Introduction
Abiotic conditions such as topography, soil fertility, humidity, temperature, and light availability are the main factors that affect plant distribution and abundance (Buckley et al., 2003; Longas et al., 2016). In addition, biotic factors (e.g. herbivores, pollinators, seed predators and dispersers) can also affect plant distribution, considering that these are associated with their dispersal capacity, germination, and recruitment (Ranieri et al., 2012; Jelbert et al., 2015). These facts agree with the regeneration niche hypothesis, which states that the distribution of each plant species is restricted to a particular set of biological conditions in which optimal germination and establishment occur (Grubb, 1977). Regeneration niche can be defined as biotic and abiotic requirements in earlier stages of the life cycles of a plant species (Grubb, 1977; Poorter, 2007). In this context, many studies have evaluated seed and seedlings responses to variables of the regeneration niche with the objective of understanding patterns of plant species distribution and their ability of habitat colonization (Poorter, 2007; Murray et al., 2005; Ranieri et al., 2012; Sõber and Ramula, 2013; Guerrero et al., 2016).
Light is an environmental factor known to exert greatest effect on seed germination and shape the regeneration niche of plant species (Benvenuti et al., 2001; Boyd and Van Acker, 2004; Gonçalves et al., 2015). Because light intensity varies temporally and spatially, several plant species have been acclimated showing different levels of plasticity as a mechanism of response to variations in these light regimes (Davidson et al., 2011; Gonçalves et al., 2015). For example, plants that occur in open areas generally show a positive relationship between seed germination and light incidence (e.g. Milberg et al., 1996; Knüsel et al., 2017). In contrast, shade tolerant tree species developed the ability to germinate their seeds under different conditions of light incidence (Simão and Takaki, 2008; Souza and Fagundes, 2014).
Morphological (i.e. size, form, hardness) and physiological (i.e. age, dormancy) seed traits can interact with their regeneration niche variables. Specifically, light can interact with seed size which, in turn, affects both the success of seed germination and seedling establishment (Milberg et al., 2000; Aud and Ferraz, 2012). Generally, smaller seeds germinate faster, but often have lower percentage of germination in comparison with large seeds (Herrera and Laterra, 2008; Souza and Fagundes, 2014). However, small seeds have a higher percentage of germination under high light availability than in darkness conditions (Moles and Westoby, 2006). Large seeds exhibit low sensitivity of light variation, suggesting more plasticity levels to light regimens (Milberg et al., 2000; Fenner and Thompson, 2005).
Light is also the main environmental factors that affects seedling survival and plant growth in different habitats. Because seed size is directly related with the amount of nutritional reserves to be allocated for initial seedling growth (Geritz, 1995; Fagundes et al., 2011), it is possible this affects the success of the recruitment of plant species across different habitats (Milberg et al., 2000; Ribeiro and Borghetti, 2014; Onyekwelu et al., 2012; Souza et al., 2015b). In general, there are positive relationships between plant size and seed biomass (Leishman, 2001; Souza et al., 2016) and larger seeds develop more vigorous seedlings (Delgado et al., 2009; Sõber and Ramula, 2013; Souza and Fagundes, 2014). Hence, plant species that produce larger seeds are favored in predictable, more competitive habitats, while those that develop greater number of small seeds have greater competitive advantage, especially in early successional habitats (Moles and Westoby, 2006; Souza et al., 2015b).
The knowledge of the regeneration niche of phylogenetically related plant species allows us to find relationships between plant species traits and the colonization probability of a particular habitat (Brown et al., 2003; Gallagher et al., 2014; Ferreras et al., 2015). In this study, we evaluated the effects of seed size and light intensity on seed germination, aerial and belowground growth of two congeneric species of Fabaceae (Copaifera langsdorffii Desf. and C. oblongifolia Mart.) to characterize traits of regeneration niche that favor plant species colonization of specific habitat. The two species share a zone of sympatry in Cerrado areas in the north of Minas Gerais State, Brazil. Copaifera langsdorffii is a heliophytic, arboreal species with wide geographic distribution (Fagundes, 2014). Copaifera oblongifolia is a shrub with restricted distribution that grows in open areas such as abandoned pastures, edges of Cerrado fragments and highways. Additionally, this species is invasive in grasslands and agricultural systems, dominating and inhibiting the development of cultivated plants, especially in northern Minas Gerais, Brazil (Fernandes et al., 2018; Coutinho et al., 2019). Therefore, we expect that widely distributed tree species produce heavier seeds and with greater variation in seed size than shrubs that present more restricted geographic distribution. We also predict that, while widely distributed tree species should be capable to germinate and develop under a variable range of environmental conditions, sun-adapted shrubs should germinate and develop better under high light intensity.
