Introduction
During the second half of the 20th century, extensive land clearing for the establishment of agricultural fields and pastures for livestock along with significant changes in agricultural practices decreased, fragmented and altered at growing rates forests in most tropical regions of the world (Gómez-Pompa et al., 1972, 1974; Janzen 1973, 1983, 1986; Kellman et al., 1998; Dirzo and Raven 2003; Brook et al., 2008; Bradshaw et al. 2009; Wright, 2010). Most current remnant tropical forests occur within protected areas surrounded by complex mosaics of vegetation with variable degrees of conservation and transformation by humans (Ricketts, 2001; Vandermeer and Carvajal, 2001; Vieira et al., 2008; Laurance et al., 2014; Chazdon, 2014). These heterogeneous landscapes include a large proportion of the world´s biodiversity which is increasingly threatened by changes in community composition, structure and dynamics, with subsequent alteration of their functioning and biogeochemical cycles (Medellín, 1994; Huston, 1994; Grubb, 1996; Lindenmayer and Fisher, 2006). Understanding the ecological consequences of forest habitat degradation and fragmentation on forest landscape dynamics is fundamental to design better agricultural, management and forest conservation practices (Harrison and Bruna, 1998; Chapman et al., 2003; Lindenmayer and Fisher, 2007; Krupnick, 2013).
The ecological changes in southeastern Mexico exemplify the consequences of social and economic policies implemented in many tropical regions of the world. De Vos (2002) documents the changes during the last 150 years in the use of natural resources in the Selva Lacandona rainforest, in eastern Chiapas. The harvest of timber from the tropical rainforest in this region was the dominant activity until the end of the first half of the 20th century. These exploitations concentrated almost exclusively on the extraction of valuable timber (mostly mahogany and tropical red cedar) and few other resources such as chewing-gum base but maintained the vegetation cover. At the beginning of the second half of the century, the Mexican government provided private enterprises with extensive areas and peasants with small parcels and initiated significant changes in the environment through an extensive colonization policy that brought people from many other regions of the country (de Vos, 2002). The new landholders deforested extensive areas for agriculture (both traditional and non-traditional) and cattle ranching compromising subsequent forest regeneration (Ochoa-Gaona et al., 2007). Forest habitat alteration and fragmentation increased at faster rates in most of the region except in the Montes Azules Biosphere Reserve (INE, 2000).
In the same decades, the region underwent major cultural changes. The traditional slash-and-burn milpa agricultural system of the local Maya Lacandon inhabitants has also been increasingly replaced with intensive agricultural practices for monocultures and pastures (Barrera et al., 1977; Levy-Tacher et al., 2002). The indigenous Lacandon Maya traditional milpa farming is based on slashing and burning to provide clearings in which crops can grow (Nations and Nigh, 1980; Nigh, 2008; Nigh and Diemont, 2013). This swidden system maintains significant larger areas vegetated since it is based in polycultures including many native woody species, and requires long fallow periods during which nutrients accumulate in the vegetation and soil, weeds are controlled, and soil properties are rehabilitated (Cowgill, 1962; Reina, 1967; Douterlungne et al., 2010, 2013). Medellín and Equihua (1998) documented in areas with large forest remnants that mammalian species richness did not differ between forests and old milpa fields and argued that traditional Maya-Lacandon use of the land in the form of small agricultural plots embedded in a large forest matrix can increase spatial heterogeneity and promote mammal diversity.
Seed dispersal and recruitment are critical demographic processes affecting species abundance and distribution (Zang et al., 2007). Habitat fragmentation limits seed dispersal affecting species distribution, local population viability and community structure (Schupp and Fuentes, 1995; Harms et al., 2000; Howe and Miriti, 2004; Hampe et al., 2008), decreasing the functionality of habitat corridors among landscape components (Levey et al., 2005; Damschen et al., 2006). Habitat fragmentation also affects seedling recruitment and survival through its effects on patch size, extent of borders, and habitat quality (McEuen and Curran, 2004; Cordeiro et al., 2009). These effects are not homogeneous across species and alter species interactions, community composition and structure, and landscape configuration and functionality (Damschen et al., 2006; Herrera and García, 2010).
