Introduction
Amaranthus L. (Amaranthaceae s.s.) is a genus comprising 70-75 species with worldwide distribution. Approximately half of these species are native to America, whereas the remaining ones occurs in the other continents. Various taxa are used as ornamentals, food, and medicine. Some species can escape from cultivation, mainly causing economic impact to agricultural systems with reduction in productivity and crop quality (see e.g., Costea et al., 2001; Hernández-Ledesma et al., 2015; Iamonico, 2015a; Das, 2016).
Amaranthus is critical from the taxonomical point of view due to its high phenotipic variability and hybridization that caused nomenclatural disorders and name misapplications (see e.g., Mosyakin and Robertson, 1996; Costea et al., 2001; Iamonico, 2015a, b, 2016b, 2017, 2020; Iamonico and Galasso, 2018).
Amaranthus polygonoides L., and A. anderssonii J.T. Howell are two taxa described from the tropical regions of America (Galápagos archipelago, the Caribbean, Mexico, and Texas (central and southern USA)) (see e.g., Bayón, 2015), which belong to Amaranthus subgen. Albersia (Kunth) Gren. & Godr. sensuMosyakin and Robertson (1996). According to phylogenetic analyses based on molecular studies (see e.g., Waselkov, 2013; Waselkov et al., 2018) and morphological data (see e.g., Bayón, 2015), these two species are highly variable and closely related. Some nomenclatural issues about these taxa, as well as taxonomic problems, need to be clarified and are dealt with in the present paper. In addition, the following names were considered and discussed: Amaranthus taishanensis F.Z. Li & C.K. Ni, Sarratia berlandieri Moq. (≡ A. berlandieri (Moq.) Uline & W.L. Bray), Scleropus urceolatus Ands. (≡ A. anderssonii J.T. Howell).
This work is part of the ongoing nomenclatural studies of all Amaranthus names, representing the eighth contribution (the first seven papers were on the Linnaean names (Iamonico, 2014a, b), the names linked to the Italian flora (Iamonico, 2016a), Amaranthus gracilis Desf. and related names (Iamonico, 2016b), Moquin-Tandon’s names published in Candolle’s Prodromus (Iamonico, 2016c), the names linked to the Australian flora (Iamonico and Palmer, 2020), and Willdenow’s names (Iamonico, 2020).
Material and Methods
This work is based on examination of specimens deposited at BM, CAS, E, FI, G, GH, IBSC, L, LG, LINN, MA, NAP, NAS, NY, P, PE, PAD, QCA, RO, and US (acronyms according to Thiers, 2020+), and analysis of relevant literature (protologues, taxonomic treatments, morphological, and molecular investigations).
The following characters, which are relevant for the studied taxa according to literature (mainly Henrickson, 1999) and personal experience, were measured on the examined specimens (see “Additional examined material”) using a millimeter ruler and an optical microscope (Carl Zeiss, Göttingen, Germany): length and width of the leaves (the width was measured at the widest part of the blade), size of the seeds (both longest and shortest diameter), ratio length/width of the leaf blades, and fruit dehiscence/indehiscence. The variability of the continuous characters (leaves and seeds) was illustrated by box plots.
The articles cited through the text follow the Shenzen Code, hereafter abbreviated as ICN (Turland et al., 2018).
Results and Discussion
Nomenclature
Amaranthus polygonoides
Linnaeus (1759: 27) validly published the name Amaranthus polygonoides through a short diagnosis (“Calycibus infundibuliformis obtusis singularis”) and the following citation: “Sloan. Jam. I. t. 92. f. 2”. This latter quotation refers to Sloane’s work (1707) about his Voyage to the Islands Madera, Barbados, Nieves, S. Christophers and Jamaica. Kellogg (1988: 160) indicated a specimen preserved at BM in Sloane’s collection (BM-SL no. 2-116) as the type of A. polygonoides. However, this specimen was never seen by Linnaeaus (see Jarvis, 2007: 157-159). The lectotype of the Linnaean name was correctly proposed by Henrickson (1999: 797) based on Sloane’s image “t. 92. f. 2”. Note that the BM specimen was the base for Sloane’s image, so it can be considered as a typotype (see Jarvis, 2007: 22-24) (the illustration is the mirror image of the specimen). The BM specimen is here indicated as the typotype for the first time.