Materials and Methods
Study species
Copaifera langsdorffii (Fabaceae) is a heliophytic tropical shrub, reaching up to 30 m height. It is widely distributed in South America, occurring from northern Argentina to southern Bolivia, and can be found in different biomes in Brazil, such as the Cerrado (Brazilian Savanna), Amazon Forest and Atlantic Forest (Souza et al., 2015a; Fagundes, 2014). Copaifera oblongifolia is a shrub of 1-2.5 m height that occurs in disturbed/open areas (i.e. abandoned pastures, edges of forest fragments and highways) of the Cerrado in central Brazil (Coutinho et al., 2019). Since the last decade, C. oblongifolia has caused economic loss for farmers of Minas Gerais State, because it has a great capacity to invade and colonize pastures and agricultural systems, and therefore, has become a dominant species capable to inhibit the development of cultivated plants (Fernandes et al., 2018; Fagundes et al., 2019).
The two Copaifera L. species share a sympatry zone in Cerrado areas in northern Minas Gerais State, Brazil. In this region, both flower from December to February and their fruits mature by September to October, coinciding with the time of their highest deciduousness (Costa et al., 2016; Fernandes et al., 2018). The fruits and seeds of both species have very similar morphology. Upon opening, each fruit exposes a single ellipsoid seed, which is black and shiny and is partially covered by a yellow-orange aril. The seeds of C. langsdorffii have orthodox behavior, with slow germination rate which extends up to 70 days after sowing (Fernandes et al., 2018). The seed size is a key factor for germination and seedling vigor (Souza and Fagundes, 2014). Although other animals attack seeds of C. langsdorffii in the pre-dispersal phase, the seed predation by the weevil Rhinochenus brevicollis Chevrolat represents the main cause of mortality of these seeds (Souza and Fagundes, 2017).
Seed collection
Seeds were collected in September 2015, which represents the period of seed dispersal peak of both species. We collected mature seeds from 17 individuals of Copaifera langsdorffii and 21 individuals of C. oblongifolia in an area of Cerrado vegetation (16˚17'20''S and, 44˚09'02''W) in Mirabela municipality, northern Minas Gerais State, Brazil. This region is characterized by semi-arid climate with well-defined dry and wet seasons. The average annual temperature is 23 ˚C and rainfall is about 1000 mm/year (Fernandes et al., 2018).
We randomly collected a total of 2212 well-formed seeds (malformed seeds and seeds with visual signals of attack by predators or pathogens were eliminated from the sampling) from both two plant species (C. langsdorffii N = 1445; C. oblongifolia N = 1775). Each seed was packed in an individually labeled paper bag and weighed with an analytical scale in order to compare mean and coefficient of variation of seed biomass between both species.
Seed germination tests
Of the total of 2212 seeds collected, we selected 225 of each species (eight to 20 seeds from each individual) to establish the germination experiment. All selected seeds were individually sown in six germination trays with 75 individual cells (2 cm length, 2 cm width and 3 cm height) and vermiculite was used as substrate for seed germination. This experimental design considers that each seed is a statistically independent experimental unit and allows for the comparisons of seed germination percentage among treatments using a binomial error distribution (see Warton and Hui, 2011; Souza and Fagundes, 2014). All seeds were subjected to disinfection by immersion in a 1% sodium hypochlorite solution for two minutes before sowing (Souza et al., 2016).
