Introduction
In the Mexican Pacific, dinoflagellate harmful algal blooms (HABs) are frequent (Band-Schmidt et al., 2011). Two of them, Gymnodinium catenatum Graham and Pyrodinium bahamense L. Plate var. compressum (Böhm) Steidinger, Tester & Taylor produce saxitoxins and cause paralytic shellfish poisoning (PSP) (Cortés-Altamirano et al., 1993, 1995, 2004; Gómez-Aguirre, 1998; Cabrera-Mancilla et al., 2000; Gárate-Lizárraga et al., 2001, 2006, 2007, 2012, 2015, 2016; Hernández-Becerril et al., 2007; Meave del Castillo et al., 2008; Díaz-Ortiz et al., 2010; Band-Schmidt et al., 2011; Meave del Castillo and Zamudio-Resendiz, 2014, 2018).
Gymnodinium catenatum (Gc) is a naked or athecate species that forms mobile chains and was described for the first time from the Gulf of California (Graham, 1943). It was initially thought that its distribution was confined to the coasts of Mexico, Japan, southern Europe and Tasmania (Hallegraeff and Bolch, 1992), but now it is considered cosmopolitan, with a worldwide distribution along the coasts of more than 23 countries. At the global level, this species began producing HABs in 1964 in La Jolla, California, USA (Holmes et al., 1967) and approximately a decade later the first European HAB was reported, in NW Spain, off the coast of Galicia (Estrada et al., 1984).
In Mexico, Gc is widely distributed in the Mexican Pacific, from Bahía Magdalena, through the Gulf of California, in the subtropical portion, and in the tropical portion to the coasts of Oaxaca (Band-Schmidt et al., 2010; Gárate-Lizárraga et al., 2015; Meave del Castillo and Zamudio-Resendiz, 2018; Fig. 1, Table 1). It is also the most studied HAB species in Mexico from ecological and physiological points of view, especially in relation to the populations of the northern Pacific region (Band-Schmidt et al., 2011).
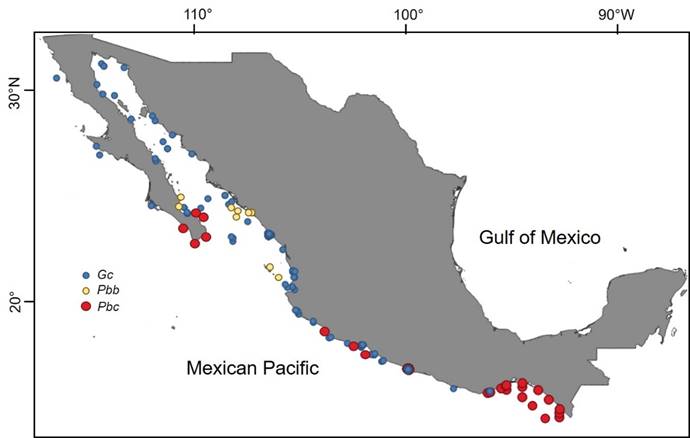
Figure 1: Distribution in the Mexican Pacific of Gymnodinium catenatum Graham (Gc, blue circles), and of the two varieties of Pyrodiinium bahamense Plate: P. bahamense var. compressum (Böhm) Steidinger, Tester et Taylor (Pbc, red circles) and P. bahamense Plate var. bahamense (Pbb, yellow circles). Prepared with own records and literature from 1935 to 2010, through the CONABIO (Comisión Nacional para el Conocimiento y Uso de la Biodiversidad database project HJ014 under the responsibility of Meave del Castillo. Map made with QGIS 3.2 (QGIS, 2018).
Table 1: HABs of Gymnodinium catenatum Graham reported for the Mexican Pacific. The date of HABs are recorded, as well as the maximum abundance, water temperature, and levels of toxicity reached evaluated by mouse bioassay. References: 1) Graham (1943); 2) Mee et al. (1986); 3) Cortés-Altamirano and Nuñez-Pastén (1991); 4) Cortés-Altamirano et al. (1995); 5) Alonso-Rodríguez (1998); 6) Ramírez-Camarena et al. (1999); 7) Cortés-Altamirano et al. (1999); 8) Cabrera-Mancilla et al. (2000); 9) Herrera-Galindo (2000); 10) Morales-Blake et al. (2000); 11) Figueroa-Torres and Zepeda-Esquivel (2001); 12) Gárate-Lizárraga et al. (2001); 13) Gárate-Lizárraga et al. (2004); 14) Cortés-Altamirano et al. (2006); 15) Rodríguez- Palacio et al. (2006); 16) Zepeda-Esquivel and Meave del Castillo (2007); 17) Gárate-Lizárraga et al. (2009); 18) Bustamante-Gil (2011); 19) Gárate- Lizárraga et al. (2011); 20) Quijano-Scheggia et al. (2012); 21) Rojas-Herrera et al. (2012); 22) COFEPRIS (2010); Ps=Present study. *=G. catenatum was present together with Pyrodinium bahamense var. compressum L. Plate, species also producing saxitoxin.
| Locality (from north to south) | Date | Abundance (cells l-1) |
Temp. (°C) |
Toxicity (μg SXTeq.100 g-1) |
Reference |
| Northern Gulf of California | March/1939 | Almost 1×106 | 14-17 | 1 | |
| Concepción Bay, Baja California | March/1990 | 1.8-3×103 | 12 | ||
| Concepción Bay, Baja California | May/1999 | >570×103 | 64-298 | 13 | |
| Libertad Port, Sonora | March/1981 | 190×103 | 7 | ||
| Kun Kaak Bay, Sonora | May/2003 | 829×103 | 14 | ||
| Mazatlán Bay, Sinaloa | 25/April/1979 | 1.15×106 | 21.6 | 20-7640 | 2 |
| Mazatlán Bay, Sinaloa | March-April/1985 | 35-544×103 | 20-21 | 3 | |
| Mazatlán Bay, Sinaloa | 01/April/1986 | 65×103 | 22.04 | 3 | |
| Mazatlán Bay, Sinaloa | Feb.-March/1988 | 170-360×103 | 20.64 | 3 | |
| Mazatlán Bay, Sinaloa | 21/April/1988 | 940×103 | 22.34 | 3 | |
| Mazatlán Bay, Sinaloa | 31/March/1994 | 1×106 | 20.94 | 4 | |
| Mazatlán Bay, Sinaloa | 05/April/1997 | 5×103 | 21 | 6 | |
| Mazatlán Bay, Sinaloa | 01/Oct./1997 | 3.8×106 | 32.9 | 6 | |
| Mazatlán Bay, Sinaloa | 09/March/1995 | 49×103 | 22.54 | 5 | |
| Mazatlán Bay, Sinaloa | 10/June/1996 | 34×103 | 21.14 | 5 | |
| Mazatlán Bay, Sinaloa | April/2001 | 15×103 | 39 | 13 | |
| Manzanillo Bay, Colima | March-May/1999 | 3.5×106 | 25 | 10 | |
| Puerto Interior, Colima | Abril/1999 | 3.8×106 | 11 | ||
| Puerto Interior, Colima | Dec./1999 | 2.5×106 | 11 | ||
| Puerto Interior, Colima | March-April/2000 | 3.3×106 | 11 | ||
| Manzanillo Bay, Colima | April/2007 | 3.8×106 | 235 | 16 | |
| Manzanillo Bay, Colima | May/2010 | 3.6×106 | 22.5 | 3648 | 20 |
| Lázaro Cárdenas, Michoacán | Nov./2005 | 560×103 | 32 | 15 | |
| Petacalco - Vicente Guerrero, Guerrero | Dec./2010 | 129×103 | 146-536* | 19 | |
| Acapulco Bay, Guerrero | March/1999 | 37.6×103 | 156 | 8 | |
| Acapulco Bay, Guerrero | Dec./2005 | 1.6×106 | 27 | 217 | 17 |
| Acapulco Bay, Guerrero | Jan.-Feb./2006 | 10×106 | 112 | 17 | |
| Acapulco Bay, Guerrero | Dec./2007 | 1.9×106 | 1152 | 17 | |
| Acapulco Bay, Guerrero | Oct./2009 | 6×103 | 18 | ||
| Acapulco Bay, Guerrero | Oct.-Nov./2009 | 14.9×103 | 29.5 | 21 | |
| Acapulco Bay, Guerrero | Nov./2010 | 188×103 | 24-27 | 392-739* | Ps, 22 |
| Huatulco Bays, Oaxaca | Oct./1998 | 10×106 | 8 | 9 |
In the Mexican Pacific, there are records of Gc causing poisonings and human deaths in the Mazatlán Bay since April 1979, with densities of 1.15×106 cells l-1 and toxicity of up to 7640 μg STXeq.100 g-1 (Mee et al., 1986). More recently, HABs with higher cell densities (3.8×106 cells l-1), and mollusc toxicity levels of 3648 μg STXeq.100 g-1 have been reported (Gárate-Lizárraga et al., 2009; Band-Schmidt et al., 2010; Quijano-Scheggia et al., 2012). Specifically, in Acapulco Bay, blooms have been reported since 1995 (Diaz-Ortiz et al., 2010) with densities of up to 13×106 cells l-1, and saxitoxin concentrations of 1165 μg STXeq.100 g-1.
Pyrodinium bahamense var. compressum (Pbc) is a thecate dinoflagellate that also forms motile chains, common in the tropical portions of the Pacific and Indian Oceans, and recognized as the cause of 97% of all cases of human illness by PSP; it was estimated that by 1995 there were 2323 people affected (Corrales and Maclean, 1995). Pyrodinium L. Plate is a monotypic genus with two varieties; var. compressum was originally described as a form of the species by Plate (1906) due to its compressed cells, i.e. shorter than wide and only one antapical spine; and later it was validated as a variety (Steidinger et al., 1980). The bahamense L. Plate variety occurred as solitary or paired organisms, with a more elongated form, and was generally considered as innocuous, occurring mainly in the subtropical-tropical portion of the Atlantic Ocean (Steidinger et al., 1980; Taylor et al., 2004). However, Landsberg et al. (2006) found that var. bahamense also produces saxitoxins and that they are accumulated in puffer fish. Recently, shellfish with PSP in Florida due to Pyrodinium bahamense were recorded (Lewitus et al., 2014).
Steidinger et al. (1980) listed six characteristics to differentiate both varieties, which also presented a geographical differentiation, e.g., var. compressum occurred in the Pacific Ocean in both western and eastern portions, while var. bahamense occurs mainly in the Atlantic and Gulf of Mexico (Badylak et al., 2004; Usup et al., 2012), although Martínez-López et al. (2007) and Morquecho (2008) have found the var. bahamense in the Mexican Pacific, in shallow and protected sites of the Gulf of California and Osorio-Tafall (1942) in offshore areas in front of the coast of the state of Chiapas. The coexistence of both varieties has also been pointed out in the Eastern Pacific HAB ( Vargas-Montero and Freer, 2003; Gárate-Lizárraga and González-Armas, 2011; Gárate-Lizárraga et al., 2015). However, in these cases, or- ganisms recognized as var. bahamense could correspond to rare morphotypes of the var. compressum.