Amaranthus taishanensis
Li and Ni (1981: 116) validly published Amaranthus taishanensis from Eastern China (Province of Shandong) on the basis of Li’s collections in the cities of Taian (locus classicus) and Jinan. The authors morphologically compared the new proposed species with A. angustifolius Lam. (currently accepted as A. graecizans L. s.s.; see Iamonico, 2015a) by the synflorescence structure, the number of the tepals in the pistillate flowers, the number of the stamens, and the length of the fruit. Twenty one years later, Li et al. (2002) clarified the identity of A. taishanensis, stating that this name is a heterotypic synonym of A. polygonoides. I examined an isotype of A. taishanensis at PE and can confirm this synonymy based on the current concept in Amaranthus (see e.g., Mosyakin and Robertson, 2003; Bayón, 2015; Iamonico, 2015a).
Sarratia berlandieri sensu stricto
The name Sarratia berlandieri was validly published by Moquin-Tandon (1849: 268-269) who provided a short diagnosis, a detailed description, and the provenance (“In Mexico inter S. Fernando ae Matamoros”); a specimen (“Berland.! N. 2279”) was also cited.
Bayón (2015: 348) indicated the “holotipo” of this name, a specimen preserved at G (code 00236968), and four “isotipos” at G (barcodes G00236969, and G00236970), GH (barcode GH00036984), and NY (barcode NY991138). Note that Bayón (l.c.), as “no visto” (= not seen) for all these five specimens. Because Moquin-Tandon (1849: 268-269) did not cite any holotype, the term “holotipo” as reported by Bayón (l.c.) is an error to be corrected to lectotype according to the Art. 9.10 of the ICN, whereas isolectotypes must replace Bayón’s “isotipos” for the other cited four specimens. Note that only the G00236969, G00236970, and GH00036984 exsiccata bear original annotations with the date of collection (year “1830”), whereas NY991138 lacks this information. However, because J.-L. Berlandier (a Belgian explorer in North America and Mexico, and traveller of A. P. de Candolle) died in 1851 (just two years later than the publication of the Moquin-Tandon’s treatment of Amaranthaceae in Candolle’s Prodromus (year 1849)) (see Stafleu and Cowan 1976: 196), it is reasonable that the NY undated specimen was also collected before 1849 and seen by Moquin-Tandon (1849). Few months later than Bayón (l.c.), Iamonico (2016c: 109) again proposed to lectotypify the name Sarratia berlandieri on the specimen G00236970 (reported by Iamonico (l.c.) under the SIB identifier 189573/3 according to the G (CHG, 2020c)), also highlighting the occurrence of other three sintypes (G00236968, G00236969 (reported by Iamonico (l.c.) under the SIB identifiers 189573/1, 189573/2 according to the G online Herbarium database), and PH00022348). Although the lectotypification by Iamonico (l.c.) was formally correct, it was superseded by that proposed (and here corrected) by Bayón (l.c.) (see Art. 9.19 of ICN). Note that the PH specimen cited by Iamonico (l.c.), and not reported by Bayón (l.c.), was not formally designated as isolectotype; therefore, this designation is made here (see “Taxonomic Treatment”). In addition, I traced further two specimens at P (barcodes P00609930, and P00609931; images available at MNHN, 2019) originally numbered as “2279” (as reported in the protologue by Moquin-Tandon (1849: 268-269)) but collected in different years than 1830 (the date of sintypes), i.e. 1832 and 1846. As a consequence, these two P specimens cannot be considered for lectotypification purposes.
Scleropus urceolatus by Andersson and Amaranthus anderssonii s.l.
Howell (1933: 95) proposed Amaranthus anderssonii as nomen novum pro Scleropus urceolatus Ands. (year 1853) based on the previously and validly published A. urceolatus Benth. (year 1844). According to Art. 7.4 of the ICN, the type of A. anderssonii is that of S. urceolatus. Andersson (1853: 162-163) provided a diagnosis and a detailed description also indicating the provenance (“Hab. locis graminosis regionis inferioris insulae Indefatigable (Ipse)”). Bayón (2015: 305) indicated the “holotipo” for this name, a specimen preserved at S (S5637), as “no visto” (= not seen). Because Andersson (1853: 162-163) did not cite any holotype, the term “holotipo”, as reported by Bayón (l.c.), is an error to be corrected to lectotype according to the Art. 9.10 of the ICN.