Our aim was to compare the arrival of propagules and their recruitment in tropical successional communities surrounded by habitat matrices with contrasting degrees of human disturbance. We are interested in understanding the potential ecological impact of policies directed to increase expansion of contiguous pastures and commercial agriculture in areas where more scattered traditional swidden agriculture was practiced. A comprehension of the ecological dynamics of agricultural traditional systems and their change provides critical information for the evaluation of current development practices and the design of sustainable use of resources (Nations and Nigh, 1978, 1980; Nigh, 2008; Nigh and Diemont, 2013; Levy-Tacher et al., 2012; Ortega-Álvarez et al., 2019). Specifically, we estimated seed rain and recruitment variation in communities that ranged from mature forests to agricultural fields and pastures within vegetation matrices dominated by old-growth forests or an array of human created habitats.
Methods
Study sites
Our study was performed in the buffer zone at the border of the Montes Azules Biosphere Reserve, Selva Lacandona, Chiapas, Mexico, centered in the Maya Lacandon homesteads of Lacanjá-Chansayab and Bonampak-Bethel (16o46'N, 91o08'W, 350-400 m elevation). Climate is warm-humid with an average annual temperature of 25-27 oC and an annual rainfall of 180-220 cm (García, 1987). Precipitation is concentrated (>80%) between summer and mid-fall (June to October). Remnants of tropical rainforest occur at basins and lower mountain rainforest on hills (Breedlove, 1981; Pennington and Sarukhán, 2005). The vegetation is characterized by a mixture of pastures, milpa fields (traditional land use of Maya Lacandon people with multiple crops including corn, squash, yuca and beans), early successional forests and mature forests (Quintana-Ascencio et al., 1996). We have a concurrent study of the dynamics of seed banks at the same sites (Quintana-Ascencio et al., 1996).
Seed traps
There was a gradient of forest transformation with larger and better-preserved forest stands near the Bonampak archaeological site and west of Lacanjá-Chansayab, and secondary forest stands, early successional forests, milpa fields and pastures concentrated around the Lacandon dwellings. We established 14 seed traps in each study vegetation association at these two localities: Bonampak-Bethel and Lacanjá-Chansayab (2 sites × 6 habitats × 14 traps; n = 168 total; Fig. 1). We used information from local peasants to select study sites and negotiated with them the access to the sites.

Figure 1: Map of the study sites in the Maya Lacandon homesteads of Lacanjá-Chansayab and Bonampak-Bethel, Chiapas, Mexico.
We sampled in six successional communities (also described in Carrillo Arreola, 1992 and Quintana-Ascencio et al., 1996):
1. Agriculturally improved pastures without grazing: this community is maintained by periodic fires during February and March. In 1990, it covered approximately 3% of the study region. It comprised a minor area in the study sites (~5%; area estimates based on Fig. 1).
2. Milpa surrounded by other milpa fields and early successional forests. Mixtures of corn, squash, beans and other annual and perennial crops are sown after the large woody species are cut and fire is applied in the remaining local community. Local peasants clear these forests at least every 10-20 years to reduce vegetation cover, enrich soils with ashes and reduce weed infestation.
3. Early successional forests with 5-15 years since abandonment: these are dense woody communities dominated by shrubs and fast-growing small trees characterized by associations of Trichospermum galeottii (Turcz.) Kosterm., Cecropia spp., Ochroma pyramidale (Cav. ex Lam.) Urb. and Piper spp. This habitat comprised <10% of the area in the study region.
4. Mid-successional forests: these are more complex forest stands with large individuals (>10 m height) of early or mid-successional tree species such as Dendropanax arboreus (L.) Decne. & Planch., Alchornea latifolia Sw., and Spondias mombin L. in the canopy (~ 10% of the area).
5. Mature forests: we chose forest stands far from human dwellings with tree strata clearly defined, diverse tree species and with average canopy >20 m height (~75% of the area).
6. Milpa fields within forest matrix: these communities were traditional multiple crop fields <1 ha in area within a matrix of forest that were cleared in preparation for planting just before the start of our study. We set traps in milpa fields within the forest matrix: seven traps were randomly located along the four milpa sides approximately 5 m from the forest border, the other seven in the center at least >20 m from the forest border. Both types of milpa fields together encompassed <2% of the study area.