Finally, each set with two trays of 75 seeds of C. langsdorffii and 75 seeds of C. oblongifolia was placed in its own germination chamber (climatic chamber MA402 Marconi, Piracicaba city, Brazil) with controlled photoperiod, temperature and light intensity as follows: T1 = high light intensity: 12 h/light at 28 ˚C and 12 h/dark at 28 ˚C, 47.5 µmol.m-2s-1 irradiation; T2 = low light intensity: 12 h/light at 28 ˚C and 12 h/dark at 28 ˚C, 23.8 µmol.m-2s-1 irradiation and; and T3 = 24 h/darkness at 28 ˚C). The humidity of the germination substrate was maintained constant daily by adding three ml of distilled water in each germination cell. Seeds were monitored daily to determine seed germination percentage and time required for germination. Seeds were considered germinated when they presented primary root protrusion.
Aerial and belowground plant growth experiment
All seedlings originated from this germination experiment were cultivated at the same experimental conditions of the seed germination test (T1 = high light intensity, T2 = low light intensity and T3 = darkness), in order to evaluate the effects of light intensity, plant species and seed mass on aerial plant growth. We estimated a time required for seedling development and biomass accumulation by seedling. The time for seedling development was calculated as the time elapsed between seed germination until seedling cotyledons fell. All seedlings were removed from the substrate soon after cotyledons fell to determine their dry biomass, and next, all seedlings were individually placed in individually labeled paper bags and transferred to an oven (QUIMIS oven Q31 4M272, Sao Paulo, Brazil) with air circulation at 60 ˚C for 72 hours. After this time, the dry weight of shoot and root systems was determined on an analytical scale. Finally, the ratio of root:shoot biomass (RSR) of all seedlings was calculated as RSR = Wroot/Wshoot, where Wroot is dry mass of the root and Wshoot is dry mass of shoots.
Statistical Analysis
To determine the differences in seed mass and coefficient of variation of seed mass between both species, we built two General Linear Models (GLMs). The models consider the species (C. langsdorffii and C. oblongifolia) as explanatory variable and average seed mass or coefficient of variation of seed mass per individual plant as response variables. The two models were tested with ANOVA, considering the Gaussian error distribution.
Variations in time required for seed germination and percentage of germination among treatments were tested with GLMs followed by ANOVA. For model constructions, time to germination or germination percentage was used as response variables and treatments of light intensity, plant species and seeds mass as explanatory variables. We used the Gaussian distribution when the time to germination was the response variable. Moreover, because data of seed germination are binary (germinated or not germinated), Binomial distribution error (corrected for Quasibinomial) was used in model construction when germination percentage was the response variable. This statistical approach provides a significant gain in analysis power (Warton and Hui, 2011).
We used five different GLMs to compare the effects of light intensity treatments, plant species and seed mass on seedling vigor. The models considered time to cotyledons shedding, total biomass, aerial biomass, root biomass or root:shoot ratio as response variables and light intensity treatments, plant species and seed mass as the explanatory variables. The models were tested with ANOVA, using Gaussian distribution. All significant models that presented an explanatory variable with more than two levels were submitted to a contrast analysis to combine levels that were not significantly different, and separation of significantly different levels.
The Akaike Information Criterion for small samples (AICc) was used for ordered explanatory variables, followed by selection of the more parsimonious model. These procedures were carried out using the MuMIn package in R software (Bartoń, 2015). All models were submitted to residual analysis to check for model fit and suitability of error distribution for each response variable (Crawley, 2007). All analyses were carried out in software R version 3.6.3 (R Core Team, 2020).
Results
We found differences in seed mass between plant species (Residual DF = 36, F = 5.193, P = 0.020). Seeds of Copaifera langsdorffii were 14% heavier than those produced by C. oblongifolia (Fig. 1A). However, the coefficients of variation of seed mass did not differ statistically between both species (Residual DF = 36, F = 1.752, P = 0.194, Fig. 1B).
