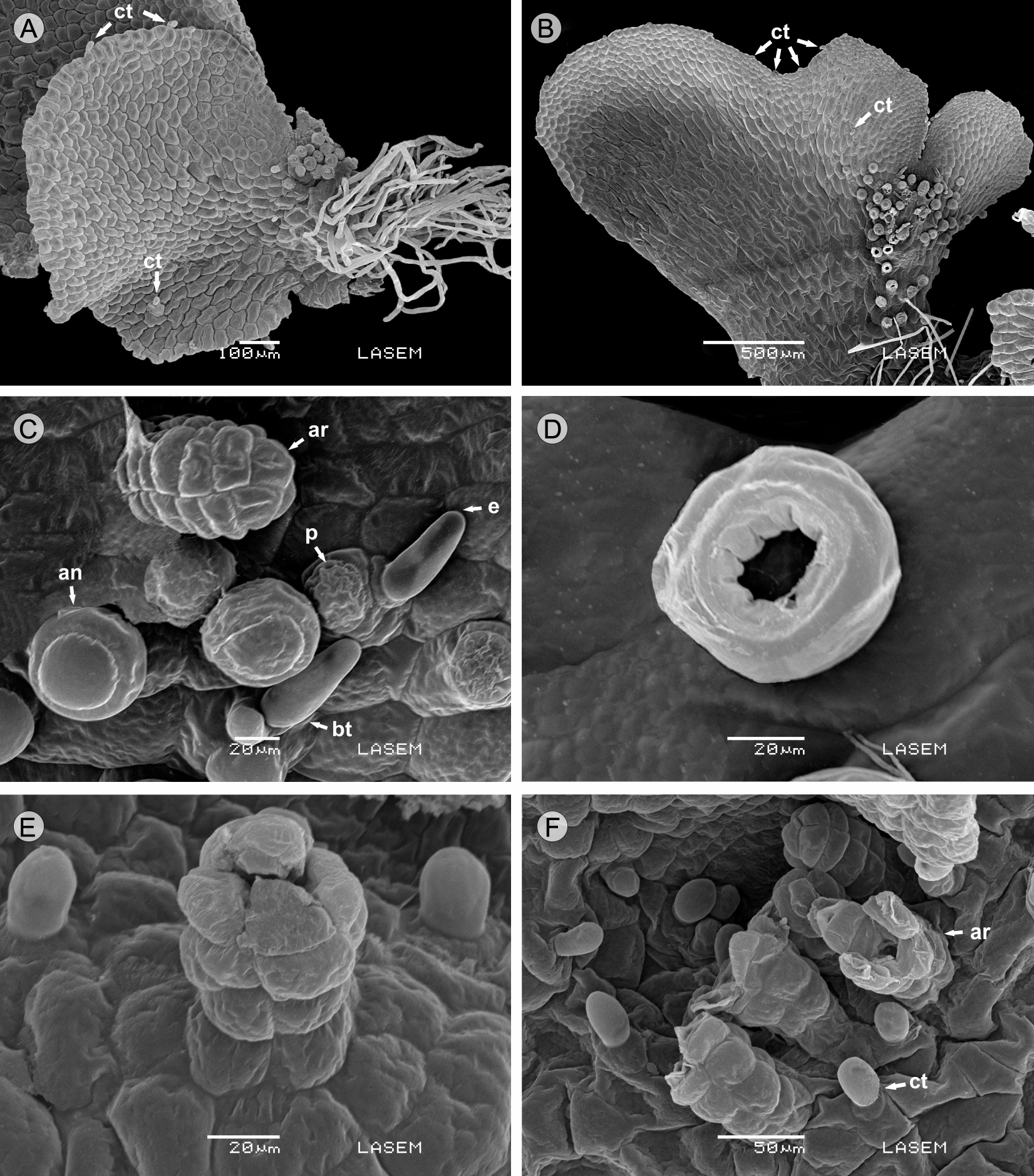
Figure 3: Microphotographs of adult gametophytes. A. Anemia tomentosa (Sav.) Sw. var. tomentosa with clavate trichomes; B. Anemia herzogii Rosenst. with clavate trichomes; C. Anemia herzogii Rosenst. showing branched trichomes, antheridia and archegonia neck; D. Anemia herzogii Rosenst. with detail of antheridium with perforated opercular cell; E. Anemia tomentosa (Sav.) Sw. var. tomentosa archegonium with four cells; F. Anemia tomentosa (Sav.) Sw. var. anthriscifolia (Schrad.) Mickel showing mature archegonia with open neck and superficial trichomes. (ct) clavate trichomes; (bt) branched trichomes; (ar) archegonium; (an) antheridium; (p) papillate cell; (e) eglandular cell.