In July 1990, we randomly distributed 14 seed traps in each replicated successional community. In each sampling station, we deployed a funnel-shaped seed trap made of tricot (50 × 50 cm) supported by a woody frame at 50 cm height. Trap content was collected in paper bags approximately every 40-60 days in August, October and November 1990, and February, April, May and July 1991. Bags were transported within 10 days to a laboratory at El Colegio de la Frontera Sur (ECOSUR), San Cristóbal de Las Casas, Chiapas. Branches and leaves were discarded and all other material screened under a dissection microscope (Leica MZ6 stereomicroscope, Heerbrugg, Switzerland). Seeds were separated by morphospecies identified with a serial number and kept in cellophane bags for botanical determination. Damaged traps were replaced as needed. Traps in pastures, milpa fields and one of the early successional forests at Lacanjá-Chansayab were burned after prescribed fires performed by local farmers (<2 per site and evaluation, total 8). Samples were pooled by replicated community to estimate composite annual estimates of seed density (abundance m-2) and composition. We adjusted estimates as necessary to account for lost samples. Seed taxonomic identity was attained using a reference catalog from plants collected around the study sites and deposited at the herbarium in the Colegio de la Frontera Sur, San Cristóbal de Las Casas, Chiapas, and in the seed catalogue at the Estación Biológica Tropical “Los Tuxtlas”, Veracruz, Universidad Nacional Autónoma de México (G. Ibarra-Manríquez and S. Sinaca, pers. communication).
New recruits’ plots
In August and October 1990 we established 90 (2 sites × 3 habitats × 15 quadrats; n = 90) permanent observation plots (0.5 × 2.0 m) to monitor abundance of seedlings and juvenile plants (<0.5 m) in the three different tree and shrub dominated vegetation associations (mature forest, mid-successional forest and early successional forest) in each location. In each replicated forest community we randomly established the 15 plots marked in the corner with wooden sticks. Plots were visited every four months for a year. Monitoring of plots at Bonampak-Bethel was two months behind those at Lacanjá-Chansayab. We identified, mapped and counted every plant <0.5 m height within the plot except prostrated plants (mostly Araceae, and other climbing herbs).
Analysis
Seeds were divided according to their species life form (herbs, vines, lianas, shrubs and trees) and size (<2 mm, 2-6 mm and >6 mm diameter). We recognized the life form of plants <0.5 m height in the plots. We assessed the significance of differences in abundance of seed assemblages between communities, months and localities with Kruskal-Wallis tests (Potvin and Roff, 1993), and the significance of differences in abundance of plant assemblages between communities and months with Monte Carlo methods (Manly, 1991), because data did not meet the assumptions of parametric tests. For the Monte Carlo tests we randomized 1000 times among communities for each species keeping fixed their rows (species) totals per evaluation. We evaluated dissimilarity based on species presence/absence for seed and plant assemblages among communities in different study sites with Non-metric Multidimensional Scaling (NMS) with Bray-Curtis dissimilarity (Sörensen’s index) and a random starting configuration. The final configuration of the ordinations was evaluated with 1000 permutations and both had a P<0.001 that similar stress values could happen by chance. Two dimensions were included in the ordination of seed assemblages after assessing the stability. Only one dimension was included for plant assemblages. We conducted analyses in R version 2.7.2 (R Core Team, 2013). We assessed differences between two areas defined by the Lacandon Maya people and successional communities pooling data within each plant association sampled.
Results
Seed rain
Overall and after a year of monitoring (August 1990-July 1991) we collected ~13,600 seeds of 144 species belonging to 48 botanical families. Rubiaceae, Fabaceae, Moraceae (includes Cecropia spp.), Araceae, Arecaceae, Piperaceae, and Poaceae comprised 32% of the species (Appendix 1). Mid-successional and mature forests had the highest species richness (60-72 species), early successional forests and milpa fields were intermediate (17-44) and pastures had the lowest species richness (11-14; Appendix 1; Fig. 2). Overall seed densities were highest in the center of the milpa fields within a forested matrix in Bethel-Bonampak (~8500 seeds m-2), in the mature forest (~7400 seeds m-2) and the early successional forest (~6200 seeds m-2) in Lacanjá-Chansayab, and lowest in the milpa fields of Bethel-Bonampak (~1700 seeds m-2), the center of the milpa within a matrix of forest in Lacanjá-Chansayab (~1000 seeds m-2), and in the pasture in Bethel-Bonampak (~100 seeds m-2; Table 1).