Howell (1933: 96) also validly described a new form of Amaranthus anderssonii, named f. erectus. The new taxon would be distinguished from the nominal one (f. anderssonii) by its habit (erect vs. spreading), length and shape of the perianth (2-2.5 mm, “tubular” vs. about 2 mm, “strongly urceolate”). Bayón (2015: 305) indicated the “holotipo” of this name, a specimen preserved at CAS (barcode CAS203293), as “no visto” (= not seen). Because Howell (1933: 96) reported a specific exsiccatum by the indication of the collection number (9837), the herbarium specimen (CAS) with the barcode (203293) and no duplicates were traced in other herbaria, I agree with Bayón (l.c.) that CAS203293 is the holotype of the name Amaranthus anderssonii f. erectus (see also the considerations by McNeill (2014) about the holotype citations).
Historical background
The taxa here considered were discussed and differently treated through time by various authors.
Moquin-Tandon (1849: 268-270) treated the taxa polygonoides and berlandieri as part of two different genera, i.e. Sarratia Moq. (a new proposed genus in Candolle’s Prodromus, with the new species S. berlandieri Moq.) and Amblogyna Raf. (including the species A. polygonoides (L.) Raf.). On the basis of Moquin-Tandon’s treatment, these two genera would differ from each other by the number of the tepals in the pistillate flowers (5 (Sarratia) vs. 3 Amblogyna)), the number of the stamens (5 vs. 3), lobes of the tepals (unequal vs. equal), the number of the stigmas (3-4 vs. 2-3), and the fruit (indehiscent vs. dehiscent).
Uline and Bray (1894: 268-270) accepted Amaranthus berlandieri and A. polygonoides as separated species. They placed A. berlandieri in the group of monoecious species with 2-3 stamens and indehiscent fruits (together with A. urceolatus Benth.), whereas A. polygonoides was included in the group with dehiscent fruits (together with A. fimbriatus Benth., A. pringlei S.Watson, and A. squarrulosum (A. Gray) Uline & W.L. Bray). Amaranthus anderssonii was not mentioned by Uline and Bray (l.c.).
Thellung (1914: 350-354) recognized Amaranthus polygonoides and A. berlandieri as separated taxa, proposing the subspecies rank for the taxon berlandieri (a new proposed combination in the Synopsis der Mitteleuropäischen Flora). Amaranthus anderssonii was not mentioned.
Standley (1917: 102, 104-105), in his treatment of Amaranthus for the North American Flora, distinguished Amaranthus polygonoides from A. berlandieri by the fruit (dehiscent vs. indehiscent, respectively) and the number and shape of the leaves (“suborbicular to obovate to oval...not crowed” vs. “oblong-lanceolate... crowed at the end of the branches”, respectively). Amaranthus anderssonii was not mentioned.
Eliasson (1985: 416-418) observed that Amaranthus anderssonii is similar to A. berlandieri sharing both vegetative and sexual characters (stem pubescence, synflorescence structure, shape and structure of the tepals, number of stamens, seed ratio length/width, and ornamentation), but differ from each other by the habit and distribution. Amaranthus polygonoides was not mentioned by Eliasson (l.c.).
Henrickson (1999: 794-799) widely discussed the names Amaranthus polygonoides and A. berlandieri, observing that the dehiscence/indehiscence of the fruits is not a constant character and stating “Specimens attributable to Amaranthus berlandieri are mostly indehiscent... but a few specimens did not show a distinct encircling line below the rugate cap of the utricle. In the 76 collections of A. polygonoides with mature fruit examined, 36 were noted to be actually circumscissile... and 35... were clearly indehiscent”. Concerning the leaves, Henrickson (1999: 796-797) highlighted a partial overlapped range of the ratio length/width of the blades, but no geographic separation occurs between the two taxa. He, therefore, concluded that the “Recognition of two taxa... would be quite arbitrary”.
Bojian et al. (2003) just mentioned Amaranthus polygonoides in a note under A. taishanensis, which was considered different from the Caribbean taxon and it would be more related to A. blitum L. (“one of us (Clemants) notes that further study might ally A. taishanensis with A. blitum, from which it seems to differ only by having five sepals”).
Mosyakin and Robertson (2003) reported Amaranthus berlandieri as synonym of A. polygonoides, following Henrickson (1999). However, they stated that “The subspecies rank may be more appropriate for A. berlandieri, as was suggested by Thellung (1914). The relationships between these taxa of the A. polygoniodes aggregate require additional study”.
In my recent paper on the Moquin-Tandon’s name in Amaranthus (Iamonico, 2016c), I provisionally accepted the Henrickson’s (1999) scheme, but I stated that “further studies are necessary to be carried out”.