HABs of Pb that occurred in the southern portion of the Mexican Pacific, including Acapulco Bay, have been reported as var. compressum due to its morphology and high toxicity (Meave del Castillo et al., 2008). Nowadays, the varieties are disputed and generally invalidated, as several taxonomists do not accept their differentiation, due in part to the result of the study by Balech (1985), who showed that there were no differences in the plates of the theca in populations of both varieties. More recently, saxitoxins production was detected in cultivated and wild Pb populations off the coast of Florida, identified as var. bahamense (Landsberg et al., 2006). Likewise, the recent detailed study of the morphology and morphometry of both varieties by Mertens et al. (2015) invalidates all the previous criteria indicated by Steidinger et al. (1980) in order to differentiate the varieties; however, the molecular sequences of the Large Subunit Ribosomal Ribonucleic Acid (LSU rRNA) show constant genetic differences (ribotypes) between the varieties.
Worldwide Pbc began to have relevance as HAB since the 1970s, when in 1972 at Port Moresby in Papua, New Guinea, it caused the death of three children (Maclean, 1989b). However, it was not until 1987 that HABs of this dinoflagellate were recorded in the American Pacific, specifically along the coasts of Guatemala (Rosales-Loessener, 1989a), where it caused the death of 26 people and 187 were hospitalized by consumption of the clam Donax (Amphichaena) kindermanni Philippi. In the American Pacific, HABs of Pbc currently occur in several countries of Central America and the southwestern portion of Mexico (Table 2). In the Mexican Pacific, Pbc was reported from 1935-1936 onwards (as P. schilleri (Matzenauer) Schiller, =P. bahamense) on the coasts of the Gulf of Tehuantepec, although at low densities (Osorio-Tafall, 1942). Its first HAB was registered from October 1989 to February 1990, in the Gulf of Tehuantepec, off the coast of Chiapas and Oaxaca (Hernández-Becerril et al., 1992; Cortés-Altamirano et al., 1993). Since that event until 2010, at least six other HABs have occurred in the southern portion of the Mexican Pacific (Meave del Castillo et al., 2008; Gárate-Lizárraga and González-Armas, 2011; Herrera-Galindo et al., 2015) (Fig. 1, Table 2). Such events tend to occur with time intervals between 3-6 years (Ronsón-Paulín, 1999, Hernández-Becerril et al., 2007, Meave del Castillo et al., 2008). Pbc HABs in Mexico generally start off the coast of Chiapas and move northwest along the coastline to the states of Oaxaca, Guerrero and Michoacán (Orellana-Cepeda et al., 1998; Meave del Castillo et al., 2008; Meave del Castillo and Zamudio-Resendiz, 2018), and they have even reached the region of Los Cabos, Baja California Sur (BCS), at the entrance to the Gulf of California (Gárate-Lizárraga and González-Armas, 2011, Fig. 1), causing the death of at least nine people (Orellana-Cepeda et al., 1998). Occurrence of Pbc HABs in Mexico coincides temporarily with the Central American events (coasts of Guatemala, El Salvador and Costa Rica, Table 2), which suggests that these are regional events that start from the Costa Rica Dome that, together with the Costa Rica current and the upwelling that occurs in the Gulf of Tehuantepec, favor the development of the Mexican HAB and its distribution throughout the Mexican South Pacific ( Vargas-Montero and Freer, 2003; Cortés-Altamirano et al., 2006; Licea et al., 2008; Meave del Castillo et al., 2008).
Table 2: HABs of Pyrodinium bahamense var. compressum (Böhm) Steidinger, Tester et Taylor, reported for the eastern tropical Pacific. The start and end date of the HAB is recorded, as well as the maximum abundance, levels of toxicity reached and kind of organisms where the toxicity was evaluated by mouse bioassay. References: 1,2) Rosales-Loessener (1989a, b); 3) Mata et al. (1990); 4) Cortés-Altamirano et al. (1993); 5) Ramírez-Camarena et al. (1996); 6) Orellana-Cepeda et al. (1998); 7) Sagastume-Cordón (2002); 8) Freer and Vargas-Montero (2003); 9) Barraza et al. (2004); 10) Ramírez-Camarena et al. (2004); 11) Licea et al. (2008); 12) Meave del Castillo et al. (2008); 13) Licea et al. (2010); 14) Gárate-Lizárraga and González-Armas (2011); 15) Gárate-Lizárraga et al. (2011); 16) Gárate-Lizárraga et al. (2012); 17) Herrera Galindo et al. (2015); 18) Amaya et al. (2018); (Ps) Present study. *=Pyrodinium L. Plate was not detected in water column but cells of this species were found inside the digestive tract of dead turtles.
| Country/locality | Start date | End date | Abundance (cells l-1) |
Toxicity (μg SXTeq.100 g-1) |
Vector organism | Reference |
| Costa Rica | ||||||
| Several locations, Pacific | Oct./1989 | 15-2000 |
Spondylus
limbatus G.B. Sowerby II as Spondylus calcifer Carpenter (1857) |
3 | ||
| Several locations Pacific | Nov./1999 | March/2002 | 8 | |||
| Several locations, Pacific | Nov./2001 | Aug./2002 | 12 | |||
| Gulf of Papagayo | Nov./2005 | Dec./2005 | 3.5×105 | 12 | ||
| El Salvador | ||||||
| Gulf of Fonseca | Nov./2005 | April/2006 | 43×106 | 2-434 | Sea turtles | 11 |
| Several locations, Pacific | Aug./2001 | Dec./2001 | 135-15468 |
Striostrea
prismatica Gray (1825) as Ostrea
iridescens
Hanley (1854) |
9 | |
| Several locations, Pacific | Nov./2009 | May/2010 | 15.3×106 | 150-1427 | Sea turtles and bivalves | 13 |
| Several locations, Pacific | Oct./2013 | 20 | 11-730 |
Lepidochelys
olivacea
Eschscholtz (1829), Chaelonia mydas Linnaeus (1758) |
17 | |
| Los Cóbanos | Nov./2017 | * | 70-1617 | Chaelonia mydas Linnaeus (1758) | 18 | |
| Guatemala | ||||||
| Las Lilas, Iztapa and Champerico | July/1987 | Oct./1987 | 30-78 | Amphichaena kindermanni Philippi (1847) | 1,2 | |
| March/1988 | Dec. 1988 | 20-51 | A. kindermanni | 2 | ||
| Jan./1989 | May/1989 | 22 | A. kindermanni | 2 | ||
| Several locations, Pacific | Aug/2001 | Oct./2001 | 62.3×103 | 90-1321 | Oysters, clams and mussels | 7 |
| Mexico | ||||||
| Gulf of Tehuantepec, Pacific | Dec./1989 | Feb./1990 | 1.7×106 | 811 |
S.
prismatica Gray (as O. iridescens Hanley), Chroromytilus palliopunctatus Carpenter (1857) |
4 |
| Acapulco, Guerrero and Caleta de Campos, Michoacán | Oct./1995 | Dec./1995 | 8549 | S. prismatica Gray (as O. iridescens Hanley (1854) | 5 | |
| Michoacán and Guerrero coasts | 1995/1996 | 520-6337 | S. prismatica Gray (as Crassostrea iridescens Hanley (1854) | 6 | ||
| Chiapas coasts | March/2001 | Feb./2002 | 180×103 | 12 | ||
| Guerrero, Oaxaca, Chiapas coasts | Aug./2001 | Feb./2002 | 3.5×106 | 48-7309 | 10 | |
| Acapulco, Guerrero | Nov./2001 | 7309 | 16 | |||
| Chiapas coasts | Dec./2005 | March/2006 | 950 | 200 | 12 | |
| Costa Grande, Guerrero | Dec./2010 | 410×103 | 416-2541 |
S.
prismatica Gray (as C. iridescens
Hanley (1843) , Donax punctatostriatus Hanley , Haliotis sp., Chiton articulatus Sowerby in Broderip & Sowerby (1832) |
15 | |
| Mexico | ||||||
| Los Cabos, Baja California Sur | Dec./2010 | 1×103 | 14 | |||
| Acapulco, Guerrero | July/2010 | Dec./2010 | 1.4×106 | 893-1388 | Chama mexicana Broderip (1835) | 16 |
| Acapulco, Guerrero | July/2010 | Jan./2011 | 778×103 | 27-2092 |
C.
mexicana Broderip (1835), Magallana gigas Thunberg (1793) as Crassostrea gigas Thunberg (1793), Pinctada mazatlanica Hanley (1856), Nodipecten subnodosus G.B. Sowerby l (1835) |
Ps |
| Oaxaca coasts | Jan./2016 | July/2016 | 131×103 | 380 |
C.
mydas Linnaeus (1758), Eretmochelys imbricata Linnaeus (1776), L. olivacea Eschscholtz (1829) |
17 |
In July 2010, a HAB of Pbc occurred in Acapulco Bay, which caused saxitoxin levels in oysters up to 1387.5 μg STXeq.100 g-1 (evaluated with mouse bioassay) and 894.5 μg STXeq.100 g-1 with high performance liquid chromatography (COFEPRIS, 2010; Gárate-Lizárraga et al., 2012). At the end of the same year, the species produced a new HAB in the bay that extended from the coasts of the state of Guerrero to the region of Los Cabos, BCS (Gárate-Lizárraga and González-Armas, 2011; Gárate-Lizárraga et al., 2012, 2015).
In this study, we report the co-occurrence of the two toxic dinoflagellates Gc and Pbc in Acapulco Bay, detected during a study that covered an annual cycle. The present was a year-round phytoplankton bimonthly monitoring study, in which the physicochemical parameters were evaluated, and the HAB of Pbc was monitored during the month that it was present (July 2010). Pbc is a very important taxon due to its production of toxic HAB; however, it has a sporadic presence in the Mexican topical Pacific. For this reason, the present study allowed us to understand some aspects of the biology and ecology of this taxon. In addition, the co-occurrence of Pbc along with Gc, another dinoflagellate that produces the same toxin (saxitoxin), and that is not common to coexist with Pbc, is the reason why we emphasize the opportunity that we had to study the biology and ecology of these two important species in the Mexican Pacific.
Materials and methods
Study area
Acapulco Bay is located in the southern portion of the Mexican Pacific, on the continental shelf of Guerrero state (99°50ˈ52"-99°56ˈ00"W and 16°47ˈ00"-16°51ˈ40" N (Fig. 2), measuring 7 × 10 km in average length and width, respectively, with a semicircular shape and depth ranging from 10-30 m inside, and more than 50 m Bay entrance (Bocana). Its sediments are thick sands, and toward the mouth the sediments are finer, corresponding to muddy sands and sandy muds (Emery, 1967; Kulm et al., 1975). The coastal relief that surrounds the bay is mountainous and during the rainy season several temporary streams drain into the bay (Mayo-Vera, 2004). The climate is Aw (rainy tropical with rain in summer), with a temperature higher than 18 °C throughout the year and average rainfall above 100 mm between June and October (rainy season) and less than 4 mm between April and May (warm dry season (Mayo-Vera, 2004). A third season (cold dry) occurs from November to March. A marine current with a velocity greater than 2 knots enters the bay, which is generated between La Roqueta Island and its western coast, generating a subsidence of water in the vicinity of Bajo Yerbabuena (Fig. 2, Anonymous, 1979; Dionni and Romo de la, 1984). Acapulco Bay is a quite diverse locality with respect to its phytoplankton community. There are currently 703 taxa recorded in its interior and adjacent coastal zone, with dinoflagellates being the most diverse group with 394 taxa (Pinzón-Palma et al., 2017; Meave del Castillo and Zamudio-Resendiz, 2018).