Figure 2: Total number of species per successional condition at the Selva Lacandona, Chiapas, Mexico (August 1990-July 1991). Lacanjá-Chansayab = l; Bonampak-Bethel= b; Vegetation associations: Mature forests = sma; Mid-successional forests = smm; Center of milpa field within forests = mcb; Border of milpa fields within forests = mbb; Early-successional forests = aca; Milpas within disturbed areas = mil; pastures = pas. Habitat perturbation increased in habitats represented by columns with lighter shades.
Table 1: Percent contribution of the most common species to the total number of seeds collected in six successional communities in the Selva Lacandona, Chiapas, Mexico (August 1990-July 1991). Lacanjá-Chansayab = l; Bonampak-Bethel = b; Successional communities: Mature forests = sma; Mid-successional forests = smm; Center of milpa field within forests = mcb; Border of milpa fields within forests = mbb; Early-successional forests (fallows) = aca; Milpas within disturbed areas = mil; grasslands = pas. *exotic.
| Species | lsma | bsma | lsmm | bsmm | lmcb | lmbb | bmcb | bmbb | laca | baca | lmil | bmil | lpas | bpas |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Brosimum sp. | 7.1 | |||||||||||||
| Cecropia spp. | 49.4 | 39.1 | 9.9 | 1.6 | 32.6 | 7.0 | ||||||||
| Ficus aurea Nutt. | 14.9 | 11.6 | 1.9 | 12.9 | 38.0 | 51.0 | 23.4 | 2.4 | 2.4 | |||||
| Ficus insipida Willd. | 6.8 | 16.1 | 2.6 | 1.2 | 4.5 | |||||||||
| Ficus jimenezii Standl. | 4.6 | 3.2 | 9.2 | 10.6 | 29.7 | |||||||||
| Heliocarpus spp. | 23.3 | 5.0 | 8.4 | |||||||||||
| Andropogon bicornis L. | 8.2 | |||||||||||||
| Hyparrhenia rufa*(Nees) Stapf | 19.2 | |||||||||||||
| Paspalum paniculatum L. | 59.4 | |||||||||||||
| Paspalum virgatum L. | 49.2 | |||||||||||||
| Bidens spp. | 10.9 | |||||||||||||
| Undetermined spp. | 12.6 | 31.2 | 32.4 | 56.6 | 64.0 | 47.9 | 15.3 | 32.4 | 11.5 | 16.6 | 28.6 | 59.3 | 8.2 | |
| Euphorbia heterophyla L. | 15.5 | 7.6 | ||||||||||||
| Iresine spp. | 2.2 | 24.0 | 9.0 | 5.5 | ||||||||||
| Piper sp. 1 | 25.1 | 24.1 | 10.9 | 2.1 | 11.2 | |||||||||
| Piper sp. 2 | 22.7 | 25.7 | 29.0 | 2.9 | 16.6 | |||||||||
| Accumulated % | 88 | 85 | 90 | 78 | 85 | 90 | 96 | 87 | 92 | 78 | 91 | 82 | 95 | 90 |
| Total seeds m-2 | 7453 | 1757 | 5843 | 1448 | 1013 | 2562 | 8528 | 2338 | 6225 | 5302 | 7344 | 1669 | 2800 | 132 |
There was seasonal variation in the number of species and seed density (Kruskall-Wallis H>19, P<0.004, for both contrasts), but there were no clear differences among localities (H = 0.49, P = 0.48). Number of species was lowest during the rainy season (August-October) and highest at the end of the dry season (April-May; Fig. 3). There was a larger change among seasons in number of species captured in the forests than in early successional forests and milpa fields. Pastures had the lowest seasonal contrast in the number of species (Fig. 3). Number of seeds of tree species was highest in July, coinciding with the start of the rainy season, while seeds of herbaceous species peaked in April at the end of the dry season. Seeds of shrubs did not show a marked variation across the year (Fig. 4).
