Introduction
Macroalgae can be considered as undesirable companions of coral reefs because they can outcompete corals, playing a significant role in coral reef degradation (Hughes, 1994). However, macroalgae do not directly kill healthy corals, but grow on dead or ill individuals (Diaz-Pulido and McCook, 2002, 2004), although some species can prevent coral settlement and growth (McCook et al., 2001). Nowadays, between 10 to 40% of the surface area of the coral reefs around the world are covered with macroalgae, making them an essential component of these communities (Bruno et al., 2014). They contribute to reef construction (Littler and Littler, 1984) and are key components of coral reef food webs (Bellwood et al., 2018).
Macroalgae are exposed to different pressures (either physical or chemical, natural or anthropogenic) that could modify their composition, distribution and abundance within the coral reef community, by changes in their habitat (Diaz-Pulido et al., 2007). Within natural pressures, there are disturbances such as cyclones that occur along and across the entire reef, particularly during the summer-autumn months; they are considered one of the most important disturbances for coral communities and they are also critical to the algal community (Diaz-Pulido et al., 2007; Beeden et al., 2015). Moreover, the increase of human activities in coastal waters, such as marine traffic, could also affect macroalgal communities through habitat destruction or sediment burial, resulting in smothering that will prevent algae to fixate to the substrate and/or cause anoxic conditions where algae cannot survive (Marshal and Edgar, 2003; Lirman et al., 2010). On the other hand, anthropogenic nutrient enrichment can cause an increase in macroalgae communities of coral reefs, and ephemeral species with rapid growth can outgrow and compete with the coral community causing a change from coral reef to macroalgae dominated reef (Lapointe et al., 2004). The extent of changes produced by natural and anthropogenic disturbances largely depends on the previous history and frequency of disturbances, geographical location, and structure of the coral reef (Gardner et al., 2005; Lirman et al., 2010; Beeden et al., 2015).
Any disturbance that causes damage to corals, such as bleaching and coral death, could result in significant increases in the available area for algal turfs and other opportunistic algae, that can be distinct to the naturally occurring algal communities in taxonomic composition and relative abundance of the different taxa (Hatcher, 1984; Diaz-Pulido et al., 2007; Schroeder et al., 2008). In some cases, the intensity of the herbivory can suppress dense macroalgal growths (Edmunds et al., 2019), and recovery of macroalgal turfs to their pre-disturbed state will occur within one year, while large seaweeds such as Sargassum C. Agardh may require longer (three-four years) if conditions remain unchanged (Diaz-Pulido et al., 2007). Nevertheless, macroalgal communities with different taxonomic composition can be permanently established because the conditions set are now favorable to overgrow previous assemblages (Hatcher, 1984).
In the Gulf of California, two events in the marine protected area, “Archipiélago of the Espíritu Santo Island” in La Paz Bay, Baja California Sur, took place in 2001, leaving considerable damage in the coral reef community. The San Lorenzo Channel coral reef was severely affected by the temporal stranding of the vessel “Lázaro Cárdenas II” in September. Later, in the same month, hurricane “Juliette” brought strong winds and currents that caused more damage to the coral reef. The removal of coral fragments and restoration of the affected areas were realized afterwards (Balart et al., 2010). From 2005 to 2007, a seasonal monitoring of macroalgae was carried out. In this study, the species richness and composition of macroalgae from the monitoring program were analyzed, comparing impacted and non-impacted zones, to evaluate whether these events affected the macroalgal assemblages in the San Lorenzo Channel coral reef.
Material and Methods
Study area
The San Lorenzo Channel coral reef is formed by an almost continuous platform of the branched coral Pocillopora spp., which, unlike more developed coral reefs, lacks distinct zones. The reef had an estimated total cover of around one hectare, a 97% cover of living coral, and is 5-10 m deep (Balart et al., 2010). It is located on the southwest coast of the Gulf of California, between the south portion of the Island Espíritu Santo and Pichilingüe, inside La Paz Bay (24°23'12.5"N, 110°18'55.5"W), in a homonymous channel with strong tidal currents (6.73 cm s-1) and a maximum depth of 20 m (Obeso-Nieblas and Jiménez-Illescas, 1989; Fig. 1). The rainfall period in the region is from August to October, September being the rainiest month of the year (180 mm) (Obeso-Nieblas, 2003). Temperature presents seasonal patterns with the minimum during winter (20 ºC) and maximum during summer (30 ºC); average salinity is 36 ups (Reyes-Salinas et al., 2003).