Sexuality
The gametophytes of A. herzogii and the two varieties of A. tomentosa are bisexual and protandric. Gametangia are of the common leptosporangiate type (Nayar and Kaur, 1971), appearing only on the abaxial surface of the gametophyte with archegonia near the notch, and many antheridia distributed mainly towards the base, mixed with rhizoids. Antheridia appeared 50 days after sowing in A. herzogii and A. tomentosa var. anthriscifolia, and after 90 days in var. tomentosa. The dehiscence of antheridia is produced in every case through perforation of the opercular cell (Fig. 3D). Archegonia originated under the notch after 60 days in A. herzogii, and after 100 days in the two varieties of A. tomentosa. The neck of the archegonia is curved away from the notched end of the gametophyte, and it is composed of four rows each with four cells long in A. tomentosa var. tomentosa (Fig. 3E), four to five cells in A. tomentosa var. anthriscifolia (Fig. 3F) and five to seven cells in A. herzogii (Fig. 3C), the mouth consists of four triangular cells. The adult gametophyte characteristics of the taxa analyzed are summarized in Table 2.
Table 2: Morphological characters of the gametophytes of Anemia herzogii Rosenst., A. tomentosa (Sav.) Sw. var. anthriscifolia (Schrad.) Mickel and A. tomentosa (Sav.) Sw. var. tomentosa.
| Taxa | Adult gametophyte |
Trichomes | Sexuality | Archegonial neck | Length adult gametophytes (mm) |
|---|---|---|---|---|---|
|
A.
herzogii Rosenst. |
asymmetrical
cordate |
branched
trichomes |
bisexual | 5 to 7 cells | 2.66±0.62 |
|
A.
tomentosa (Sav.) Sw. var.
anthriscifolia
(Schrad) Mickel |
symmetrical
cordate |
clavate | bisexual | 4 to 5 cells | 2.58±0.45 |
|
A.
tomentosa (Sav.) Sw. var.
tomentosa |
asymmetrical
cordate |
clavate | bisexual | 4 cells | 1.84±0.21 |
Young sporophytes
The formation of sporophytes is only evident in A. herzogii after 180 days (Fig. 4A). The young sporophytes have spatulate laminae with entire margin, hypostomatic, with desmocytic and pericytic stomata, and dichotomous venation (Fig. 4B), with eglandular and clavate trichomes similar as those present in the gametophytes (Fig. 4C).
Discussion
The palynological features, such as shape, dimension, ornament and color of the spores in Anemia herzogii, A. tomentosa var. anthriscifolia and A. tomentosa var. tomentosa, recorded in this work, are consistent with the descriptions by Tryon and Lugardon (1991), Ramos Giacosa et al. (2012) and Labiak et al. (2015). De la Sota and Mickel (1968) and Ramos Giacosa (2014) cited irregular shapes and dimensions in the spores of both varieties of A. tomentosa, however, these irregularities were only found in var. anthriscifolia. Mickel (1962), attributed the irregular shapes of the spores of this variety to hexaploidy.