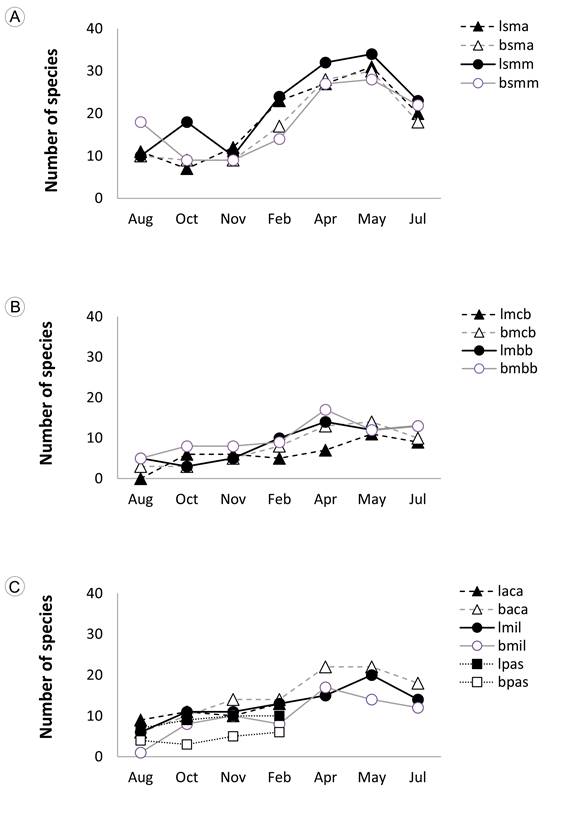
Figure 3: Number of species per month and successional condition in the Selva Lacandona, Chiapas, Mexico (August 1990-July 1991). A. mature (ma) and mid-successional (mm) forest; B. center (cb) and border (bb) of the milpa surrounden by forest; C. early successional forest (aca), milpas (mil) ans pastures (pas). Lacanjá-Chansayab = l; Bonampak-Bethel= b; Vegetation associations: Mature forests = sma; Mid-successional forests = smm; Center of milpa field within forests = mcb; Border of milpa fields within forests = mbb; Early-successional forests = aca; Milpas within disturbed areas = mil; pastures = pas.

Figure 4: Seed density per month by life-form in the Selva Lacandona, Chiapas, Mexico (August 1990-July 1991; vines and unknown species had small contributions and were not plotted).
Seeds of different life forms varied across successional communities. Of the 144 species recorded in our seed collection 42 were trees, 25 herbs, 21 shrubs, 19 vines, and 40 could not be determined. Seeds of trees and vines were frequent in forest communities and milpa fields surrounded by forests, and scarce or absent in open communities in disturbed areas. Seeds of shrubs were more abundant in early successional forests and milpa fields in disturbed areas. Seeds of herbaceous species were most abundant in the milpa fields and in early successional forests but were frequent in all communities.
The seed rain was dominated by few species (Table 1). Tree species in the genera Brosimum Sw., Cecropia Loefl. and Ficus L. (included here all three within the Moraceae) contributed with 22-76% of the seeds in the forests, 13-81% in disturbed habitats surrounded by forests, 11-33% in the early successional forests, less than 3% in the milpas within disturbed matrix, and were absent in the pastures. Seeds of Piper spp. were only found in early successional forests and in milpa fields, where they contributed with 2-25%. Seeds of Asteraceae were relatively abundant in all communities (8.2-57%). Exotic invasive grasses were exclusive of pastures, where they contributed with (49-87%).
Distribution of seeds in the three size groups varied among successional communities and seasons (Kruskall-Wallis H>55, P<0.001, for both contrasts), but there was no clear evidence of heterogeneity among localities (H = 0.08, P = 0.77). In our sample, 38 species had seeds <2 mm, 76 were 2-6 mm, and 28 were >6 mm in diameter. The smallest seeds were relatively homogeneously distributed across communities. Seeds of intermediate size were more abundant in early successional forests than in forests and almost absent in open communities. The largest seeds were most abundant in the border of the forest with the milpa fields in the forest matrix, followed by forests, early successional forests, and were scarce in disturbed and open habitats. The peak of dispersal of small and large-sized seeds happened during the end of the dry season. Mid-sized seeds did not show a clear seasonality of dispersal.
NMS ordination indicated the dissimilarity among assemblages (axis 1 eigenvalue = 0.99, P = 0.02, axis 2 eigenvalue = 0.03, P = 0.04; Fig. 5). This ordination explained 0.896 of the variance. Axis one summarized most of the dissimilarity variation among successional communities studied. The dissimilarity pattern was consistent between Lacanjá-Chansayab and Bonampak-Bethel. Mature and mid-successional forest communities were highly similar from each other and formed a cluster. Milpa fields within a matrix of forest were closest to the forest communities and in a central position among forests, early successional forests, and milpa fields in the disturbed habitat matrix. In the ordination space, associations in the borders of the milpa surrounded by forest were closest to the forests. Associations in the milpa fields within the matrix of disturbed habitats were equally distant from the early successional forests and milpa in the forests. Pastures were the most distant from all other communities. Axis two mostly summarized differences among early successional forests and the rest of the communities.