Phytoplankton collection
Phytoplankton was collected ten times during an annual cycle in October 2009, March, May, June, July (several dates), August, September, November 2010 and January 2011, at five sites inside the bay (Muelle, Centro, Naval, Punta Bruja and Bocana) and three sites in the adjacent coastal area (Sinfonía, Caleta and Puerto Marqués, Fig. 2, Table 3).
Table 3: Study locations within Acapulco Bay, Guerrero, Mexico, and adjacent coastal zone, where phytoplankton collections were conducted from October 2009 to January 2011. Georeference, dates and number of samples collected by location are indicated.
| Locality | Latitude N | Longitude W | Zone | 2009 | 2010 | 2011 | |||||||
| (m) | 24/Oct. | 6/March | 15/May | 10/July | 17/July | 24/July | 5/Aug. | 10/Sept. | 19/Nov. | 15/Jan. | |||
| Sinfonía | 16°50'23.81" | 99°55'20.03" | 31.2 | 5 | 5 | 5 | 5 | 5 | 1 | 5 | 5 | 5 | |
| Caleta | 16°49'43.48" | 99°54'19.54" | 28.4 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | ||
| Muelle | 16°50'29.10" | 99°54'05.16" | 13.0 | 4 | 4 | 4 | 4 | 4 | 3 | 2 | 4 | 5 | 4 |
| Centro | 16°50'59.45" | 99°52'30.96" | 35.6 | 5 | 5 | 5 | 5 | 5 | 3 | 3 | 5 | 5 | 6 |
| Naval | 16°50'18.33" | 99°51'27.16" | 14.7 | 4 | 4 | 5 | 4 | 4 | 3 | 3 | 5 | 4 | 4 |
| Punta Bruja | 16°49'19.45" | 99°51'16.24" | 23.4 | 5 | 5 | 5 | 5 | 5 | 3 | 1 | 5 | 5 | 5 |
| Puerto Marqués | 16°48'15.76" | 99°51'16.24" | 27.4 | 5 | 5 | 5 | 5 | 5 | 3 | 5 | 5 | 5 | |
| Bocana | 16°49'14.46" | 99°53'25.97" | 47.9 | 4 | 5 | 6 | 5 | 5 | 3 | 6 | 5 | 6 | |
| Total | 37 | 38 | 40 | 38 | 38 | 18 | 10 | 40 | 39 | 40 | |||
The collections were made with a Van Dorn bottle (at different depths depending on the depth of the site: 1, 3, 5, 10, 20, 30, 50 m, or bottom). The samples were preserved with a neutral lugol solution (Throndsen, 1978). Since Pbc is a dinoflagellate that sporadically produces HAB in the Mexican Pacific, at the time of its detection in Acapulco Bay, as of 7/July/2010 (by LESP-Guerrero staff, COFEPRIS, 2010), it was decided to realize weekly collections to track the HAB (July/10, 17 and 24 and August/5).
Gc is a naked dinoflagellate, whose morphology is affected by fixatives; in order to evaluate its morphological characteristics, in vivo samples were collected, with a Van Dorn bottle at 1, 3 and 5 m depth, in 10 l plastic containers during the sampling, and as soon as possible concentrating the material in the Ecology Laboratory, Facultad de Ecología Marina, Universidad de Guerrero in Acapulco, through a reverse filtration system (Dodson and Thomas, 1978), followed by immediate observation of the concentrated material with an optical microscope (Motic BA310, Kowloon Bay, Hong Kong). Gc material used for quantification was fixed with lugol solution, but for its observation in an electron microscope, it was fixed with 2.5% glutaraldehyde using filtered seawater.
Observation of organisms with SEM
Samples for observation with the electron microscope were rinsed with distilled water several times to remove the fixative. Gc samples were allowed to settle by gravity in the successive rinses, while Pbc samples were centrifuged (SOLBAT J600, Puebla, Mexico) at 1000 rpm for 15 minutes (Throndsen, 1978). Subsequently, the material was dehydrated using an acetone gradient series, leaving the material to rest for 15 minutes at each step (Boltovskoy, 1995). When the samples were finished, they were placed in small microporous chambers, covered with filter paper and dried with the method of critical point drying in a Critical Point Dryer (SAMDRI-780B, Tousimis, Rockville, USA). Once the samples were perfectly dry, the material was placed on aluminum stubs covered with sticky carbon polka dots and finally coated with a gold layer (Ferrario et al., 1995) using a Sputter Coater Equipment (Denton Vacuum Desk III, Moorestown, New Jersey, USA) and observed in scanning electronic microscopes (JEOL JSM-5900LV, Tokyo, Japan; Hi- tachi I2360N, Tokyo, Japan).
Phytoplankton abundance evaluation
The density and distribution of the toxic dinoflagellates of Gc and Pbc in Acapulco Bay, as well as the Shannon-Weaver diversity of the phytoplankton community, were evaluated. All the phytoplankton species contained in 338 bottle samples were identified and counted. Ten collections, made in the months of October 2009, March, May, July (days 10, 17 and 24), August, September and November 2010 and January 2011 (Table 3), were analyzed by the Utermöhl method (Edler and Elbrächter, 2010), using an inverted microscope (Motic AE31, Carlsband, Canada), and sedimentation chambers of 25 or 50 ml, depending on the concentration of phytoplankton in the samples.
Additionally, we evaluated the density of dinoflagellate organisms reported as Gc predators, such as Noctiluca scintillans (Macartney) Kofoid et Swezy (Holmes et al., 1967; Alonso-Rodríguez et al., 2005; Bustillos-Guzmán et al., 2013) and Polykrikos kofoidi Chatton (Holmes et al., 1967), as well as of Gyrodinium fusus (Meunier) Akselman, an active predator of vegetative cells of Pbc and of Chytriodinium affine (Dogiel) Chatton, which parasites its cysts, according to observations made in the present study.
From the density data, the relative abundance of each of the species was obtained considering the total phytoplankton of each sample as 100% and classifying each species as rare <10%, scarce >10-25%, common >25-50%, abundant >50-80%, or dominant >80%. For Gc the monthly averages were obtained from the abundance values of 1 to 10 m and for Pbc from 3 to 5 m. These depths were chosen based on the observations of the distribution of dinoflagellate abundances at different depths and sites in Acapulco Bay and the adjacent coastal area obtained by Bustamante-Gil (2011), to avoid underestimating the monthly abundance data of the species in question. With data obtained in that study and those reported by other authors for the same bloom of Pbc in July 2010, we calculated the negative rate of growth or rate of decline of the species (-r), according to the equation modified by Guillard (1973, in Wood et al., 2005).
In addition, the Shannon-Weaver diversity index (H'=-Σpi log pi) was calculated, where pi is the proportion of each of the species in the sample according to the count of cells, considering the total sum of cells counted in a sample, giving the value in bits (Margalef, 1978).
Morphometric analysis
To evaluate the Gc morphometry, live samples were examined immediately after their collection, with the optical microscope (Motic BA310, Kowloon Bay, Hong Kong). Of the living organisms, videos were taken when their movement was still active; later, with aid of the the software Video to Picture version 5 (Aoao Digital Studio, 2008-2019), the videos were transformed into photographs to make the measurements. To study the morphometry of Pbc, the samples were observed with a Leica optical microscope (DMLB, Wetzlar, Germany) in bright field, with integrated digital camera, using trypan blue staining to facilitate the observation of the plates of its cellulose theca (Boltovskoy, 1995).
For both species a random selection of the specimens measured was made according to the availability and proper conservation of the material. In Gc the length of the cell body corresponded to the total length of the organism (LT, Fig. 3A), while for Pbc, the length was taken without considering the apical horn or spines, and therefore the value of the length corresponded only to the length of the cell body (Lc; Fig. 3B); in the case of the planozygotes, much scarcer cells, 30 cells were measured. For both species, the transdiameter (Td, width of the organism at the level of the cingulum) was measured without taking into account the cingular veils, since both species are cavozone (with excavated edges cingulum) (Figs. 3A-B). For Pbc the thickness of the cell was also measured in the apical or antapical views of the organisms (Fig. 3C). In the case of Pbc cysts, the diameter was measured since they were spherical, as well as the length of the processes, including the longest and the shortest (Fig. 3D). The dimensions were obtained by measuring the photographs taken with the optical or scanning electronic microscope with the software Able Image AnalyzerTM version 3.6 (Mu-Labs, 2008-2012).
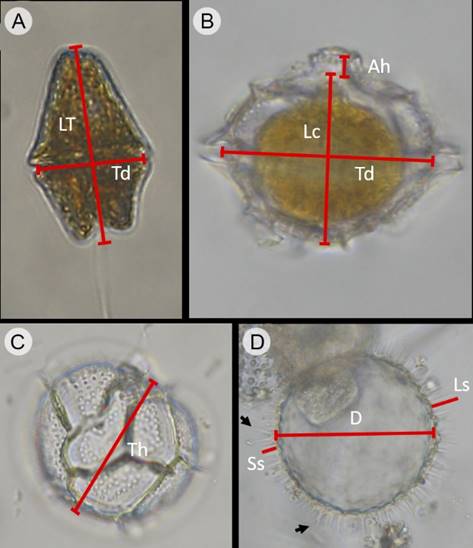
Figure 3: Way to evaluate the dimensions in organisms of G. catenatum Graham (Gc) and Pyrodinium bahamense var. compressum (Böhm) Steidinger, Tester et Taylor (Pbc), collected in Acapulco Bay, Mexico. A. cell of Gc in ventral view; B. cell of Pbc in dorsal view; C. cell of Pbc cell in antapical view; D. empty Pbc cyst collected in water column; arrows indicate the bifurcated processes at the base or towards the apex. LT=Total length, Lc=Length of the cellular body (without apical horn or antapical spines), Td=Transdiameter, Ah=Apical horn, Th=Thickness of the cell, D=Diameter of the cyst, Ls=Length of long spine-shaped processes, Ss=Length of short spine-shaped processes.
To analyze whether the forms of Pbc found in Acapulco Bay resembled the literature reports for var. bahamense or var. compressum, correlation analyses (NCSS Data Analysis, 2015) were made of the Lc/Td relationship vs the total cell length (Lc), as well as the relationship LT/Td vs transdiameter (Td). The Lc/Td ratio was classified into two groups: forms with Lc/Td<1 (compress organisms) and Lc/Td≥1 (spherical or more elongated forms). The number of data included in the analysis was n=632, divided into four groups as follows: a) Acapulco organisms Lc/Td<1, n=481; b) Acapulco organisms Lc/Td≥1, n=70; 51 organisms belonging to var. bahamense taken from the following references (Osorio-Tafall, 1942; Steidinger et al., 1980; Balech, 1985; Gómez-Aguirre and Licea, 1997; Steidinger and Tangen, 1997; Badylak et al., 2004; Martínez-López et al., 2007; Morquecho, 2008; Gárate-Lizárraga and González-Armas, 2011; Martínez-Tecuapacho, 2011; Usup et al., 2012) and 30 cells belonging to var. compressum taken from the following references (Osorio-Tafall, 1942; Taylor, 1976; Steidinger et al., 1980; Balech, 1985; Badylak et al., 2004; Gárate-Lizárraga and González-Armas, 2011; Martínez-Tecuapacho, 2011; Usup et al., 2012).
Due to the lack of sufficient data, an analysis of the Gc morphometry in relation to physicochemical parameters was not performed. However, given that there were enough data on Pbc cell measurements, for this taxon we evaluated the relationship of the morphometry with physicochemical parameters.