Taxonomic remarks
Morphology
The results obtained in the present study confirm that dehiscence/indehiscence of the fruit cannot be considered as a constant character, as already highlighted by Henrickson (1999). On the contrary, the box plots show a separation of the studied taxa based on leaf blade and seed sizes.
Concerning the size of the leaf blades, a difference in the ratio length/witdh occurs among Amaranthus anderssonii, A. berlandieri, and A. polygonoides. The lower ratio charaterizes A. anderssonii (range 1.25-1.67), followed by A. polygonoides which ratio value (1.30-2.60) partially overlaps with those of A. berlandieri (2.60-4.20) (Fig. 1). Between A. polygonoides and A. berlandieri there is also a difference in the shape of the leaves which is, respectively, ovate to obovate and lanceolate.

Figure 1 Box plots for the ratio leaf length/leaf width. Medians (horizonthal bar), 25th and 75th percentiles, and maximum and minimum (wiskers) of selected features are shown. ande = Amaranthus anderssonii J.T. Howell; berl = A. berlandieri (Moq.) Uline & W.L. Bray; poly = A. polygonoides L.
Amaranthus anderssonii displays smaller seeds, having the longest diameter as 0.69-0.83 mm long and the shortest diameter ranging from 0.50-0.63 mm (Fig. 2A-B). On the other hand, A. berlandieri and A. polygonoides are more similar to each other, because the 25th and 75th percentiles of the longest diameter of the seed slightly overlap between them (total ranges are 0.81-0.87 mm for A. berlandieri vs. 0.80-1.00 mm for A. polygonoides); the variability of the shortest seed diameter of A. polygonoides is almost completely included in that of A. berlandieri (vs. 0.63-0.71 mm for A. berlandieri, and 0.68-0.71 mm for A. polygonoides). Note, however, that A. berlandieri shows narrower seeds (Fig. 2B).

Figure 2 Box plots for the seeds: A. seed lenght (measurements in mm); B. seed width (measurements in mm). Medians (horizonthal bar), 25th and 75th percentiles, and maximum and minimum (wiskers) of selected features are shown. ande = Amaranthus anderssonii J.T. Howell; berl = A. berlandieri (Moq.) Uline & W.L. Bray; poly = A. polygonoides L.
Phylogenetic analysis of molecular data
According to the recent molecular phylogenetic study by Waselkov et al. (2018), the only one among the published molecular papers on Amaranthus that includes Amaranthus anderssonii (A. berlandieri was considered as synonym of A. polygonoides), it was clearly shown that A. polygonoides (a Caribbean species) is the most closely related taxon to the endemic A. anderssonii (from Galapágos). In fact, these two species form a strongly supported clade (informally named “Anderssonii clade”; bootstrap values = 100 (nuclear markers), and 98 (chloroplast regions); see Waselkov et al., 2018: 446, Fig. 1 A-B). Both A. anderssonii and A. polygonoides are part, in turn, of the unresolved “Galapágos clade” as informally named by Waselkov et al. (2018). The phylogenetic tree based on nuclear markers (Waselkov et al., 2018: 446, Fig. 1A) shows that the species related to the “Anderssonii clade” were A. tamaulipensis Henrickson (the earliest divergent lineage in the clade formed by tamaulipensis+anderssonii+polygonoides, with a bootstrap value = 95), and A. crassipes (three accessions forming a strongly supported clade (bootstrap values = 100)). On the other hand, the tree derived from the analysis of the chloroplast regions (Waselkov et al., 2018: 446, Fig. 1B), places A. tamaulipensis as separated species, but related to both Anderssonii and crassipes clades.
The nuclear markers tree (Waselkov et al., 2018: 446, Fig. 1A) shows the “Anderssonii clade” + A. tamaulipensis and the crassipes-clade forming a moderately supported clade (bootstrap value = 88). The whole group (tamaulipensis+anderssonii+polygonoides+crassipes) is sister group to the large clade (bootstrap values = 84-100) informally named “ESA + South American” (ESA = Eurasian/South Africa/Australian; see Waselkov et al., 2018: 447, caption of Fig. 1). Note that the other taxa belonging to the “Galápagos Clade” sensuWaselkov et al. (2018: 446) form a well-supported clade that is not closely related to the tamaulipensis+anderssonii+polygonoides+crassipes group. The tree derived from the chloroplast regions shows the Anderssonii+crassipes+tamaulipensis clade with low support; however, relationships among major clades within Amaranthus are not resolved and/or have moderate to low support values. Simplified phylogenetic relationships within Amaranthus based on Waselkov et al. (2018) are shown in Fig. 3.