Figure 5: Non-metric multidimensional scaling with Bray-Curtis dissimilarity (Sörensen’s index) for associations of seeds of species in successional communities in Lacanjá-Chansayab, Chiapas, Mexico. Black symbols: Bonampak-Bethel; Gray symbols: Lacanjá-Chansayab; Vegetation associations, open circles: Mature forests; open squares: Mid-successional forests; filled squares: Border of milpa fields within forests; filled circles: Center of milpa field within forests; diamonds: Early-successional forests; up-pointed triangles: Milpas within disturbed areas; down-pointed triangles: pastures; Size of the site symbols was adjusted by the proportion of tree species in the sample; crosses: species.
Establishment of new recruits
Overall, we recorded ~3416 new recruits with a height <0.5 m belonging to 238 morphospecies in 48 botanical families, 98 were identified to genus and 76 to species (Appendix 2). Among these plants, the largest number of species occurred in mature and mid-successional forests compared to early successional forests in both locations (Table 2). The number of species was higher in the mid-successional and mature forests of Bonampak-Bethel compared to Lacanjá-Chansayab. The number of plants <0.5 m in height was highest in the forests and lowest in the early successional forests (Table 2). Plant density was in general higher in the mid-successional and mature forests of Bonampak-Bethel compared to Lacanjá-Chansayab. Survival of plants <0.5 m was higher in mid- and late successional forests than in early successional ones (Table 2).
Table 2: Density of plants < 0.50 cm tall (m-2 ± standard error), number of species per evaluation and total (in three evaluations) and plant survival of plants in control plots at three replicated forested successional communities in the Selva Lacandona, Chiapas, Mexico (August 1990-June 1991). Lacanjá-Chansayab = l; Bonampak-Bethel= b; Successional communities: Mature forests = sma; Mid-successional forests = smm; Early-successional forest (fallows) = aca. Monitoring of plots at Bonampak-Bethel was two months behind those at Lacanjá-Chansayab. We do not have evidence of significant differences between communities with the same letter in the same column after Monte Carlo tests with 1000 simulations.
| Sampling months | Plant survival (± standard error) | |||||||
|---|---|---|---|---|---|---|---|---|
| Site | Variable | August/October 1990 | December/ February | April/June 1991 | Aug/Oct-Dec/Feb | Dec/Feb -Apr/Jun | Aug/Oct-Apr/Jun | Total of species |
| lsma | plants m-2 | 14.5 (1.03) b | 16.0 (1.22) b | 15.9 (1.56) b | 0.43 (0.07) c | 0.46 (0.07) c | 0.30 (0.06) b | |
| bsma | plants m-2 | 18.5 (0.85) a | 23.4 (0.95) a | 36.1 (3.62) a | 0.63 (0.05) a | 0.32 (0.05) e | 0.33 (0.05) b | |
| lsmm | plants m-2 | 18.1 (1.33) a | 16.7 (1.12) b | 18.3 (1.45) b | 0.54 (0.06) b | 0.50 (0.07) b | 0.43 (0.06) a | |
| bsmm | plants m-2 | 20.6 (1.07) a | 25.9 (1.07) a | 33.6 (2.12) a | 0.53 (0.06) bc | 0.61 (0.06) a | 0.36 (0.05) b | |
| laca | plants m-2 | 7.67 (1.09) c | 10.9 (1.01) c | 17.6 (1.68) b | 0.47 (0.07) c | 0.40 (0.08) d | 0.26 (0.07) c | |
| baca | plants m-2 | 9.07 (1.03) c | 10.5 (1.19) c | 15.5 (1.56) b | 0.37 (0.08) d | 0.38 (0.07) d | 0.23 (0.06)c | |
| lsma | species | 43bc | 45 b | 40 b | 69 b | |||
| bsma | species | 66 a | 68 a | 61 a | 96 a | |||
| lsmm | species | 53 ab | 58 ab | 53 ab | 74 b | |||
| bsmm | species | 55 ab | 68 a | 64 a | 96 a | |||
| laca | species | 34 c | 42 b | 45 b | 67 b | |||
| baca | species | 28 c | 31b | 47 b | 59 b | |||
Most small plants belonged to a few species. Seedlings of Chamaedorea spp. were abundant in the mid-successional and mature forest communities (7-11% of the total number of seedlings in a given community), and rare in early successional forests. Seedlings of Pseuderanthemum verapazense Donn. Sm., an unidentified taxon of Sapindaceae, and an unidentified species were common in the forests of Lacanjá-Chansayab (26-38%), while seedlings of Ampelocera hottlei (Standl.) Standl., Rinorea guatemalensis (S. Watson) Bartlett, Brosimum alicastrum Sw., Paullinia costata Schltdl. & Cham., and an unidentified species of Sapindaceae were common in the forests of Bonampak-Bethel (25-41%). Plants of Iresine spp. were abundant in early successional forests in both locations (17-32%). Seedlings of Dendropanax arboreus, Piper glabrescens (Miq.) C. DC., and a Heliconia species were abundant in the fallow fields at Lacanjá-Chansayab (22%), while seedlings of Acalypha diversifolia Jacq., Psychotria pubescens Sw. and a grass were common in the fallow fields at Bonampak-Bethel (28%). We recognized the life form of the small plants for 143 species: three were annual herbs, 30 perennial herbs, 15 vines, nine ferns, 44 shrubs, 8eight palms, and 32 trees. Seedlings of trees, palms and vines were more abundant in mid- and late successional forests. Small individuals of shrubs were common in all forest stands. Herbs were more abundant in early successional forests.