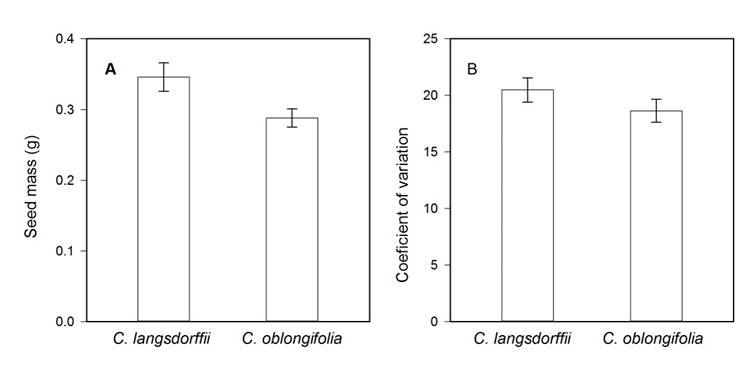
Figure 1: A. variations in seed mass; B. coefficient of variation of seed mass between Copaifera langsdorffii Desf and C. oblongifolia Martius species in Minas Gerais, Brazil.
The time required for seed germination varied in function of light intensity and seed mass (Table 1). Contrast analysis showed that time for seed germination was greater in high light intensity treatment, followed by low light intensity, and darkness. Seed mass affected positively time required for seed germination (y = 22.93 + 19.37x).
Table 1: Summary of minimal adequate models showing the effects of explicative variables (light intensity, seed mass and plant species and their interactions) on the variables’ responses (aerial mass, root mass and root:shoot ratio) of seedlings of Copaifera langsdorffii Desf. and C. oblongifolia Mart. in Minas Gerais, Brazil.
| Response variables | Explicative variables | Error distribution | Deviance | Df from residue | Residual deviance | F-values | P-values |
| Time to seed germination | Light intensity
Seed mass Species Light intensity × seed mass Light intensity × species |
Gaussian | 12109.1 1248.5 23.1 1960.2 2193.4 |
352 351 350 348 346 |
52083 50834 50811 48851 46658 |
44.899 9.258 0.171 7.268 8.1329 |
<0.0001 0.0026 0.6793 0.0008 0.0003 |
| Percent seed germination | Light intensity
Species Seed mass Light intensity ×species Light intensity × species × seed mass |
Binomial | 12.3916 4.7335 5.4008 18.746 10.545 |
447 446 445 443 438 |
432.37 427.64 422.24 403.49 389.59 |
6.453 4.930 5.625 9.763 5.491 |
0.0017 0.0269 0.0181 <0.0001 0.0044 |
Moreover, the interaction between light intensity and plant species suggests that C. langsdorffii seeds germinate faster under high light intensity (C. langsdorffii: 32.72 ± 1.46, C. oblongifolia: 39.78 ± 1.82, Fig. 2A), while C. oblongifolia seeds required less time to germinate under low light intensity (C. langsdorffii: 36.25 ± 2.02 SE, C. oblongifolia: 27.63 ± 1.38 SE, Fig. 2B) and darkness (C. langsdorffii: 23.27 ± 0.77 SE, C. oblongifolia: 20.86 ± 0.72 SE , Fig. 2C). Finally, the interaction between light intensity and seed mass suggested that seed mass affected the time to seed germination, especially under low light intensity and darkness.
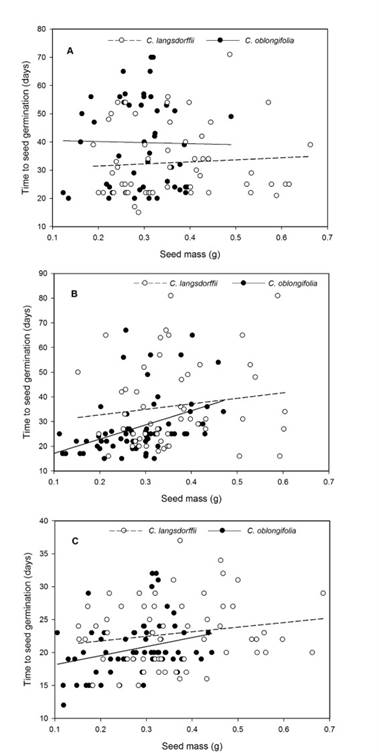
Figure 2: A. relationship between seed mass and time required to seed germination of Copaifera langsdorffii Desf. and C. oblongifolia Mart. plants in high light intensity in Minas Gerais, Brazil; B. low light intensity; C. darkness.