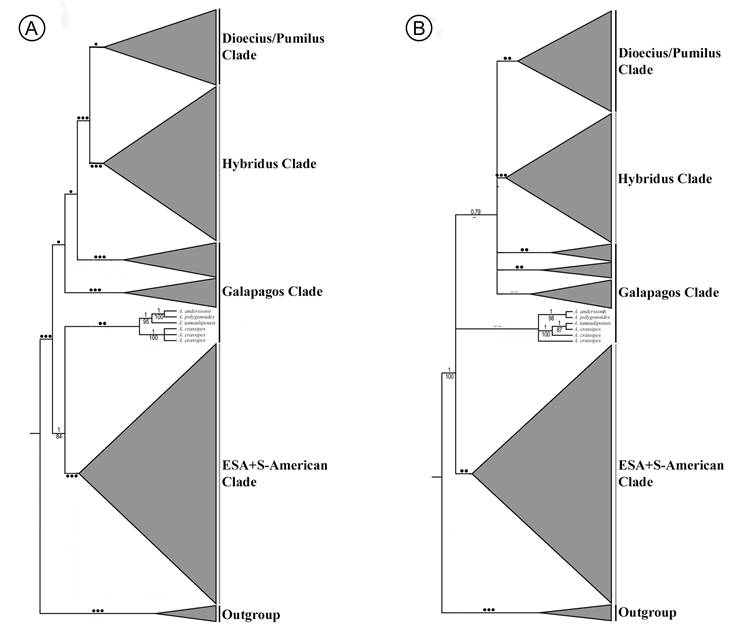
Figure 3 Phylogenetic relationships within Amaranthus L., based and simplified from Waselkov et al. (2018): A. nuclear markers (ITS, A36, G3PDH, and Waxy), B. chloroplast regions (trnL5′-trnL3′ and matK/trnK). Triangle size is proportional to sample size. Legend: ••• = high support (bootstrap values: 100-95), •• = medium support (bootstrap values: 94-75), • = low support (bootstrap values: 7-50), -- = no bootstrap value provided by Waselkov et al. (2018).
Taxonomic conclusion
The three taxa here studied are currently recognized as two separated species, A. anderssonii and A. polygonoides (see e.g., Bayón, 2015: 305, and 348, respectively). The taxon berlandieri is synonymized with the latter name, although various authors (e.g., Mosyakin and Robertson, 2003; Iamonico, 2016c) highligthed that additional studies are required to clarify the correct relationship between these taxa.
The results obtained in the present study showed that the taxa anderssonii, berlandieri, and polygonoides can be distinguished based on some morphological characters (i.e., leaf blade shape and length/width ratio, as well as seed size). The ranges of these characters just partially overlap among the taxa, especially between berlandieri and polygonoides. Molecular phylogenetic analyses by Waselkov et al. (2018) revealed a relationship between A. anderssonii and A. polygonoides. Moreover, there is a geographic separation: the taxon anderssonii is endemic to Galápagos archipelago, the taxon polygonoides occurs in the Caribbean and coastal areas of Texas, and the taxon berlandieri occurs in Mexico and inland areas of Texas.
Based on the current definition of infraspecific ranks, subspecies, varieties, and forms, those taxa can be treated as groups that are distinguishable based on morphology (despite having overlapping ranges), with a geographical, ecological and/or reproductive isolation (see e.g., Hardion et al., 2017). The group anderssonii-berlandieri-polygonoides represents a case that can be treated as a single species with different infraspecific taxa. Because there is high morphological similarity among them, and considering the adjacent distribution areas between berlandieri and polygonoides as compared to anderssonii, I here propose to treat the latter taxon at subspecies rank of A. polygonoides (the earliest published name at species level), whereas the taxon berlandieri will be recognized at variety level (new nomenclatural change, see below) of the subsp. poligonoides (automatically estabilished according to Art. 26.3 of the ICN). Note that there is an earlier basionym for A. anderssonii (Scleropus urceolatus), so it must be used as the base of my proposed combination (see below).
Amaranthus polygonoides L. subsp. urceolatus (Ands.) Iamonico, comb. et stat. nov.
≡ Scleropus urceolatus Ands., Kong. Svenska Vetensk. Acad. Handl., n.s. 3(41): 162-163. 1853.