Evaluation of physicochemical parameters
Simultaneously with the phytoplankton survey, water samples were collected at different depths, to measure the phytoplankton biomass (chlorophyll-a) and physico-chemical parameters. The water temperature, salinity, and dissolved oxygen were evaluated with multiparameter probes (YSI-556 MPS, YSI-550A, Yellow Springs, USA; Thermo-OrionStarTM, Thermo Fisher Scientific Inc., Waltham, USA). With a white Secchi disc, the transparency of the water (Zsd) was measured and from this, the thickness of the euphotic layer (Zeu) was calculated according to the following formula: Zeu=-Zsd ln (0.01)/1.44 (Kirk, 1994). In addition, monthly changes in temperature were estimated, dividing the average temperature value of each month between the average annual temperature, indicating decreases in water temperature values below 1.0.
The determination of nutrients was carried out on in situ filtered water samples through Whatman GF/F filters of 0.7 μm of pore (Sigma-Aldrich, Toluca, Mexico), frozen (0 oC) until processed at the laboratory by the following techniques: reduction by Cd-Cu columns for nitrates+nitrites (Strickland and Parsons, 1972), indophenol blue for ammonium (Solórzano, 1969), ascorbic acid-molybdate for orthophosphates (Murphy and Riley, 1962) and p-silicomolybdic acid for silicates (Schwartz, 1942). In addition, the phytoplankton biomass (Chl-a) was also evaluated using the spectrophotometric method (Parsons et al., 1984).
HAB toxicity evaluation
In this study we did not evaluate the toxicity of the species when they generated the HAB. However, saxitoxin concentrations in molluscs collected during the HAB (Health alert of COFEPRIS (2010)) were considered.
Evaluation of climatic parameters
To assess the influence of climate on the HAB occurrence, data obtained from meteorological station 76850 located in Acapulco were used to graph the monthly average of temperature and precipitation for 2010, as well as the monthly averages during the previous 35 years (1973-2010; Anonymous, 2010). It has been pointed out that the presence of HABs is related, at least for Pbc, to the “El Niño” Southern Oscillation (ENSO) (Maclean, 1989a). Therefore, in order to know whether the bloom of Pbc occurred in Acapulco Bay during 2010 coincided, or not, with events “El Niño” or “La Niña”, the dates were placed on a graph of variations in surface water temperature anomalies, made with National Oceanic and Atmospheric Administration (NOAA) standardized values of Multivariate ENSO Index (MEI), from January 1989 to January 2011 (Wolter, 2012).
Statistical analyses
To test significant differences in mean species abundance (Gc and Pbc) among the collection depths (vertical distribution), the localities (horizontal distribution), and the months (seasonal distribution), one-way analysis of variance was applied, ensuring that the assumption of equality of variances was fulfilled (Levene’s test 95%) (Zar, 2010). To determine whether the morphology of Pbc organisms is affected by environmental conditions, simple Spearman or Pearson correlation analyses (ρ (Rho) and r respectively) were used to measure the degree of association between the morphometric variables of this species and the physicochemical parameters. Correlation assumptions were evaluated by residual analyses (Chatterjee et al., 2000). These statistical procedures were performed using the NCSS statistical program version 10 (NCSS Data Analysis, 2015).
Canonical correspondence analysis (CCA) was applied to the abundance of Gc, Pb, total phytoplankton and Chl-a data matrix (dependent set) and the physico-chemical data matrix (independent set) in order to elucidate relations between biological assemblages of species and environmental variables. This method describes and allows the visualization of the differential habitat preferences (niches) of taxa via an ordination diagram (Ter Braak and Verdonschot, 1995). Interset correlations of this analysis were used to determine the environmental variables that were most important in determining species abundance (McGarigal et al., 2000). CCA was performed using the MVSP program (Kovach Computing Services, 2017).
Evaluation of Satellite images
To determine whether HABs found in Acapulco Bay during 2010 started inside the bay or rather corresponded to regional events from the ocean and impacted the bay, 52 images were analyzed corresponding to the weekly average of satellite Chl-a of the year 2010 in the Mexican Pacific, obtained from CONABIO (2015).
Results
Quantification of phytoplankton
From the analysis, a total of 501 phytoplankton taxa were recognized, of which 217 were diatoms (43%), 265 dinoflagellates (52%) and 19 (3.8%) belonged to other groups (Cyanophyta, Heterocontophyta, Prymnesiophyta, Euglenophyta and Chlorophyta (Prasinophyceae)). The abundances of phytoplankton ranged from 5.1×103 to 2.45×106 cells l-1, with the highest values found from November 2010 to January 2011. Diatoms dominated throughout the year, except for the month of May (Fig. 4). The phytoplankton biomass varied from 1.07 to 46.3 mg Chl-a l-1 (Table 4), with the monthly average being lower in August 2010 (3.73 mg l-1) and the highest in July/10/2010 (10.39 mg l-1). The H' index showed that the phytoplanktonic community of Acapulco Bay had an average annual interval of H'=1.9-4.9 bits, and an annual average of 3.8 bits (Table 4).
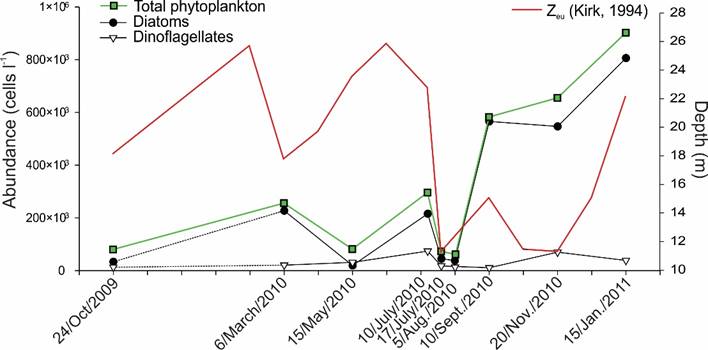
Figure 4: Average abundance of total phytoplankton and diatoms and dinoflagellates, as well as the depth of the euphotic zone (Zeu)obtained from the samples taken from October (2009) to January (2011) in Acapulco Bay, Mexico.
Table 4: Values (monthly average (
| H'(bits) | Chl-a (mg l-1) | |||||
| Date | Range | x̅ | S | Range | x̅ | S |
| Oct./2009 | (3.0-4.8) | 4 | 0.54 | (4.65-9.70) | 6.48 | 0.99 |
| March/2010 | (1.9-3.9) | 3 | 0.48 | (4.02-11.6) | 6.57 | 1.95 |
| May/2010 | (3.1-4.8) | 4.1 | 0.49 | (4.3-20.02) | 9.01 | 2.46 |
| 10/July/2010 | (1.9-4.0) | 3.4 | 0.46 | (4.93-46.3) | 10.39 | 9.31 |
| 17/July/2010 | (2.6-4.0) | 3.6 | 0.58 | (1.07-16.2) | 4.50 | 3.19 |
| Aug./2010 | (2.8-4.4) | 4.1 | 0.48 | (2.54-9.60) | 3.73 | 1.27 |
| Sept./2010 | (2.9-4.7) | 3.8 | 0.43 | (2.30-6.80) | 4.07 | 1.19 |
| Nov./2010 | (3.4-4.8) | 4.3 | 0.32 | (3.15-11.5) | 6.54 | 2.51 |
| Jan./2011 | (4-4.9) | 4.5 | 0.2 | (1.62-8.36) | 4.72 | 1.45 |
| x̅ Year | (1.9-4.9) | 3.5 | 0.68 | (1.07-46.3) | 6.22 | 2.7 |
| CV=0.19 | CV=0.43 | |||||
Abundance and distribution of toxic species
Gymnodinium catenatum
Gc was a constant component of the phytoplankton community of Acapulco Bay during 2010, having a frequency of 50% (considering the total of 338 samples analyzed) and a temporal frequency of 100% (considering the total of 10 collections). This means that the species was present in Acapulco during the entire study period, at least at one site of each collection date. The abundance of Gc varied between 20-189×103 cells l-1 (maximum value on 20/11/2010, in Punta Bruja locality, 5 m depth), and an average abundance of 5277 cells l-1; that represented a relative abundance, from 10.01% in the month of January (Puerto Marqués locality, to 1 m depth), up to its greatest abundance, 27.17% in November; being considered as a scarce to common species in the bay. The mean monthly values of Gc abundance showed significant differences among months (ρ=0.19, p=0.00069, n=315). Based on the convention of 5×103 cells l-1 to consider a HAB of species that cause PSP because shellfish would become toxic to human consumption after 24 hrs (Martínez et al., 1991, Reguera, 2002), Gc surpassed this value in some localities during the months of June and July 2010 and January 2011. However, the condition of HAB was only reached in November 2010 due to the presence of shellfish toxicity, although it was present together with P. bahamense (COFEPRIS, 2010), with a maximum abundance of 188×103 cells l-1 in the localities Muelle (5 m) and Bocana (10 m) and a mean abundance of 38.4×103 cells l-1. Their abundance values were low during the rest of the year (Table 5, Fig. 5), and there were no reports of toxicity in shellfish for Acapulco. With respect to average abundances by depth, the highest values were found in November between 3 and 5 m, with 11.6×103 and 17.1×103 cells l-1 respectively (Fig. 2, in Meave del Castillo and Zamudio-Resendiz, 2014), that did not discolor surface waters. Regarding the horizontal distribution (calculating the average density between 1-10 m deep, Figs. 6A-I), high values (≥5×103 cells l-1) were observed in November 2010 in the whole bay (Figs. 6H), and in Naval and Caleta locations in July 2010 (Figs. 6E-F) and January 2011 (Fig. 6I), respectively. In September 2010 (Fig. 6G), the species was found only outside the bay (in Sinfonía and Caleta locations).
Table 5: Abundance of Gymnodinium catenatum Graham in
Acapulco Bay, Guerrero, Mexico, from May 2009 to January 2011.
The date of collection (Date), minimum (Min.), maximum (Max.),
average (
| Date | Min (cells l-1) | Max (cells l-1) | x̅ | S± | Reference |
| May/2009 | - | 3.8×103 | - | - | Rojas-Herrera et al. (2012) |
| June/2009 | - | 1.3×103 | - | - | Rojas-Herrera et al. (2012) |
| June/2009 | - | 54.4×103 | - | - | Gárate-Lizárraga et al. (2016) |
| July/2009 | - | 133 | - | - | Rojas-Herrera et al. (2012) |
| Aug./2009 | - | 3.1×103 | - | - | Rojas-Herrera et al. (2012) |
| Oct./2009 | - | 12.5×103 | - | - | Rojas-Herrera et al. (2012) |
| 24/Oct./ 2009 | 20 | 6×103 | 987 | 1.4×103 | Present study |
| Nov./2009 | - | 17.2×103 | - | - | Rojas-Herrera et al. (2012) |
| Dec./2009 | - | 1.9×103 | - | - | Rojas-Herrera et al. (2012) |
| 6/March/2010 | 40 | 3.9×103 | 851 | 1×103 | Present study |
| 15/May/2010 | 64 | 1.9×103 | 487 | 645 | Present study |
| 10/July/2010 | 40 | 10×103 | 1.3×103 | 2.5×103 | Present study |
| 17/July/2010 | 59 | 6.5×103 | 1.7×103 | 2.1×103 | Present study |
| 10/Sept./2010 | 54 | 185 | 129 | 67 | Present study |
| 19/Nov./2010 | 993 | 188×103 | 38.4×103 | 5.3×103 | Present study |
| 15/Jan./2011 | 64 | 72.2×103 | 6×103 | 16×103 | Present study |
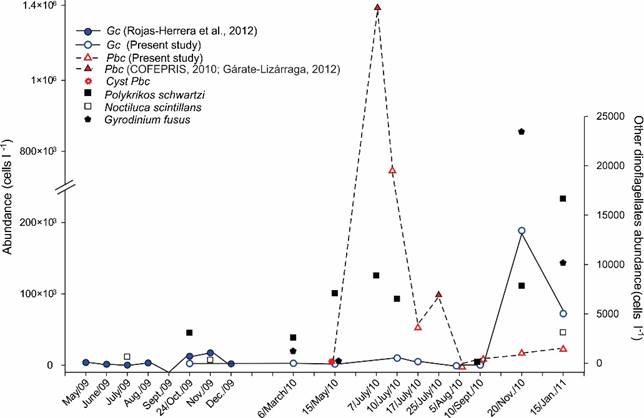
Figure 5: Average abundance per date of sampling of the toxic dinoflagellates Gymnodinium catenatum Graham (Gc) and Pyrodinium bahamense var. compressum (Böhm) Steidinger, Tester et Taylor (Pbc) and their predators: Polykrikos schwartzii Bütschli, Noctiluca scintillans (Macartney) Kofoid et Swezy, and Gyrodinium fusus (Meunier) Akselman, in Acapulco Bay, Mexico; during the period from May 2009 to January from 2011. Modified from Meave del Castillo and Zamudio-Resendiz (2014).