Figure 1: San Lorenzo Channel coral reef, La Paz Bay, Gulf of California, Mexico. Z1=zone 1, Z2=zone 2, Z3=zone 3.
After the disturbances occurred, three different zones could be observed in the San Lorenzo Channel coral reef, depending on the damage suffered. The first zone (zone 1) corresponded to the reef area that was directly impacted by the vessel “Lázaro Cárdenas II”, on September 22, 2001, which removed all the coral cover by leaving only a sandy bottom. The total damaged area was 847 m2. In this zone, as part of a restoration program that was implemented, concrete modules (30 units of 1 m3) were placed in October 2002 and used to cement coral fragments, being the only hard substrates available. At the end of September 2001, the adjacent area (zone 2) was damaged by the huge number of coral fragments (over 50 tons) swept to it, from the previous damage zone 1, by the force of the currents originated by the hurricane “Juliette”. Dead coral fragments buried live coral and the total area damaged was of approximately 7625 m2. After both disturbances, zone 2 preserved a substantial part of its physical and biological characteristics, but with new bare spaces and rubble of dead coral. Zone 3 corresponded to the non-impacted reef area with live coral heads, with a total area of 1217 m2; for this reason, it was considered the control zone.
Field and laboratory work
Surveys were done every three months from February 2005 to December 2007, completing three annual periods, except in the first year when only three surveys were possible (N=11). In each zone, surveys were done within an area of 900 m2 that was covered by the same scuba diver for 60 minutes. The area was measured using a compass and a 100 m line.
In each zone, three to five thalli of all conspicuous algae were collected. Conspicuous algae (CA) were identified as larger (~>10 cm height) and anatomically complex erect algal forms that were found as sparse aggregations or as individuals upon rocks and fragments of dead coral. Additionally, five rocks and five fragments of dead coral covered with macroalgal turfs were collected. Macroalgal turfs (T) refer to a ubiquitous and multispecies assemblage of short algae (<10 cm height), that comprised different morphologies as filamentous (corticated and uncorticated), foliose and calcareous articulated, among others (Connell et al., 2014).
Algae were rinsed with marine water, transported to the laboratory, and then preserved in formaldehyde 4%. They were identified to the lowest taxonomic level possible by means of their morphological characteristics, using the references for the region (Setchell and Gardner, 1924; Dawson, 1944; Norris, 2010, 2014). Current species names were revised in AlgaeBase (Guiry and Guiry, 2020); with this information a presence-absence matrix was elaborated, and a taxonomic list was made.
Daily records of surface ocean temperature (°C) of the San Lorenzo Channel were obtained for 2005-2007 from the NOAA (National Oceanic Atmospheric Administration), using 0.1-degree quadrants. Seasonal averages were calculated with the data of the three months before the collection date.
Analysis
To evaluate whether the sampling effort was representative, species rareness cumulative curves were obtained with the presence-absence matrix data. The species richness estimators used were Chao 2, Jackknife 1, and Bootstrap, which could be utilized when only presence-absence data are available. The calculations were done in the package PRIMER version 6.0 (Clarke and Warwick, 2001).
Changes in species richness between zones and years were assessed using a two-way ANOVA test (Zar, 2009). Differences in the proportion of species growing as conspicuous algae or in macroalgal turfs between zones and years were probed through a chi-square test (Zar, 2009). Distributional patterns of species for each zone and season were analyzed using multivariate techniques applied to the presence-absence data. A Sørensen similarity matrix was obtained; no previous transformations were required. An ordination analysis by means of a non-metric multidimensional scaling (MDS) to the matrix data was carried out. Finally, a two-way analysis of similarities (ANOSIM) was performed to determine the presence of differences in general algal structure, analyzing zones and years. As a complement, a similarity percentage test (SIMPER) was used to determine which species contributed to defining the groups depicted in the ordination; these tests were run with the software PRIMER ver. 6.0 (Clarke and Warwick, 2001).
Results
Accumulation curves of species showed that the estimated number of macroalgae species in the San Lorenzo Channel coral reef were 132 for Bootstrap, 151 for Jackknife 1, and 164 for Chao 2 (not shown). A total of 117 species were found during the sampling period, which means that the observed number of species represented between 71 and 89% of the potential maximum species richness.