Dissimilarity in floristic composition among locations and vegetation associations was highest between mid-successional and mature forests and early successional forests (>0.91 for all comparisons), and lowest between mid- and late successional forests within locations (0.43 and 0.47 for Bonampak and Lacanjá-Chansayab, respectively). Distance between locations was 0.67 for early successional forests, 0.60 for mid-successional and 0.68 for mature ones. Distances between mid-successional and mature forests across localities were 0.54 and 0.66.
Discussion
We document pervasive effects of the increasing predominance of humanized landscapes in the buffer zone of the Montes Azules Biosphere Reserve, Selva Lacandona, Chiapas, Mexico. We observed a significant decline in species richness and a change in dominance of life forms in the seed rain assemblage along the floristic successional gradient. At the extremes, mature forest included seeds of diverse tree species while the assemblage in pastures was dominated by seeds of a few grasses, mostly introduced exotic species. Areas characterized by intensive agriculture and pastures nearby human dwellings were associated with the most simplified and homogeneous floristic composition of seed assemblages. Seeds of tree species were present in milpa fields surrounded by forest but were absent in milpa in humanized environments where there was a dominance of seeds of generalist and widespread herbaceous species, many of them exotic species. The arrival of tree seeds into milpa fields surrounded by forests promotes faster recovery of the forest (Gómez-Pompa and Vázquez-Yanes, 1981).
Increasing dominance of open and disturbed habitats facilitates dispersal and colonization by invasive and weedy species (Uhl et al., 1981; Uhl and Clark, 1983; Saulei and Swaine, 1988; Drake, 1998). Because most of these herbaceous species have long distance dispersal and long-lived seed banks, as their seeds accumulate in the soil of mature remnant forest stands within the humanized mosaic where they are readily available to establish after disturbances ( Guevara and Gómez-Pompa, 1972; Demel and Granström, 1995; Quintana-Ascencio et al., 1996; López-Toledo and Martínez-Ramos, 2011). This compositional change in the seed bank can increase the interference of these species with crops in recently prepared milpa fields and may alter successional sequences (del Castillo and Pérez-Ríos, 2008). Notwithstanding the observed influence of the humanized landscapes on the recovery of the original associations of the tropical rainforest, it should be noted that we do not expect to have a complete representation of the tree species composition in the forest based on seeds arriving to our traps within a year. Whether the temporal and methodological limitations of our study are sufficient to make our point regarding the pernicious effects of extensive humanized habitats, will depend on future long-term studies in the same region aimed to assess a possible extinction debt in the coming decades (Wearn et al., 2012) and the recovery potential contained in widespread secondary growth vegetation (Gómez-Pompa et al., 1972; Chazdon, 2014).