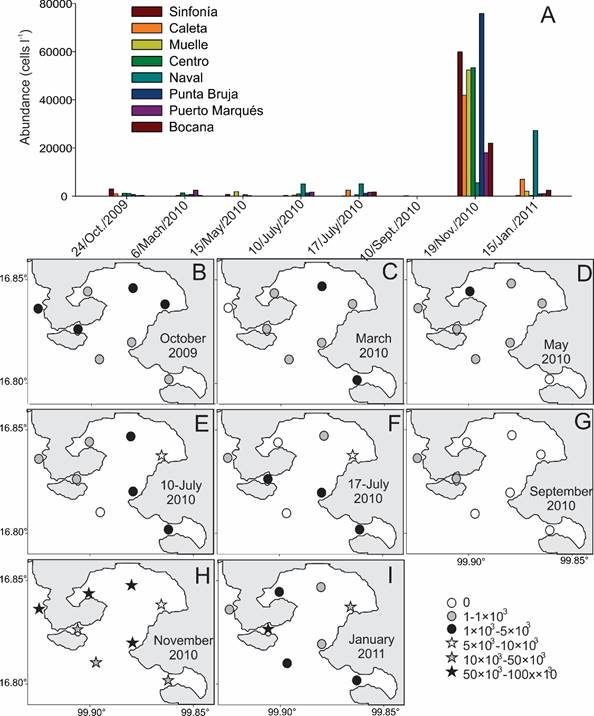
Figure 6: Distribution of the average abundance of Gymnodinium catenatum Gaham (Gc) in the different localities studied in eight collections made in the Acapulco Bay, Guerrero, Mexico. A. average abundance values by location and date of sampling; B-I. horizontal distribution of abundance between 1-10 m depth considering abundance intervals on different collection dates: B. collection of October 2009; C. collection of March 2010; D. collection of May 2010; E. collection of 10/July/2010; F. collection of 17/July/2010; G. collection of September 2010; H. collection of November 2010; I. collection of January 2011.
Pyrodinium bahamense var. compressum
Pbc was present during the study period between the months of July 2010 to January 2011, and during this whole period (except in August) it reached abundance values to be considered HAB (Fig. 5). Thus, there were significant differences among the mean monthly values of abundance (p<0.0005). It had a frequency of 47% considering the total of samples analyzed (338) and a temporal frequency of 62.5% of the samples obtained at the time the HAB occurred. Its abundance varied between 20- 773×103 cells l-1 (Table 6), representing 0.003% of the relative abundance in the locality Sinfonía, at 1 m depth in September, and reaching a maximum of 67.6%, when it formed a HAB (Centro, 3 m, on July/10/2010), classifying it during the whole study year as a rare to abundant species in Acapulco. The highest average values of Pbc abundance were observed at 3-5 m depth, with values of 41.2×103 and 44.5×103 cells l-1, respectively (Fig. 2 in Meave del Castillo and Zamudio-Resendiz, 2014).
Table 6: Abundance of Pyrodinium bahamense var.
compressum (Böhm) Steidinger, Tester et
Taylor, in Acapulco Bay, Guerrero, Mexico, from May 2010 to
January 2011. *=Only cysts with content present in the water
column; average=
| Date | Min cells l-1 | Max cells l-1 | x̅ | S± | S/√ˉn | r | Reference |
| 15/May/2010 | - | 350* | - | - | Present study | ||
| 7/July/2010 | - | 1.4×106 | - | - | COFEPRIS (2010) | ||
| 10/July/2010 | 20 | 773×103 | 65.1×103 | 159×103 | 25×103 | -0.085 | Present study |
| 17/July/2010 | 326 | 52×103 | 9.2×103 | 9×103 | 1.4×103 | -0.385 | Present study |
| 24/July/2010 | 65×103 | 101×103 | 83×103 | 25.5×103 | 5.6×103 | 0.082 | Gárate-Lizárraga et al. (2012) |
| 4/Aug./2010 | 40 | 320 | 140 | 156 | 20 | -0.575 | Present study |
| 10/Sept./2010 | 21 | 7.2×103 | 1.1×103 | 1.8×103 | 339 | Present study | |
| 19/Nov./2010 | 40 | 16.5×103 | 1.7×103 | 3.4×103 | 561 | Present study | |
| 15/Jan./2011 | 76 | 22×103 | 3.8×103 | 7.1×103 | 585 | Present study |
Table 6 summarizes the abundance values of Pbc and shows that the standard deviation in some dates is very high, probably due to its occurrence in the form of patches, both horizontally and vertically. In the present study the highest average value was observed on July/10/2010 (65.1×103 cells l-1), followed by July/17/2010 (9.2×103 cells l-1, Table 6, Fig. 5, Fig. 7A). Although Gárate-Lizárraga et al. (2012) indicate average abundance values higher than those recorded by us. In Figure 7B, it is observed that when the Pbc HAB ocurred, it developed simultaneously throughout the interior of the bay, with the highest density recorded in Centro locality, at 3 m depth. A week later the values had already decreased, with a maximum density of 52.3×103 cells l-1 in La Bocana at 5 m deep (Fig. 7C). For the following week, the taxon apparently had a upturn, because Gárate-Lizárraga et al. (2012) indicated a maximum density of 101×103 cells l-1 in the interior of the bay at July/25 and up to 606×103 cells l-1 in the western portion of the bay, in the vicinity of Punta Pilares (see Fig. 2). By August its abundance was already very low (40 to 320 cells l-1), and it was practically restricted to Naval site (Fig. 7D). As of September/10, the Pbc abundance increased again, with a maximum of 7.2×103 cells l-1, in the Centro locality at 10 m deep, and for November it reached a maximum abundance of 16.5×103 cells l-1, at 10 m depth, in Punta Bruja (Table 6), indicating that this taxon is shade adapted. In this second HAB, the highest abundances of Pbc were concentrated in the eastern portion of the bay (Figs. 7G, H), possibly due to the prevailing winds.
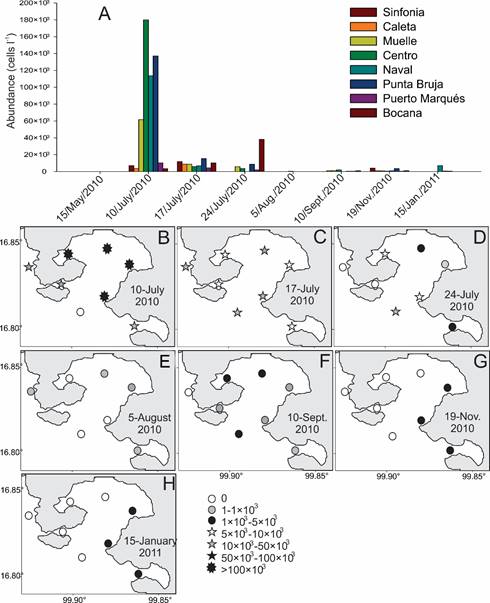
Figure 7: Distribution of average abundances of Pyrodinium bahamense var. compressum (Böhm) Steidinger, Tester et Taylor (Pbc) in the different localities studied in seven collections in Acapulco Bay, Guerrero, Mexico. A. average abundance values by location and date of sampling; B-H. horizontal distribution of abundance between 3-5 m depth considering abundance intervals on different collection dates: B. collection of 10/July/2010; C. collection of 17/July/2010; D. collection of 24/July/2010; E. collection of August 2010; F. collection of 10/September/2010; G. collection of 19/ November/2010; H. collection of 15/January/2011.
Regarding the horizontal distribution for Pbc in Acapulco Bay, the average abundances per date, considering only values between 3 to 5 m depth, showed that the conditions of the Bay led to the massive development of Pbc and higher densities (>100×103 cells l-1) occurred in its interior, on July/10/2010, while in the adjacent coastal portion the values were at most one-fourth of the abundance in the interior (6.6×103-23.4×103 cells l-1; Fig. 7B).
The presence of Pbc in Acapulco as vegetative cells in the water column ended definitively in January 2011, when only agglomerations of solitary and immobile organisms were found at 1 m depth in Naval station, with a maximum density of 22.2×103 cells l-1. Given that the HAB of Pbc was monitored during all months and the maximum abundance evaluated on July/7/2010, July/17/2010, July/24/2010 and August/4/2010, the negative growth rate (-r) calculated from the decrease of HAB varied from -0.082 to -0.575 (Table 6). The HAB of Pbc affected the diversity values of the phytoplankton community, since the interval obtained throughout the study was H'=1.9-4.9 bits, with an annual average of 3.8 bits (Table 4), and the lowest value obtained just in July/10/2010 (1.9 bits in the Centro locality, 3 m deep; Fig. 8), where the highest Pbc abundance value was recorded (773×103 cells l-1), and therefore the decrease of the average of H' in the month of July to H'=3.36 bits.
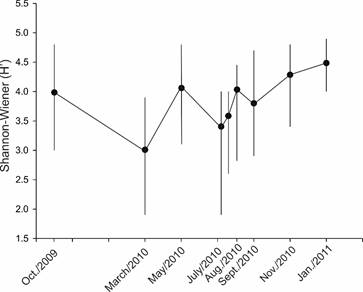
Figure 8: Monthly values of ecological diversity (H') with averages (dots), and its variation considering minimum and maximum values, of the phytoplankton community in Acapulco Bay, Guerrero, Mexico, during the period studied from October 2009 to January 2011. In July 2010, phytoplankton collections were carried out on two dates 10/July/2010 and 17/July/2010.
On the other hand, the phytoplankton Chl-a estimated biomass increased. The annual average of Chl-a in all the depths had an interval of 1.07-46.28 mg l-1 and an annual average of 6.31 mg l-1 (Table 6). In July 2010, when Pbc produced a HAB, the average value of Chl-a increased to 10.39 mg l-1. Just at the site and date where Pbc reached its maximum abundance, the highest value of Chl-a for the study period was obtained (46.28 mg l-1).