From the 117 species, 32 were identified as conspicuous algae and 85 were part of the turfs growing on rocks or on coral fragments. Of the total number, 70 species belonged to the phylum Rhodophyta (60%) with 37 genera, 20 families, 10 orders and one class; 25 species corresponded to Ochrophyta-Phaeophyceae (21%) with 13 genera, 7 families, 4 orders and one class, and 22 species to Chlorophyta (19%) with 10 genera, 10 families, 5 orders and one class. The most abundant families were Rhodomelaceae with 20 species and Ceramiaceae with 13 (Appendix). From the total number of species, five had been previously reported as endemic to the region: Codium amplivesiculatum Setchell & N.L. Gardner, Sargassum horridum Setchell & N.L. Gardner, Sphacelaria brevicornis Setchell & N.L. Gardner, Gracilaria marcialana E.Y. Dawson and Palisada pedrochei J.N. Norris.
In general terms, zone 2 had the highest number of species (87), with 50 genera, 33 families, 18 orders and three classes, followed by zone 1 (77), with 43 genera, 28 families, 14 orders and three classes; and zone 3 (76), with 44 genera, 28 families, 15 orders and three classes (Fig. 2). The year with the highest number of species was 2007 (95), followed by 2006 (77) and 2005 (61) (Fig. 2). Accordingly, significant differences between 2007 and 2005 were found (F2,24=3.6; p<0.04), but not between zones (F2,24=0.3; p=0.7) or in the years in relation to the zone (F4,2=1.3; p>0.2). In 2007 a change in seasonal patterns was observed, especially in zone 2, where the richness increased during the warmer months, unlike previous years (Fig. 3). The conspicuous algae proportion oscillated between 26% and 31% of the total richness by zone or year (Fig. 4). No significant differences were found in the proportions of conspicuous algae or macroalgae growing on turfs between zones (χ2=2.3; p =0.7) or years (χ2=0.2; p=0.9).

Figure 2: Macroalgae species number by phylum in the San Lorenzo Channel coral reef, La Paz Bay, Gulf of California, Mexico.
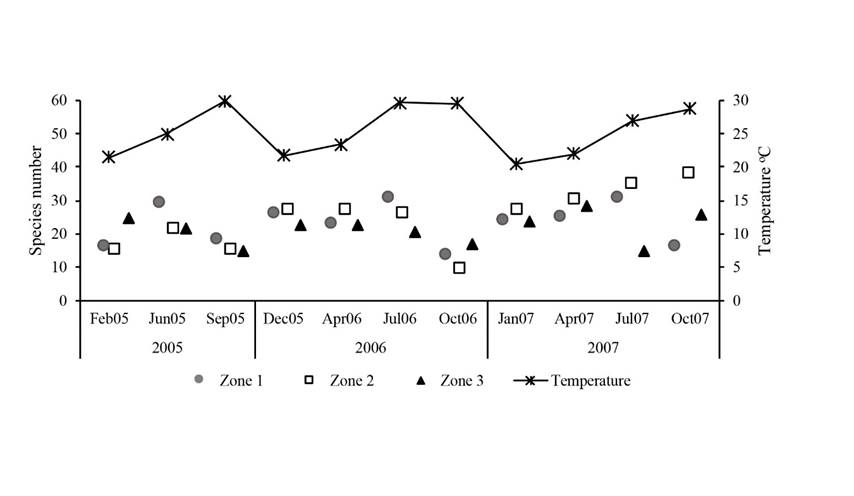
Figure 3: Macroalgae species number by zone and year in the San Lorenzo Channel coral reef, La Paz Bay, Gulf of California, Mexico.

Figure 4: Percentage of conspicuous algae (black) and macroalgae forming turfs (white) in the San Lorenzo Channel coral reef, La Paz Bay, Gulf of California, Mexico.
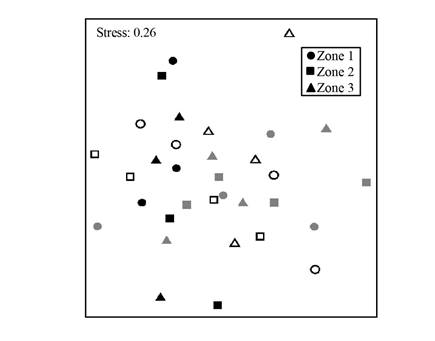
The MDS showed no aggregation pattern by zone or year (Stress: 0.26; Fig. 5). Moreover, no significant differences were detected in the composition of species between zones (R=0.05, p=0.8), and years (R=0.06, p=0.2). SIMPER analysis estimates that the average similarity inside zones or years was low, from 36% to 39%, and dissimilarity was high, between 60% and 63%. Since no aggregations between the species composition of each zone or year were found, no more SIMPER results are shown.