Site successional condition and landscape configuration affected community composition. Species composition of seed dispersal assemblages in the Lacandon rainforest was more similar to species composition of local vegetation than previously reported similarities between soil seed banks and vegetation from a concurrent study (Quintana-Ascencio et al., 1996). As it has been documented in other studies in tropical forests (Franklin and Rey, 2007; Gomes-Freitas et al., 2013; Weerasinghe et al., 2019), small and wind dispersed seeds of generalist herb species dominated seed assemblages in human-controlled environments while large fleshy seeds or fruits of woody species were more common in continuous forest stands or in their border with milpas. Fast decaying seed dispersal kernels of many woody species can help to explain the similarity between seed rain and vegetation (Álvarez-Buylla and Martínez-Ramos, 1990; Drake, 1992; Burrows, 1994). Human impacts on species interactions can alter and reduce dispersal kernels. Seed removal, seed dispersal distances and seed predation processes shape seed dispersal kernels and influence the location and density of seeds deposited (Dirzo et al., 2007). Growing anthropogenic impact including hunting, habitat alteration and fragmentation changes abundance of vertebrates and affects their behavior (Babweteera and Brown, 2010; Bagchi et al., 2011; Bravo, 2012; Brodie and Aslan, 2012; Carrara et al., 2015).
Forest loss and habitat configuration have direct effects on plant recruitment, survivorship and fecundity of many species and indirect long-term negative effects on their population viability through decreasing growth rates and loss of genetic variation (Heywood and Iriondo, 2003; Gagnon et al., 2011). Changes in mortality, growth and recruitment and in forest stand structure with succession in a neighboring region in the Selva Lacandona were rapid during the first five years of succession, decreasing rapidly afterwards and negatively related with initial stand basal area, but relatively independent of initial tree density (Van Breugel et al., 2006). Reduced diversity and differences in species composition have been documented between large forest fragments and smaller fragments within humanized habitat mosaics (Arroyo-Rodríguez and Mandujano, 2006). In these impoverished landscapes pioneer, weedy and alien species increase in abundance in the remnant smaller fragments and along forest edges (Tabarelli et al., 2010; Lôbo et al., 2011). Larger borders with agricultural fields and pastures increase risks of seed predation by scatter-hoarding rodents in disturbed forests (Gutiérrez-Granados, 2011). The extent of forest fragments in the Brazilian Amazon influenced seed abundance and composition of soil seed banks and clearly influenced the potential resilience and regeneration of these sites in the event of natural or anthropic disturbances (Sousa et al., 2017).
We found consistent seed rain assemblages among the two studied localities and seasonal variation in the identity, number of species and seed density captured. Patterns of variation in seed number and abundance among successional communities were commensurate between the relatively independent landscapes of Bonampak-Bethel and Lacanjá-Chansayab, strengthening the generality of our findings. We also observed that species richness was significantly higher during the dry season (April-May) and lowest during the rainy season (August-October). Similar seasonal variation in seeds captured has been documented in other tropical rainforests (Sheldon and Nadkarni, 2013). Habitat and life form affected the seasonal abundance and richness of seeds caught. The change in number of species captured was higher in the forests than in early successional forests and milpa fields, while pastures had almost no variation. Peak abundance of seeds of tree species coincides with the start of the rainy season, while seeds of herbaceous species peak during the dry season. Variation in abundance of seeds of shrubs was lower across seasons.
Cumulative replacement of tropical forest and scattered milpa fields by pastures and extensive commercial agriculture that use exotic agrochemicals and intensive soil management will increasingly compromise forest regeneration and conservation of biodiversity. Until recently, forest regeneration neighboring a traditional agricultural landscape has been dependent on the configuration of a highly diverse vegetation matrix. Our results add to prior evidence indicating that progressively smaller forest fragments surrounded by more homogeneous matrices of increasingly simplified habitats will reduce seed exchange of mid- and late successional species among remnant stands and alter environmental conditions necessary for their establishment. Simultaneously, growing abundance of widespread weedy generalist herbaceous species will modify species interactions in recently disturbed and regeneration habitats which will negatively affect recruitment and establishment of late successional species. The traditional Mayan milpa system relies on successional processes restocking soil nutrients and reducing nuisance plants (Collier, 1975; Hernández-Xolocotzi, 1993; Douterlungne et al., 2010; Nigh, 2008; Nigh and Diemont, 2013). Shorter return times associated with intensive agriculture will decrease crop harvesting rates because of changes in vegetation composition and reduction in the accumulation of plant biomass and nutrients. Growing dominance of intensive productive systems hinders the regeneration potential of secondary habitats linked to tropical rainforest and undermines the sustainability of traditional practices and the economic balance of native people in tropical regions.











 text new page (beta)
text new page (beta)