Morphology and morphometry of vegetative cells and cysts of the species
Vegetative cells of Gymnodinium catenatum
In general, within the Acapulco Bay, Gc cells were brownish-golden, solitary or forming chains of up to 32 cells, with an average of six cells per chain (Figs. 9A-J). It was evident that cells swelled with lugol or formalin fixation; therefore, only living cells were measured (n=31). The total variation interval found was: LT=22.2-51.4 μm, and Td=14.3-32.1 μm, (Table 7). The average of the LT/Td ratio was 1.6 (Table 7). Thus, the morphology of the cells varied considerably from practically round cells (LT/Td=1, Figs. 9E, I, J, 10B-D), to elongated (LT/Td=2.4; Table 7, Figs. 9A, B, F-H).

Figure 9: Organisms photographed live of Gymnodinium catenatum Graham (optical microscope) (Gc). A. solitary cell, dorsal view; B. Terminal cell of a chain, ventral view; C. chain with four cells in ventral view with flagella; D. chain of eight cells, some in ventral and others in dorsal view; E. rounded apical cell, dorsal view; F. three elongated cells of a chain, ventral view; G. solitary cell, ventral view; H. chain with 8 cells, dorsal view; I. chain with 32 cells with compressed cells; J. chain of three rounded cells, ventral view. Scales: A, B, E, F, G=20 µm; C, D, H, I, J=50 µm.
Table 7: Morphometry of cells of Gymnodinium
catenatum Graham collected in Acapulco Bay,
Guerrero, Mexico (n=31). The range of measurements, the average
value (
| Date | LT (µm) | Td (µm) | LT/Td | n |
| March/2010 | (36-38) |
(22-25) |
(1.5-1.7) |
4 |
| May/2010 | (30-32) |
(26-29) |
(1.0-1.2) |
3 |
| July/2010 | (36.4-45.1) |
(19.2-26.2) |
(1.5-2.4) |
11 |
| Nov./2010 | (22.2-51.4) |
(14.3-32.1) |
(1.2-1.7) |
13 |
| Annual Data | (22.2-51.4) |
(14.3-32.1) |
(1.0-2.4) |
31 |
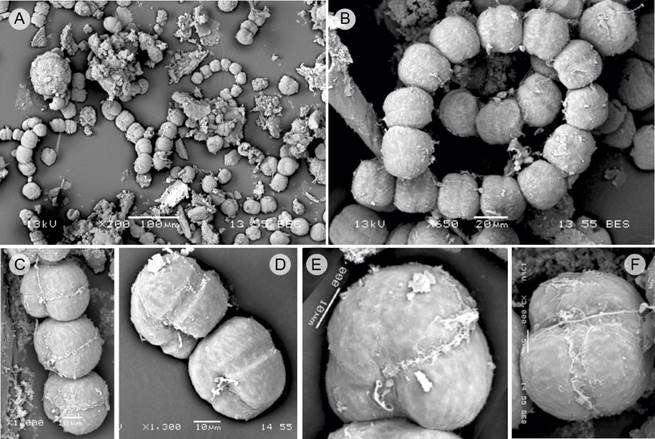
Figure 10: Gymnodinium catenatum Graham (SEM) (Gc). A. image of a bloom, showing solitary cells and in chains of up to eight cells; B. spiral chain of 14 cells, dorsal view; C. chain of four cells, latero-ventral view; D. chain of two cells, latero-ventral view; E-F. apical cells, ventral view. Scales: A=100 µm; B=20 µm; C, D, E=10 µm; F=5µm.
The photographs of Gc obtained with scanning microscopy showed that the glutaraldehyde fixation also slightly swelled the cells (Figs. 10A-F). However, it was possible to recognize the characteristic features of the species, including the width of the cingulum of 6 μm (Fig. 10D).
Gymnodinium catenatum cysts
Although Gc produces a characteristically reticulated cyst of 36-62 μm diameter (Bolch et al., 1999) and is often found at the sites where the species produces HABs, in the present study no cysts were observed in the water. Sediment samples were not taken to analyze for cysts.
Vegetative cells of Pyrodinium bahamense var. compressum.
In the case of Pbc, both solitary cells (Figs. 11A, E; 12A, D) and chains of up to 16 cells (Figs. 11B-D) were observed, with an
average of eight cells per chain. Polyhedral cells with high sutures, often
anterioposteriorly compressed (Lc/Td=0.71; Table 8), especially those cells located in the intercalary
position of the chain. Cells were almost spherical (Lc/Td=1.0) when solitary
or occupying the final position in a chain (Figs. 11A, B, D), or even slightly longer than wide (Lc/Td=1.16;
Table 8 (Fig. 11E). Cell measurement data for vegetative cells
(discarding cells with the appearance of planocygotes) of
Pbc (with n=466) are presented in Tables 8 and 9. Vegetative cells had a cell length interval (Lc) of
31.2-48 μm, with an

Figure 11: Pyrodinium bahamense var. compressum (Böhm) Steidinger, Tester et Taylor (optical microscope) (Pbc); A. solitary cell, ventral view, with long left antapical spine (arrow); B. chain of 4 compressed cells, the terminal cell more rounded and with long left antapical spine (arrow); C. chain of eight compressed cells, the terminal cell more rounded and with long left antapical spine; D. chain of 16 cells, the terminal cell more rounded and with long left antapical spine; E. solitary cell, dorsal view, showing long apical spine (arrow); F-H. chains with larger cells intercalated (maybe planocygotes); I. conglomerate of cells found at the end of the bloom at a depth of 10 m (probably temporary pellicle cyst stage). Scales: A-B, E-H=20 µm; C-D, I=50 µm.
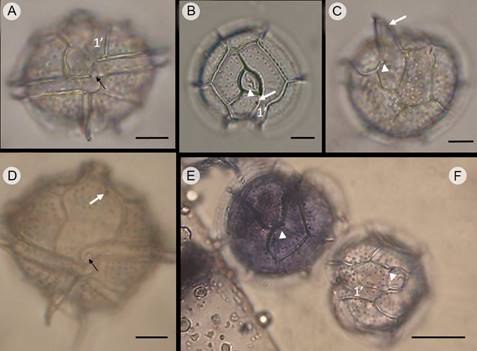
Figure 12: Pyrodinium bahamense var. compresssum (Böhm) Steidinger, Tester et Taylor (Pbc), showing organisms in different views in light microscope. A, D. ventral view showing the displacement of the descending cingulum, the height of the broad and flat apical horn and the left and right antapical spines; in A of similar size, in D of different size, with the left being longer. In A and D the complex of small sulcal plates and flagellar pores is observed (black arrow); B, F. apical view, showing the apical pore complex (APC) with the cover plate (Pl) as a comma, the pore plate (Po) and the accessory pores (arrow head). In A, B and F it is clearly observed that plate 1’ is very narrow and does not touch the APC; C, E. antapical view; in C the arrow indicates the very long right antapical spine. In C and E the accessory pore of the posterior sulcal plate (Sp, arrow head) is evident. In B and D the ventral pore (white arrow) is observed. In B and E the arrangement of the trichocyst pores in the apical plates of the epitheca plates are observed, and in C and E trichocyst pores in the antapical plates of the hypotheca are observed. Cells in E and F stained with Trypan blue. Scales: A-D=10 µm; E-F=20 µm.
Table 8: Morphometry of organisms of Pyrodinium
bahamense var. compressum (Böhm)
Steidinger, Tester et Taylor, in different dates of collection
in Acapulco Bay, Guerrero, Mexico (N=551), considering all types
of cells found. The range of measurements (in brackets), the
average value (
| Date | Lc (µm) | Td (µm) | Lc/Td | n |
| 10/July/2010 | (32-62) |
(38-69) |
(0.74-1.16) |
75 |
| 17/July/2010 | (31.2-52.8) |
(36-60) |
(0.71-1.12) |
214 |
| Aug./2010 | (31.2-50.4) |
(36-52.8) |
(0.71-1.06) |
146 |
| Sept./2010 | (33.6-48) |
(38.4-52.8) |
(0.77-1.13) |
63 |
| Nov./2010 | (38.4-55.2) |
(43.2-55.2) |
(0.76-1.16) |
47 |
| Jan./2011 | (36.4-45.9) |
(41.7-48.8) |
(0.80-1.05) |
6 |
| Annual Data | (31.2-62) |
(36-69) |
(0.71-1) |
551 |
Table 9: Summary of cell measurements (total range, mean and standard deviation) of Pyrodinium bahamense var. compressum (Böhm) Steidinger, Tester et Taylor, from Acapulco Bay, Guerrero, Mexico, and its comparison with data from Pyrodinium bahamense Plate var. bahamense of lagoon and mangroves of San José, Island, B.C.S, Mexico. *=The average values were obtained by calculating them with the maximum and minimum values. Lc=Cell length (without apical horn, or spines); Td=Transdiameter (width of the cell at cingulum level); Lc/Td=Ratio cell length/transdiameter; L=Length.
| Present study |
Morquecho (2008)
Morquecho et al. (2014) |
||||
| Vegetative cells | Range | x̅ | S | Range | x̅ |
| Lc (µm) | (31.2-48) | 41.0 | 4.0 | (33-55) | 42 |
| Td (µm) | (36-55.2) | 45.4 | 4.0 | (37-56) | 43.8 |
| Lc/Td | (0.71-1) | 0.9 | 0.07 | - | - |
| Thickness (without list, µm) | (33.8-44) | 39.9 | 3.4 | - | - |
| Cingulum | |||||
| Width (µm) | (2.5-4) | 3.4 | 0.4 | - | - |
| Displacement (x) | (1.4-1.8) | 1.6 | 0.14 | (1.0) | - |
| Apical horn | |||||
| L (µm) | (3.0-6.7) | 4.5 | 1.4 | (4-16) | 7 |
| Apical spine | |||||
| L (µm) | (2.5-14.3) | 7.8 | 4.9 | ||
| Left antapical spine | |||||
| L (µm) | (4.3-24.6) | 16.8 | 5.4 | (12-26) | 20 |
| Right antapical spine | |||||
| L (µm) | (6.6-13.9) | 9.4 | 2.2 | (7.2-13) | 9.6 |
| Ventral pore | |||||
| Diameter (µm) | (1.2-1.5) | - | - | (0.8-1) | - |
| Trichocyst pores | |||||
| Diameter (µm) | (0.3-0.7) | 0.6 | - | (0.4-0.7) | - |
| Planocigotes | |||||
| Lc (µm) | (48.8-62) | 51.9 | 2.7 | - | - |
| Td (µm) | (44.8-69) | 53.9 | 5.6 | - | - |
| Lc/Td | (0.7-1.15) | 0.96 | 0.08 | - | - |
| Cysts | |||||
| Diameter (µm) | (67.2-73) | 70.1 | 2.5 | (43-56) | 49.5* |
| L process (µm) | (3.9-13.4) | 8.4 | 2.1 | (5.0-13) | 9* |
The plate formula of the theca was: Po, Pl, 4’, 0a, 6”, 5”’, 1p, 1””, following the terminology of Mertens et al. (2015) for the designation of the postcingular plates. First apical (1’) is a narrow rectangular plate that does not touch Po, so that the apical plates can be written as (3’+ 1’) instead of 4’ (Fukuyo and Taylor, 1989).