The explanatory variables light intensity, plant species, seed mass and the interactions: light × plant species and light × plant species × seed mass affected the percentage of seed germination (Table 1). The time for cotyledons shedding varied in function of light intensity and plant species (Table 2).
Table 2: Summary of minimal adequate models showing the effects of explicative variables (light intensity, seed mass and plant species and their interactions) on the variables’ responses (aerial mass, root mass and root:shoot ratio) of seedlings of Copaifera langsdorffii Desf. and C. oblongifolia Mart. in Minas Gerais, Brazil.
| Response variables | Explicative variables | Error distribution | Deviance | Df from residue | Residual deviance | F-values | P-values |
| Time to cotyledon falls | Light intensity
Species Light intensity × species |
Gaussian | 2249.28 1560.79 329.45 |
234 233 231 |
20808 19247 18918 |
13.733 19.059 2.011 |
<0.0001
<0.0001 0.1361 |
| Shoot mass | Light intensity
Species Seeds mass Light intensity ×species Light intensity ×seed mass |
Gaussian | 0.049 0.053 0.198 0.001 0.013 |
232 231 230 228 226 |
0.408 0.356 1.158 0.157 0.144 |
43.671 92.869 347.15 0.698 11.385 |
<0.001
<0.001 <0.001 0.4991 <0.001 |
| Roots mass | Species Seeds mass |
Gaussian | 0.001 0.006 |
240 239 | 0.015 0.014 |
22.07 104.72 |
<0.001
<0.001 |
| Root: shoot ratio | Species | Gaussian | 0.036 | 238 | 2.123 | 4.126 | 0.0433 |
Contrast analysis showed that seed germination percentage was lower in darkness, but germination percentage did not change between treatments of low and high light intensity (Table 3). In general, seed mass showed a negative relationship with germination percentage (Residual DF = 448, F = 10.264, P <0.001), and seeds of C. oblongifolia (0.83 ± 0.02: mean ± SE) had greater percentage germination than those of C. langsdorffii (0.74 ± 0.03: mean ± SE). However, the significance of the interaction between light intensity × plant species indicate that the effects of light intensity on seed germination is species-dependent. In fact, in high light intensity, germination percentage of C. langsdorffii and C. oblongifolia seeds did not vary (Residual DF = 148, F = 3.625, P = 0.062), but C. oblongifolia seeds presented greater germination percentage than those of C. langsdorffii in low light intensity (Residual DF = 148, F =10.982, P <0.001) and darkness (Residual DF = 148, F = 10.907, P <0.001). Finally, the significance of the interaction of third order suggests the effect of light available on the relationship between germination and seed mass is species-dependent (Fig. 3A-C).
Table 3: Results of contrast analysis showing the effects of different light intensity treatments on response variables (time to seed germination, percent of seed germination, time required to cotyledons shedding, and shoot mass) of Copaifera langsdorffii Desf. and C. oblongifolia Mart. in Minas Gerais, Brazil. *The levels of low light intensity and darkness were grouped and then compared with the high light intensity treatment.