The germination of spores, in the three taxa, corresponds to the Anemia-type, described for other species of this genus by Atkinson (1962), Nayar and Kaur (1971), Raghavan and Huckaby (1980), Nester and Schedlbauer (1981), Nester and Coolbaugh (1986) and Takahashi et al. (2012). Several authors mentioned that the ability to germinate depends on environmental factors that can influence the process, such as light, pH and hormones (Nester and Coolbaugh, 1986; Miller, 1968; Von Aderkas and Cutrer, 1983). Raghavan and Huckaby (1980) ensured that spore germination of Anemia hirsuta, A. munchii Christ and A. phyllitidis starts within 24 hours with the incidence of red light. In natural conditions, it happens within 3-4 days in A. mexicana (Nester and Schedlbauer, 1981), and within 30 days in A. munchii (Escamilla-Aquino et al., 2008). The spores of the three taxa studied here, germinated 4-5 days after sowing with high germination percentages of over 70%.
The number of cells that constitute the filamentous gametophytes of Anemia tomentosa var. tomentosa does not exceed 10; in the case of A. herzogii and A. tomentosa var. anthriscifolia this may be up to 20. In this phase gametophytes match with the one recorded in A. munchii, in which the filaments have up to 20 cells (Escamilla-Aquino et al., 2008) and A. phyllitidis with filaments from three to seven cells (Takahashi et al., 2012).
The development of the gametophytes’ lamina corresponds to the Ceratopteris-type pattern proposed by Nayar and Kaur (1971); a morphological pattern shared with many genera in the large Pteridaceae family (Martínez, 2010; Martínez et al., 2017). The three taxa studied developed a lateral marginal meristem on one side of the laminar gametophytes, this which also described by previous authors (Atkinson, 1962; Nayar and Kaur, 1971; Nester and Schedlbauer, 1981; Raghavan, 1989; Escamilla-Aquino et al., 2008). Pray (1971) described that A. colimensis a developed lateral marginal meristem on both sides of the laminar gametophyte, although one of them remains without activity. The laminar phase is also characterized by the development of clavate trichomes in the three studied taxa, which were observed in other species of Anemia as well (Nayar and Kaur, 1971; Nester and Schedlbauer, 1981; Escamilla-Aquino et al., 2008). However, the branched trichomes of A. herzogii have not been previously described for gametophytes or sporophytes of Anemia; similar structures were mentioned by Nayar and Kaur (1971) for Ctenopteris suspensa (L.) Copel. (= Lellingeria suspensa (L.) A.R. Sm. and R.C. Moran), a Polypodiaceae.
The shape of adult gametophytes is principally cordate, symmetric and asymmetric. In Anemia gametophytes were reported as bisexual and unisexual (Atkinson, 1962; Mickel, 1962; Pray, 1971; Reynolds, 1979; Nester and Schedlbauer, 1981; Nester, 1985; Escamilla-Aquino et al., 2008; Takahashi et al., 2012), in this study bisexuality prevails.
The structure of gametangia, antheridia and archegonia corresponds partially to those cited for other Anemia species by Atkinson (1962), Nayar and Kaur (1971) and Nester (1985). The authors mentioned that the dehiscence of antheridia is produced by the partial or complete detachment of the opercular cell. In this case, it was observed that in A. herzogii and in the two varieties of A. tomentosa it is produced by the perforation itself.
The archegonia in the three taxa studied have the same location and quantity of cells, they do not have trichomes on the necks. Nevertheless, Nester (1985) mentioned the presence of unicellular trichomes in A. mexicana, such as marginal trichomes observed in gametophytes of these species.
In young sporophytes of A. herzogii, the laminae have simple, eglandular and clavate trichomes, as the ones observed in the adult sporophytes of A. australis (Mickel) M. Kessler and A.R. Sm. (Luján et al., 2011). The type of stomata observed in the studied species coincide with those of Luján et al. (2011).
The molecular phylogenetic studies from Labiak et al. (2015) determine that Anemia is a monophyletic genus, and they distinguish, among others, the Tomentosae clade (including A. tomentosa var. tomentosa and A. tomentosa var. anthriscifolia) and the Phyllitidis clade (including A. herzogii and A. phyllitidis), both differentiated by the spores ornamentation. The gametophytic contribution obtained in this study differentiates the varieties of A. tomentosa by its morphology of young laminar gametophyte, and A. herzogii by having branched trichomes exclusive of the gametophytic phase.











 nueva página del texto (beta)
nueva página del texto (beta)