In the images obtained with SEM in this study, plates C1 and C6 could be recognized in ventral view (Fig. 13A) and plates C3 and C4 in dorsal view (Fig. 13B). In addition, SEM showed the Sa plate (Figs. 13A, 14) and the Ssa and Sda plates (Fig. 14). Plate Sp is large, with an irregularly quadrangular shape (Fig. 13F) and a small posterior attachment pore (or connecting pore) on the right side. The sutures and especially the crests between the 1’ and 4’ plates are often absent (Fig. 12D), probably as a result of the asexual division with an oblique or diagonal cleavage furrow without ecdysis ( Taylor and Fukuyo, 1989). Plate 4’ has an obvious ventral pore, 1.2-1.5 μm in diameter (Table 9), located in the upper portion, close to the suture with the plate 1’ (Figs. 13A, D). The cingulum is cavozone (or excavated) (Figs. 11A, 12A, 13A-B), prominent and without ribs, measuring 2.5-4 μm wide, descending, with a displacement of 1.4-1.8×. Cells have wide cingular lists (3.5-6.4 μm, Figs. 11A, E; 12A-C), divided by the sutures of the pre- and postcingular plates, and generally unequal sulcal fins, with the right one more developed than the left one (4.2-5.2 μm wide, Figs. 13A- C). All the plates, as well as the cingular and sulcal lists, are covered by very fine teeth called pustules/tubercules by Taylor and Fukuyo (1989) (Figs. 13C-F). The sutures of the plates develop as raised flanges of 2-2.4 μm wide. The majority of the Acapulco cells had a short apical horn, broad at the base, and truncated at the apex, with a length of 3-6.7 μm (Table 9, Figs. 11A, E; 12A, B; 13A, B, E), and commonly a very short apical spine (Figs. 13A,B) and rarely a long one (Fig. 11E, Table 9), located dorsally, between plates 3’ and 4’ (Fig. 13E). Frequently, cells presented an evident spine on the hypotheca, which arised from an antapical position, on the left side of the organism, on the suture of the 1’’’’ plate with the union of 1p plate. This left antapical spine has a vertical radius and a broad list, and it can be very long, especially in the terminal cells of the chains (Figs. 11A, B; 12D; 13A), or shorter, with a length variation of 4.3-24.6 μm (Table 9, Figs. 11E-H, 12A, 13B). The organisms also present another shorter spine on the right side of the hypotheca (Table 9, Figs. 11A, 12A, D; 13A, B), although these sometimes also are quite developed (Fig. 12C), which corresponds to the development of the right sulcal list. The apical pore complex (APC) has a circular-triangular shape and is perfectly visualized both with LM and SEM; it is composed of the pore plate (Po) and a second plate that covers it partially (Pl), called cover plate by Steidinger et al. (1980), especially on the right side, with a comma shape, ornamented with spinules and with an evident rim on its periphery. The pore plate (Po), located below Pl, has 9-15 pores, the same size or sometimes slightly smaller than the trichocyst pores of the rest of the plates (diameter=0.25-0.5 μm, Fig. 13D) and in the left portion it usually has an attachment pore, much larger and generally elongated (2 μm long × 0.65 μm wide, Fig. 13D). The cells form chains that connect through the fixation pores located on the Po and Sp plates. It was observed that when the heat of the microscope lamp stresses the cells that form chains, they are easily separated.
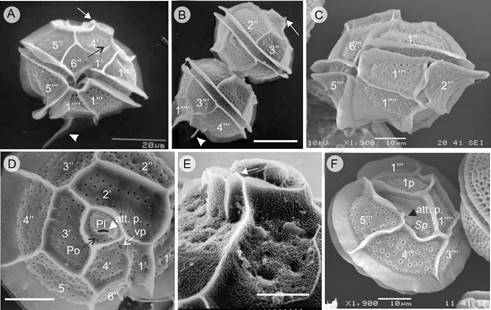
Figure 13: Pyrodinium bahamense var. compressum (Böhm) Steidinger, Tester et Taylor (SEM). A. solitary cell, ventral view showing the narrow 1’ plate, the ventral pore (black arrow), the small apical spine (white arrow) and the long left antapical spine (white arrow head); B. two cells in chain, dorsal view, showing short apical spine (white arrow) in the upper cell and the left antapical spine in the posterior cell (white arrow head); C. solitary cell, latero-ventral view showing the width of the crests and sulcal lists and the trichosyst pores in all the plates, including the cingular ones; D. cell in apical view, with four apical plates and detail of the Po with pores (black arrow), Pl plate with comma shape, the accessory pore (att.p. white arrow head), and the ventral pore (vp: white arrow); E. detail of the apical pore complex in epitheca, showing the short and flat apical horn and the small apical spine (white arrow); F. cell in antapical view, showing the posterior sulcal plate (Sp) with a small accessory pore (att.p. black arrow head). Nomenclature of plates of the theca according to Mertens et al. (2015): (‘)=Apical plates, (‘’)=Precingular plates, (‘’’)=Postcingular plates, (‘’’’)=Antapical plates; p=Intercalar posterior plate, C=Cingular plates. Po= Pore plate; Pl= Cover plate; Sp=Sulcal plates. Scales: A, B=20 µm; C, D, F=10 µm; E=5 µm.
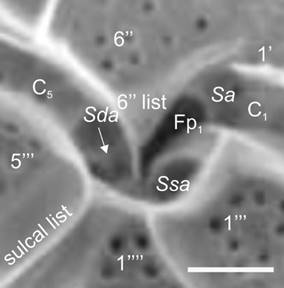
Figure 14: Pyrodinium bahamense var. compressum (Böhm) Steidinger, Tester et Taylor (SEM). Amplification of the ventral region of the cell evidencing three sulcal plates; Sa=Anterior sulcal plate, Sda=Right accesory sulcal plate (white arrow); Ssa=Left accessory sulcal plate, Fp1=Flagelar pore 1, C=Cingular plates; (‘)=Apical plates; (‘’)=Precingular plates; (‘’’)=Postcingular plates; (‘’’’)=Antapical plates. Mertens et al. (2015) was followed for the appointment of sulcal plates. Scale bar=5 µm.
The electron microscopy images revealed large numbers of trichocyst pores in
the theca plates and even in the cingular plates (0.3-0.7 μm in diameter;
Table 9), observable with the
optical microscope (Figs. 13B,C). An
observation that attracted attention during the HAB in July was the presence
of solitary cells or cells interspersed in a chain with much larger
dimensions, which could be gamete producing cells (gametangium) or
planocygotes (Figs. 11F-H). The
measurement of 30 cells yielded the following data: Lc=48.8-62 μm,
An important variation was found in the morphology and dimensions of the Pbc cells, both between the collection sites of the same sample, and in the different dates in which the HAB was monitored (Figs. 11A-I). The results of the dispersion graph of data between the dimensions (Lc) or Td of the Pyrodinium organisms from Acapulco with the cell length/transdiameter ratio (Lc/Td) (Figs. 15A-B), which also included data obtained from the literature for the two varieties of the species (Pbb and Pbc), showed that the majority of Acapulco organisms (87%) are concordant with that reported for var. compressum.
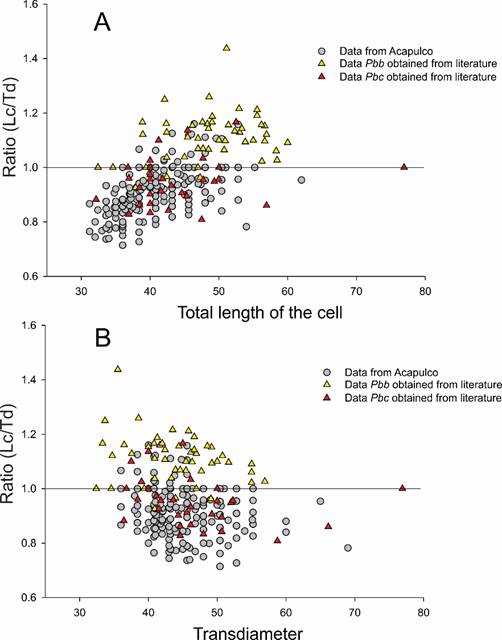
Figure 15: Graph of dispersion of data between the length (A) and the transdiameter (B) of the cells of Pyrodinium bahamense var. compressum (Böhm) Steidinger, Tester et Taylor, and the relationship between the rate of both measures. The analysis included data from highly compressed Acapulco cells (Lc/Td<1, n=481); and round or more elongated cells (Lc/Td≥1, n=70); as well as the measures reported in the literature or obtained from the published schemes or photographs of the literature of var. bahamense (Pbb, n=51) and from var. compressum (Pbc, n=30).
It was interesting to note that when the Pbc HAB began to decrease, the proportion of rounded or elongated cells increased, and similarly, the number of solitary or short-chain cells increased. On the contrary, when the population of Pbc grew, the number of compressed cells, typical of this variety increased, while cells with a morphology similar to var. bahamense (Pbb) were scarcer (Fig. 16). The analysis of variance showed significant differences in the dimensions of the cells by month of collection and locality (p<0.01), but not by depth (p>0.136), which translates into changes in the morphology of the individuals throughout the sampling cycle and between the localities. Given that the Lc/Td ratio may be due to the change in either the length of the cell, its width at the transdiameter level, or a mixture of both measurements, the variation of each of these characters (Lc and Td) was analyzed, finding that both had a coefficient of variation (VC) that was practically the same (VC=0.12 and VC=0.1 respectively, Table 8), which may mean that in the case of Pyrodinium, the distinct morphology of its varieties is due to the morphometric differences of both cellular dimensions (Lc and Td).
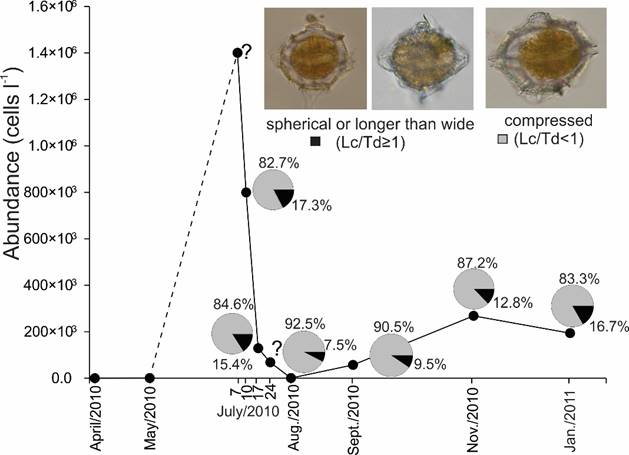
Figure 16: Maximum abundance of Pyrodinium bahamense var. compressum (Böhm) Steidinger, Tester et Taylor, and proportion of morphotypes (compressed cells or rounded and elongated cells) during different samplings in the Acapulco Bay, Guerrero, Mexico, in the HAB period (July 2010 to January 2011). The question mark on July 24 means we did not collect organisms that today, but the abundance data were taken from literature.
Relating cell dimensions with the environmental parameters, significant inverse correlation values were obtained between the cellular transdiameter of Pbc with the temperature (r=-0.166, p=0.0007, n=405) and dissolved oxygen in the water (r=-0.189, p=0.0001, n=405).