| Response | Interaction levels (mean ± standard error) | Deviance | Residual DF | F | P | ||
| variables | Darkness | Low light | High light | ||||
| Time to seed | 21.93 ± 0.45 | 31.42 ± 1.27 | 5586.3 | 250 | 45.306 | <0.001 | |
| germination | 31.42 ± 1.27 | 35.87 ± 1.27 | 1125.9 | 221 | 5.101 | 0.024 | |
| Percent seed | 0.80 ± 0.03 | 0.69 ± 0.03 | 5.082 | 298 | 5.048 | 0.025 | |
| germination | 0.88 ± 0.04 | 0.80 ± 0.03 | 2.972 | 298 | 2.953 | 0.087 | |
| Time to | 33.15 ± 1.02 | 29.72 ± 1.19 | 589.96 | 140 | 6.793 | 0.010 | |
| cotyledon falls | 36.20 ± 0.98 | 33.15 ± 1.02 | 391.21 | 170 | 4.537 | 0.035 | |
| Shoot mass | 0.092 ± 0.003 | 0.091 ± 0.004 | 0.0006 | 170 | 0.051 | 0.821 | |
| 0.092 ± 0.003* | 0.124 ± 0.006 | 0.051 | 239 | 29.005 | <0.001 | ||
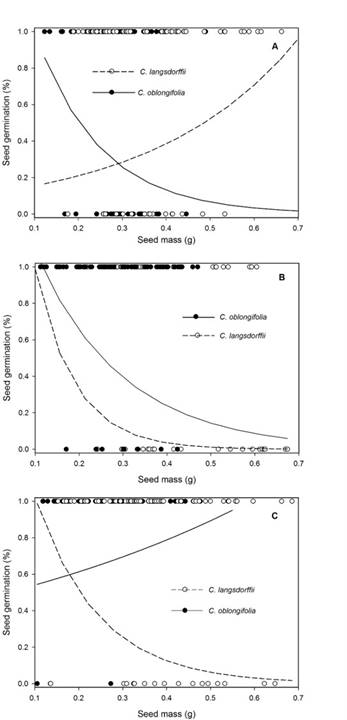
Figure 3: A. effects of seed mass on percent of seed germination of Copaifera langsdorffii Desf. and C. oblongifolia Mart. plants in high light intensity in Minas Gerais, Brazil; B. low light intensity; C. darkness.
Contrast analysis showed that the time to cotyledons shedding was different among the three treatments of light intensity, being greater in darkness, followed by low light intensity and high light intensity treatments (Table 3). Moreover, the time to cotyledons shedding varied between species (Residual DF = 239, F = 24.27, P <0.001), with cotyledons of C. langsdorffii and C. oblongifolia presenting a mean delay of 30.24 ± 1.3 SE and 36.25 ± 1.1 SE days to their fall, respectively (Fig. 4).
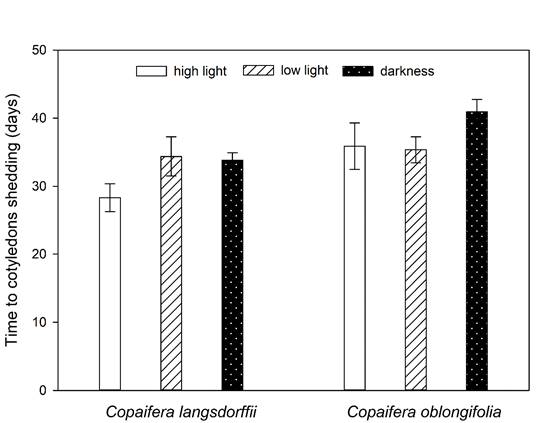
Figure 4: Variations in time required to cotyledons fall (mean ± ED) of Copaifera langsdorffii Desf. and C. oblongifolia Mart. seedling in high light intensity, low light intensity, and darkness in Minas Gerais, Brazil.
The shoot dry mass of seedlings was affected by light intensity, plant species, seed mass, and by interaction light intensity × seed mass (Table 2). Shoot dry mass of C. langsdorffii seedlings was 29.4% greater than dry mass of those of C. oblongifolia (Residual DF = 239, F = 44.52, P <0.001). According with contrast analysis, seedling growth under higher light intensity showed greater shoot dry mass in comparison with seedling growth on low light intensity and darkness (Table 3). Furthermore, shoot dry mass showed a positive relationship with seed mass (Residual DF = 239, F = 322.83, P <0.001, y = 0.002 + 0.305x). Finally, the significant interaction between light intensity and seed mass suggests that curve slopes vary among the treatments of light intensity. In fact, the contrast analyses suggest that the curve slope of low and high light intensity treatments did not vary between them (Residual DF = 169, F = 0.6460, P = 0.422), but was greater than that of darkness treatment (Residual DF = 237, F = 25.783, P <0.01) (Fig. 5).