≡ Amaranthus anderssoniiJ.T.Howell, Proc. Calif. Acad. Sci., ser. 4 21: 95. 1933 (Fig. 4). TYPE: ECUADOR. Galápagos, Galapágos-öarna, Indefatigable (Isla Santa Cruz), X.1852, N. J. Andersson and J. Nils s.n. (S-R5637!, lectotype designated by Bayón, 2015: 305 (as “holotipo”, here corrected according to the Art. 9.10; image of the lectotype available at JSTOR, 2019a).

Figure 4 Specimen of Amaranthus polygonoides subsp. urceolatus (Ands.) Iamonico (Galápagos, Island Española, J. T. Howell 8703, CAS368651).
= Amaranthus anderssoniiJ.T. Howell f. erectus J.T. Howell, Proc. Calif. Acad. Sci., ser. 4, 21: 96. 1933. TYPE: ECUADOR. Galápagos, Isla Duncan, 7.V.1832, J. T. Howell 9837 (holotype: CAS203293!; image of the holotype available at CAS Botany Collection Database (CAS, 2020)).
Distribution: endemic from Galápagos archipelago (Islands Baltra, Española, Santa Cruz, and Santiago; Eliasson, 1985: 432; Bayón, 2015: 305). Not known elsewhere.
Amaranthus polygonoides L., Pl. Jamaic. Pug.: 27. 1759 subsp. polygonoides var. polygonoides
≡ Roemeria polygonoides (L.) Moench, Methodus (Moench): 341. 1794.
≡ Amblogyna polygonoides (L.) Raf., Fl. Tellur. 3: 42. 1837.
≡ Albersia polygonoides (L.) Kunth, Fl. Berol. ed.2, 2: 144. 1838.
≡ Amblogyna polygonoides Danzell & A. Gibson., Bombay Fl.: 219. 1861, nom. illeg. (Art. 53.1 of ICN) (Fig. 5). TYPE: (icon) t. 92, f. 2 “Blitum polygonoides viride seu ex viridi et albo variegatum polyanthos” in Sloane (1707: 144) (lectotype designated by Hendrickson (1999: 797); image of the lectotype available at BHL, 2020); typotype (here indicated) BM-SL no. 2: 166 (NHM, 2020).
= Amaranthus taishanensisF.Z. Li & C.K. Ni, Acta Phytotax. Sin. 19(1): 116. 1981. TYPE: CHINA. Shandong, Taian, 26.VI.1979, F. Z. Li 00116 (holotype: SDFS non vidi fideLi et al. (2002: 384); isotypes: PE00024069! (CVH, 2004-2015), and SDNU non vidi fide Li and Ni (1981: 116, reported as “herbarium of Forestry School of Shantung Province”), and Li et al. (2002: 384)).
= Amaranthus verticillatus Pav. in Moquin-Tandon (1849: 268, 270), nom. inval. pro syn. of Amblogina polygonoides (≡ Amaranthus polygonoides) (Art. 36.1b of ICN).

Figure 5 Specimen of Amaranthus polygonoides L. subsp. polygonoides var. polygonoides (Antigua, St. John’s, J. A. Shafer 35, NY1373866).
Distribution: native to the Caribbean and the coastal areas of Texas (Eliasson, 1985: 432). Out of the native range Amaranthus polygonoides s.l. was recorded in Europe as a casual alien species (Iamonico, 2011), in Africa as naturalized in Egypt (Iamonico, 2015b), and in Asia (China; Bojian et al., 2003 under A. taishanensis).
Amaranthus polygonoides L. subsp. polygonoides var. berlandieri (Moq.) Iamonico, comb. et stat. nov.
≡ Sarratia berlandieri Moq., Prodr. (DC.) 13(1): 268-269. 1849.
≡ Amaranthus berlandieri (Moq.) Uline & W.L. Bray, Bot. Gaz. 19: 268. 1894.
≡ Amaranthus polygonoides L. subsp. berlandieri (Moq.) Thell., Syn. Mitteleur. Fl. 5(1): 352. 1919 (Fig. 6). TYPE: MEXICO. Tamulipas, Ranchos del Mojete de Matamoros à San Fernando, X.1830, J.-L. Berlandier 2279 (G00236968!, lectotype designated by Bayón, 2015: 348 (as “holotipo”, here corrected according to the Art. 9.10; image of the lectotype available at CHG, 2020a); isolectotypes at G00236969 (CHG, 2020b), G00236970 (CHG, 2020c), GH00036984 (CHG, 2020d), NY991138 (NYBG, 2020) (designated by Bayón, 2015: 348 (as ”isotipos”, here corrected according to the Art. 9.10), and PH00022348 (here designated; JSTOR, 2019b).