Discussion
A total of 117 marine benthic algal species were found in the coral reef of the San Lorenzo Channel, with 85% of the potential species richness of the reef according to the bootstrap method for species richness estimation (Petersen and Meier, 2003), suggesting that this is an area of high species richness, as previously discussed by Riosmena-Rodríguez and Paul-Chávez (1997) and Casas-Valdez et al. (2000). The number of species reported in this study represented 43% of the total registered species of the La Paz Bay, which is similar to that reported for nearby sites as Espíritu Santo Island (106 spp., Paul-Chávez and Riosmena-Rodríguez, 2000) and Punta Galeras (117 spp., Rodríguez-Morales and Siqueiros-Beltrones, 1999).
From the 117 species, the highest percentage belonged to red algae, followed by brown and green algae. In a previous study for the same channel, the highest percentage of the algae corresponded to the red algae (70%), while only 10% corresponded to green algae (Iglesias-Prieto et al., 2003). Other authors found the same tendency in adjacent places such as Balandra, Punta Galeras and Espíritu Santo Island (Rocha-Ramírez and Siqueiros-Beltrones, 1991; Rodríguez-Morales and Siqueiros-Beltrones, 1999; Riosmena-Rodríguez and Paul Chávez, 1997; Casas-Valdez et al., 2000). Several authors have discussed that this pattern might be linked with the high diversity of red algae, as well as the great variety of life forms and more efficient reproductive strategies, for example, the formation of spores that allows them to resist and manifest in all seasons of the year (Mathieson, 1989; Woelkerling, 1990; Mateo Cid et al., 1993).
The relative dominance of turf forming algae in the San Lorenzo Channel has also been observed in other reef systems that have been impacted by hurricanes or storms, such as in a tropical reef community in the Mexican Caribbean (van Tussenbroek and Collado-Vides, 2000), as well in the Gulf of California (Scrosati, 2001). In the case of the Gulf of California, turfs are well-represented in number of species, which could be associated with the high seasonal variation in the temperature (i.e., Littler and Littler, 1981), irradiance and nutrient availability (Pacheco-Ruíz and Zertuche-González, 1999), and hurricanes (Tello-Velasco, 1986), among other factors. This growth form allows them to persist in areas with high environmental availability (Hay, 1981), such as the San Lorenzo Channel, a zone with high energy (high-velocity currents), which may favor the presence of macroalgal turfs. In other coral reefs impacted by a shipwreck, changes in macroalgal assemblages have been attributed to the establishment of unpalatable assemblages of macroalgae that were avoided by grazers (Hatcher, 1984), or to the addition of nutrients by the remains of the wreckage and the decrease in abundance by some herbivores (Marshal and Edgar, 2003).
Despite the fact that a differential damage was caused to the reef by the stranding of the vessel and later by the hurricane, no significant differences were found in species richness and composition between the impacted and non-impacted zones or between years, as was expected. Macroalgal assemblages between zones were similar from the beginning of the monitoring program, possibly because the recovery of macroalgal assemblages occurs within the first years after the disturbance took place (Diaz-Pulido et al., 2007). In our case, sampling began three years after the disturbances. Additionally, despite the changes in substrate caused by the vessel “Lázaro Cárdenas II” and the hurricane, the three zones had suitable substrate for macroalgae attachment. It is important to note that we did not evaluate the abundance of macroalgae in the three zones, as it is possible that there could be differences in macroalgal abundances among them as found in other studies (Rogers, 1996, 1997).
The only appreciable change was observed in 2007, when richness was higher during the warmest months, especially in zone 2, contrasting what was observed in other years and other localities of the Gulf of California, in which the richness was low. In this regard, water temperature is a critical factor that limits growth rate and species distribution (e.g., Lüning, 1993; Hurd et al., 2014) and could be, in part, responsible for the deviation observed in 2007. During this year, lower temperatures than those reported for previous years were observed (-2 oC) (Fig. 3) in the La Paz Bay, a situation which was related to the influence of the cold phase of the El Niño-Southern Oscillation (ENSO), being 2007 the coldest year in the first decade of the 21st century (Guevara-Guillén et al., 2015).
Macroalgal assemblages in the San Lorenzo Channel coral reef were able to recover, regardless of the modification of the substrate caused by the stranding and the hurricane “Juliette” in 2001. Therefore, it is important to implement monitoring efforts in case of anthropogenic or natural disturbance occurs to document the extension of damage, recovery of the communities, and propose mitigation strategies if necessary.











 nueva página del texto (beta)
nueva página del texto (beta)