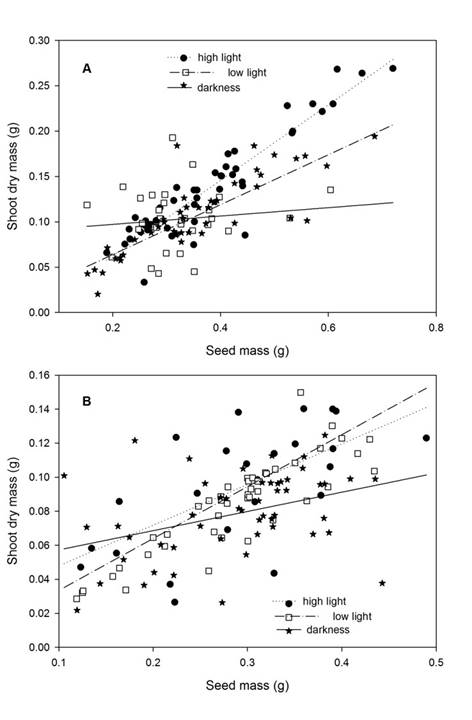
Figure 5: A. relationship between seed mass and shoot dry mass of Copaifera langsdorffii Desf.; B. C. oblongifolia Mart. seedling in high light intensity, low light intensity, and darkness in Minas Gerais, Brazil.
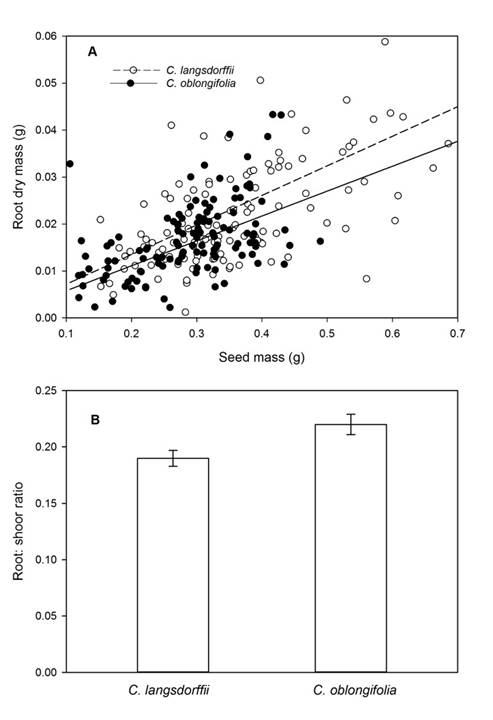
Root dry mass of seedlings was affected by plant species and seed mass (Table 2). Root mass of C. langsdorffii seedlings was 22.2% greater than root mass of C. oblongifolia. Besides, we found a positive relationship between seedling mass and seed mass (Fig. 6A). Finally, we observed that root:shoot biomass ratio of C. oblongifolia was approximately 13.2% greater than root:shoot biomass of C. langsdorffii (Table 2, Fig. 6B).
Discussion
Our results showed that light intensity and seed size were important factors that interact to shape the regeneration niche of Copaifera langsdorffii and C. oblongifolia. It is generally accepted that the niche of an adult plant species reflects its regenerative niche (Quero et al., 2009). According to this approach, the wide geographical distribution of C. langsdorffii suggests that its seeds can germinate successfully under a wide range of environmental conditions as observed experimentally in our study. On the other hand, C. oblongifolia occurs in open areas under conditions of high incidence of sun radiation. However, in our study, we found that its seeds had high germinability under low light intensity and darkness. These different physiological responses between seeds and adults of C. oblongifolia suggest a regenerative-adult niche conflict during the ontogenetic stages of these plant species. This agrees with other studies that have shown that seeds of some species of weeds adapted to environments of high incidence of solar radiation present high levels of seed germination in the absence of light or after being buried in the soil (Ohadi et al., 2010; Batlla and Benech-Arnold, 2014; Wang et al., 2016; Hu et al., 2017).