Figure 6 Specimen of Amaranthus polygonoides subsp. polygonoides var. berlandieri (Moq.) Iamonico (USA, Texas, Cameron, W. R. Carr 29880, NY3042458).
Distribution: native to Mexico and inland areas of Texas (Eliasson, 1985: 432).
A diagnostic key for the infraspecific taxa of Amaranthus polygonoides, as recognized in the present paper, is shown here. The distribution areas are also reported.
1a. Ratio length/width of the leaf blades 1.30-4.20; seeds with longest diameter 0.80-1.00 mm long, and shortest diameter 0.63-0.71 mm long ..................................................................................... subsp. polygonoides
2a. Ratio length/width of the leaf blades (1.30-)1.60-2.10(-2.60), blades ovate to obovate; seeds with longest diameter 0.80-1.00 mm long, and shortest diameter 0.68-0.71 mm long; Caribbean and coastal areas of Texas ................................................ var. polygonoides
2b. Ratio length/width of the leaf blades (2.60-)2.90-3.90(-4.20), blades lanceolate; seeds with longest diameter 0.81-0.87 mm long, and shortest diameter 0.63-0.71 mm long; Mexico and inland areas of Texas .................................. var. berlandieri (Moq.) Iamonico
1b. Ratio length/width of the leaf blades (1.25-)1.30-1.60(-1.67); seeds with longest diameter 0.69-0.83 mm long, and shortest diameter 0.50-0.63 mm long; Galápagos Islands ................. subsp. urceolatus (Ands.) Iamonico
Additional examined material: Amaranthus polygonoides subsp. polygonoides var. berlandieri. MEXICO. De S. Fernando à Matamoros, 1832, J.-L. Berlandier 2279 (P00609930); 1846, J.-L. Berlandier 2279 (P00609931). UNITED STATES OF AMERICA. Texas, Travis, 20.V.1872, E. Hall 534 (NY3364372); Nueces, 23-30.III.1894, A. A. Heller 1487 (NY3364377); Duval, 1888, M. B. Croft 223 (NY3364371); Austin, 15.VII.1936, B. C. Tharp s.n. (GH01929204); Starr, 1 mile E. of Davis Oil Fileld Cafe, 11.XII.1940, V. L. Cory 36134 (GH01929193); Hidalgo, Rio Grand Valley, 02.VIII.1942, M. L. Walker 32 (NY3364367); Aransas Refuge, 10.I.1944, V. L. Cory 45967 (GH01929197); Cameron, on waste grounds in The Lower Rio Grande Valley at Brownsville, 02.X.1965, R. Runyon 5936 (GH01929194); Cameron, agricultural landscape on delta of Rio Grande, 20-25 ft., 19.VII.2011, W. R. Carr 29880 (NY3042458).
Amaranthus polygonoides subsp. polygonoides var. polygonoides. ANTIGUA AND BARBUDA. Antigua, near St. John’s, I.1907, J. A. Shafer 35 (NY1373866); Antigua, Gunthorpes, 06.X.1937, H. E. Box 1140 (US01084457). BAHAMAS. Great Inagua, Mathew town, 15.X.1904, G. V. Nash 1079 (NY1373847). BELGIUM. Wallonia, Béthane (Goé), Adv. Laineir dans la cour du lavoir, 16.IX.1959, N. Cnops s.n. (sub. A. vulgatissimus Spegaz.), rev. J. Duvigneaud and J. Lambinon (LG). CHINA. Su Xian, 1983, Xuewen Wang (王学文) 1604 (IBSC0190235); Xiyan Yanzhou, Shandong, in fields, 50 m, 28.VI.2004, C. Yong Guo 15030101 (P00871046); ibidem (MA896135); Shimenshan, Qufu, Shandong, near water, 300 m, 06.VII.2004, Cheng Yong Guo 1501116 (P00871059); Jiangsu, Xuzhou, 18.VIII.2011, L. Qixin, Xiong Yuning (刘启新,熊豫宁) 10-1-117 (NAS00591444, NAS00591445); Jiangsu, Xuzhou, 01.VII.2013, L. Qixin, Xiong Yuning (刘启新,熊豫宁) 3291 (NAS005914429, NAS00591446). CUBA. La Habana, La Universidad, 09.XI.1921, E. L. Eckman 13470 (NY1373862). CURAÇAO. Mt. Pleasant, 20-27.III.1913, N. L. Britton and J. A. Shafer 3123 (US01099924). DOMINICAN REPUBLIC. Hispaniola, Santiago de los Caballeros, 17.IV.1954, J. J. Jiménez Almonte 2653 (US00847576); Pedernales, Aguas Negras, 24-27.VI.1975, A. H. Liogier 23282 (NY1373850); La Altagracia, in town of Boca de Yuna, at W side of Rio Duey (Rio Yuma) at Mar Caribe, 8 m, 24.II.1981, T. A. Zanoni 10603 (NY13738653); La Altagracia, Isla Sona, 29.VI.1981, T. A. Zanoni 15162 (NY1373857). GUADALOUPE. Terrain sablonneux de la Desirade, 1893, A. Duss 2793 (NY1373852). HAITI. L’Artibonite, Southeast of Gros Morne, Vicinity of Gros Morne, Départment de l’Artibonite, 235 m, 1926, E. C. Leonard 9973 (NY1373861). ITALY. Abruzzo, Giulia, sine die (ante 1831), M. Tenore s.n. (NAP); Campania, Golfo di Napoli, sine die (ante 1817), G. Gussone s.n. (NAP-GUSS); Puglia, Barletta, 1822, A. Bruni s.n. (G00189974); Marche, Ascoli, sine die (century XIX), sine collectore s.n., det. G. Lusina (RO); Marche, Porto d’Ascoli, VII.1839, P. Sanguinetti s.n., det. G. Lusina (RO); Marche, Senigallia, sui binari della stazione ferroviaria, 30.VI.1946, A. Bettini s.n. (FI(3)); Marche, da semi di piante di Senigallia sul mio terrazzo in vaso, 22.VII.1949, A. Bettini s.n. (FI); Lombardia, nasce nell’Orto botanico di Mantova spontaneo, sine die (XIX century), sine collectore s.n. (PAD); Lombardia, nelle sabbie e negli incolti nel mantovano, III.1877, Barbieri s.n. (FI); Toscana, Giardino dei Semplici, 1820, sine collectore s.n. (FI); Toscana, Hort. Bot. Bonon, 1829, P. Bubani s.n. (FI); Toscana, H Pisano, 1839 ect., sine collectore s.n. (FI); Toscana, H. Bot. Mus. Flor., 27.VI.1857, sine collectore s.n. (FI); Veneto, Orto Botanico di Padova, coltivato, VIII.1896, Adr. Fiori s.n. (FI). THE NETHERLANDS. Brabant, Tilburg, 19.IX.1955, J. H. Kern et al. 0823314 (L), 0823316 (L), 0823317 (L); Brabant, Tilburg, 01.IX.1958, J. H. Kern, S. J. van Ooststroom and T. V. Reichgelt 0823315 (L); Brabant, Stortterrein afval Tilburg e Wolwasserij, 13.IX.1962, J. Dorgelo 0823313, det. F. Adema (L); Sine loc., 1964, P. Aellen 00295340 (E). UNITED STATES OF AMERICA. Florida, Key West, II.1846, F. Rugel 31 (GH01929190); Texas, 7.5 miles SSW of Woodsboro... in disturbed clay at the gate to the lake Trap, river Pasture, 24.IX.1978, S. R. Hill 7905 (NY3364376).
Amaranthus polygonoides subsp. urceolatus (≡ A. anderssonii). ECUADOR. Galápagos, Isla Española, Punta Suarez, 3 m, V.1975, H. H. Werff 2055 (QCA4061!); Galápagos, Galapágos - öarna, Indefatigable (Santa Cruz Island), 1853, N. J. Andersson s.n. (S07-12528!); Galápagos, Island Española, Gardner Bay, 20.IV.1932, J. T. Howell s.n. (CAS368651!); Galápagos, Santiago Island, James Bay, James Island, 05.VI.1932, J. T. Howell s.n. (CAS368652!); Galápagos, Santiago Island, Sullivan Bay, James Island, 13.VI.1932, J. T. Howell 10053 (CAS368653!); Galápagos, Isla Española, Punta Suarez, 10 ft, V.1975, van der Werff 2055 (CAS368656!).











 text new page (beta)
text new page (beta)