Seed mass showed a positive relationship with the time required for seed germination and conversely, presented a negative relationship with germination percentage in both plant species. Seed size is directly related with volume ratio and coat thickness of the seeds. Hence, greater seeds have low capacity of water absorption, resulting in increase of time for germination (Souza et al., 2015b). In contrast, increase in time to seed germination can raise the probability of attack by microorganisms, resulting in loss of seed viability (Fagundes et al., 2011; Souza and Fagundes, 2014). This fact may explain the negative relationship between seed size and germination percentage observed in this study. In fact, four saprophytic fungus species (Aspergillus niger P.E.L. Van Tieghem, Aspergillus flavus Link, Mucor sp. and Rhizopus stolonifer Vouillemin) were observed developing on seeds during the final phases of the seed germination test.
Following germination, light had a positive effect on time required to cotyledons shedding. In the absence of light, all nutrients available for seedling growth come from cotyledon reserves as photosynthesis is light limited. Therefore, we can expect that seedlings growing under low light intensity and darkness are more dependent of cotyledon reserve, exhausting this resource early. However, despite C. langsdorffii had heavier seeds, which implies higher cotyledon reserves than C. oblongifolia, the seedlings of C. oblongifolia retained their cotyledons for a longer time. These results suggest that C. langsdorffii can use cotyledon reserves more intensely in order to reach greater initial growth. In several seedlings of arboreal species, a fast-initial growth generally is considered as an adaptive strategy which promotes greater light capture at understory level, increasing the competitive ability and therefore, more probability of survival in environments of low light availability (Aud and Ferraz, 2012).
Seedlings of both species, under conditions of high light intensity, presented greater shoot mass than those subjected to low light intensity and darkness, suggesting that both study species share the same needs for light availability (i.e. high light intensity) for initial development. Therefore, similar physiological processes should be regulating this pattern of initial development in both species. Moreover, seed mass affected positively aerial plant growth (i.e. seedlings shoot mass), where seedlings of C. langsdorffii had more mass than seedlings of C. oblongifolia. Our results support the general predictions that seeds with greater mass develop larger and vigorous seedlings (He et al., 2007). The interspecific variation in seed/seedling size observed in this study probably reflects the adult niche of each plant species. In fact, plant species producing larger seeds have more reserves, having vigorous seedlings with greater competitive ability in predictable habitats. In contrast, plants developing smaller seeds should present greater percentage of germination and germinate more quickly in order to colonize more efficiently habitats in initial stages of succession (Moles and Westoby, 2006; Sõber and Ramula, 2013).
Root dry mass of both species was not affected by light intensity but was positively affect by seed mass, suggesting that initial growth of root system is dependent of seed reserves. Moreover, greater root mass of C. langsdorffii probably is associated with greater seed mass of this species. Finally, we observed that C. oblongifolia allocated proportionally more resource to root development than C. langsdorffii. It is important to highlight that resource allocation for roots or shoots is seed size dependent and smaller seeds must allocate proportionally a greater amount of resource to root development (Yang and Midmore, 2005). High investment in the root allows the root system to reach deeper levels of substrate with more water and nutrients, increasing the seedling survivorship with poor cotyledon reserves in stressful environmental conditions (Canadell and Zedler, 1995).
We conclude that light represents a key resource that interacts with seed mass to shape the regenerative niche of two Copaifera species. This information allows the understanding of the factors that affect the distribution of adult plants. Our results show the importance of seed size and light availability as factors affecting germination rates, as well as aerial and below ground growth of two Copaifera species. In the case of C. langsdorffii, our study shows experimental evidence of the importance of these factors for their ability to colonize different environments, which explains their wide geographic distribution. Adult shrubs have restricted distribution and occur in disturbed areas under high level of light incidence, while their seeds present high germinability under available low light. In order to justify this apparent seed-adult conflict and local pattern of plant distribution, we hypothesize that the technique of revolving soil before sowing of seed crops could bury and stimulate seed germination of the seed bank of C. oblongifolia.











 nueva página del texto (beta)
nueva página del texto (beta)