Pyrodinium bahamense cysts
Since February, and especially in May 2010, in the water column of the interior and exterior of the bay, Pb cysts were found (Fig. 5) with a density of up to 350 cysts l-1, when vegetative cells were not yet observed (Fig. 7A). During the HAB in July no cysts were observed in the water column in which the collections were made, in contrast to August and November. These spinous cysts were always spherical, with a diameter of 67.2-73 μm (Table 9 and commonly with bifurcated processes and slightly flattened open tip (Figs. 3D, 17A,B, also Figs. 8-9 in Meave del Castillo and Zamudio-Resendiz, 2014), called oblate and with scabrate wall, according to Matsuoka and Fukuyo (2000). The spinous processes have different lengths in the same cyst with a range of variation of Ls=3.9-13.4 μm, =8.4 (Table 9). Tables 9 and 10 present a summary of the morphometry of the vegetative cells and cysts of the Pbc population studied in Acapulco during the 2010 HABs and their comparison with morphometric data from other Pbc and Pbb populations recorded by other authors. It can be clearly seen that the morphotype of the organisms of Acapulco correspond better with the var. compressum in all the characters evaluated. In January 2011 agglomerations of solitary and immobile cells were found (Fig. 11I) at 1 m depth in Naval station, whose morphology resembles the temporary pedicle cysts of Pbc studied by Onda et al. (2014).
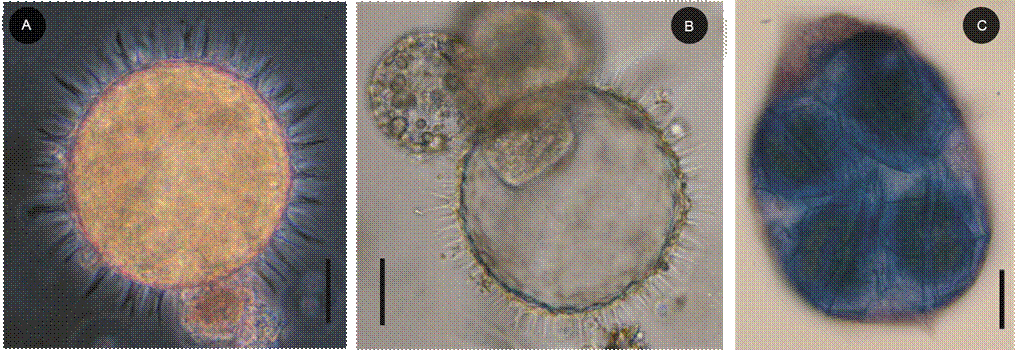
Figure 17: Cysts and predators (both of cysts and vegetative cells) of Pyrodinium bahamense var. compressum (Böhm) Steidinger, Tester et Taylor. A. cyst with content of Pyrodinium bahamense var. compressum photographed with optical microscope using phase contrast; B. empty Pyrodinium bahamense cyst parasitized by the dinoflagellate Chytriodinium affine (Dogiel) Chatton; C. vegetative cell of Gyrodinium fusus (Meunier) Akselman, dyed with Tripan blue, completely swollen due to the presence of four vegetative cells of Pyrodinium bahamense var compressum in its interior that were phagocytosed. Scale bars: A-C=20 µm.
Table 10: Summary of average dimensions of Pyrodinium bahamense var. compressum (Böhm) Steidinger, Tester et Taylor, obtained in Acapulco in the present study and other data obtained by different authors for both varieties: Pbc (Pyrodinium bahamense var. compressum), Pbb (P. bahamense var. bahamense). *=The average values were obtained by calculating them with the maximum and minimum values of different populations indicated in the literature. Veg. cell=Vegetative cells; Lc=Length of the cell (without apical horn, neither antapical spines); Td=Transdiameter; Lc/Td=Rate cell length/transdiameter); D=Diameter; Ls=Length of the cysts spine-shaped processes with spine-shaped.
| Cortés-Altamirano and Hernández-Becerril (1992) | Mertens et al. (2015) | Matsuoka (1989); Matsuoka et al. (1989); McMinn (1992) | Steidinger et al. (1980) | Morquecho (2008); Morquecho et al. (2014) | Martínez-López et al. (2007) | Wall and Dale (1969) | Present study | General average | ||
|
Veg.
cell Pbc | ||||||||||
| x̅, Lc (µm) | 41* | 48? | 42.84 | 39.2 | 39.6 | 40.6 | ||||
| x̅, Td (µm) | 49.5* | 44 | 47.97 | 44.1 | 44.9 | 46.1 | ||||
| Lc/Td | 0.83* | ¿? | 0.89 | 0.89 | 0.88 | 0.87 | ||||
| Pbb | ||||||||||
| x̅, Lc (µm) | 51* | 51.11 | 42.5 | 41.9 | 44 | 46.10 | ||||
| x̅, Td (µm) | 46.7* | 49.09 | 39.9 | 43.8 | 43 | 44.50 | ||||
| Lc/Td | 1.09* | 1.04 | 1.06 | 0.95 | 1.02 | 1.03 | ||||
| Cysts | ||||||||||
| Pbc | ||||||||||
| x̅, D (µm) | 53.56* | 61.7 | 70.1 | 61.8 | ||||||
| x̅, Ls (µm) | 10* | 15.5 | 8.3 | 11.3 | ||||||
| Pbb | ||||||||||
| x̅, D (µm) | 50.35* | 49.5* | 40* | 49.6 | ||||||
| x̅, Ls (µm) | 8.7* | 9 * | 9* | 8.9 | ||||||
Predation
It has been reported that some species of phagotrophic dinoflagellates consume Gc as is the case of Noctiluca scintillans and Polikrikos kofoidii Chatton (Holmes et al., 1967; Alonso-Rodríguez et al., 2005; Bustillos-Guzmán et al., 2013). Since such dinoflagellate species were recorded in the analyzed samples, their density was plotted along the year with the density of Gc (Fig. 5), observing that when the abundance of Gc increased, those of N. scintillans also increased in January 2010, and Polykrikos schwartzi Bütschli in November 2010 (Fig. 5), reaching abundance values of up to 3×103 cells l-1, coinciding with the highest values of Gc observed.
On the other hand, during the Pbc HAB in the samples analyzed in the Acapulco Bay, one to four cells of this dinoflagellate were observed inside the heterotrophic-phagotrophic dinoflagellate Gyrodinium fusus (Meunier) Akselman (Fig. 17C, also Figs. 6-7 in Meave del Castillo and Zamudio-Resendiz, 2014), especially in the months of July and November 2010, meaning that 1-3% of the Pbc cells registered in the month of November 2010 were being phagocytosed by G. fusus. In that month this phagotrophic species reached a maximum of 7.9×103 cells l-1 in the Centro locality, 3 m deep and 9×103 cells l-1 in January 2011 (Fig. 5), probably being an important biological factor for the decrease of Pbc abundance. It was also observed that from February 2010 and until November 2010, live Pbc cysts, and even empty cysts, had attached trophonts of the parasite dinoflagellate Chytriodinium affine (Fig. 17B, and Figs. 8-9 in Meave del Castillo and Zamudio-Resendiz, 2014).
Toxicity
Although the toxicity of the two species was not measured in the present study, at the moment of the highest Gc abundances (November 2010) and during the HAB of Pbc in July 2010 in Acapulco Bay, the Laboratory of Public Health of Guerrero State (COFEPRIS, 2010) reported saxitoxin present in mussels from localities both within the bay, and nearby coastal areas, corroborating that when the Gc HAB co-occurred with Pbc in November 2010, toxicity values of 392-739 μg SXT eq.100 g-1 were recorded (Gárate-Lizárraga et al., 2012). Meanwhile a HAB of Pbc (July 2010) caused a higher toxicity: 893-1388 μg SXT eq.100 g-1 in Chama mexicana Broderip (Gárate-Lizárraga et al., 2012).
Evaluation of physicochemical and climatic parameters
The maximum, minimum, average, and standard deviation values of the physicochemical data are presented in Tables 11-12, where it is observed that, according to the coefficient of variation of annual values, the physico-chemical parameters with greater variation were ammonium (1.04), nitrites+nitrates (1.0) and silicates (0.73). The water temperature fluctuated throughout the year, from 20.5 °C (January 2011, Bocana, 50 m depth) to 33 °C (October 2009, Puerto Marqués, 5 m depth), with a mean value of 27.9 °C, S=1.06 °C (Table 11). The highest average values occurred in October 2009 (30.66 °C, Table 11) and from July to September 2010 (28.8-29.7 °C, Table 11), and the lowest temperatures at the end of the year and the beginning of the following one (November 2010 to January 2011) (25.48-25.97 °C, Table 11). Salinity varied between 30.0 (October 2009, Puerto Marqués, 1-30 m depth; 10/July/2010, several places, 1-20 m depth; 17/July/2010, Puerto Marqués, 1-3 m depth) and 38.3 (September 2010, Bocana, 1 m depth), with x=32.9 and S=0.68. The lowest salinity (30.8-31) occurred in the rainy season (July 2010). Surprisingly, in September (month in which the rains are still important (200 mm), the average salinity value was high (34.83, Table 11), which may indicate the intrusion of a hotter and more saline oceanic current into Acapulco Bay.
Table 11: Monthly ranges, averages and standard deviations of physical parameters of the Acapulco Bay, Guerrero, Mexico, in the period of October/2009 to January/ 2011. Coefficient of variation (CV) for annual data is also determined. Zeu=-Zsd ln (0.01)/1.44 (Kirk, 1994). Temp.=Water temperature, O2=Disolved oxygen, Zeu=Depth of the euphotic zone.
| Date | (Min-Max) x̅ | Salinity (Min-Max) x̅, S | O (ml/l-1) 2 (Min-Max) x̅, S | Zeu (m) (Min-Max) x̅, S | |
| 2009 October | (29-33) 30.66±1.002 |
(30-35) 32.46±1.66 | - | - | |
| 2010 | |||||
| March | (20.9-27.2) 25.67±1.39 |
(33.9-34.5) 34.13±0.1 |
(0.9-7.9) 5.85±1.6 |
(12.8-22.4) 17.8±3.4 |
|
| May | (23.5-28.2) 27.31±0.74 |
(34.4-34.7) 34.50±0.05 |
(4-7.4) 6.67±0.54 |
(16.3-30.4) 23.6±4.9 |
|
| 10/July | (23.5-29.7) 28.50±1.42 |
(30-33) 30.82±0.93 |
(3.1-10.9) 7.24±2 |
(12.8-39.7) 22.8±10.7 |
|
| 17/July | (25.5-30.2) 29.07±0.7 |
(30-32) 30.97±0.68 |
(5-7.5) 6.38±0.31 |
(6.6-17.6) 11.3±3.7 |
|
| August | (26.6-30.6) 29.69±0.85 |
(32.3-33.5) 32.70±0.26 |
(3-6.1) 5.30±0.69 |
(9.6-21.4) 15.1±4.6 |
|
| September | (24.5-30.2) 28.80±1.24 |
(33.5-38.3) 34.83±0.93 |
(2.1-10) 7.13±1.7 |
(7.4-16.0) 11.5±3.1 |
|
| November | (23.9-27.2) 25.97±0.82 |
(30-37) 31.45±1.39 |
(1.9-8) 4.79±1.6 |
(12.8-16.6) 15.1±1.5 |
|
| 2011 | |||||
| January | (20.5-27.4) 25.48±1.34 |
(33.8-34.4) 34.08±0.13 |
(0.79-6.7) 3.50±1.2 |
(16-25.6) 21.6±3.6 |
|
| Annual data | (20.5-33) 27.91±1.06 CV=0.03 |
(30-38.3) 32.9±0.68 CV=0.02 |
(0.79-10.9) 5.85±1.2 CV=0.2 |
(6.6-39.7) 17.35±4.4 CV=0.25 |
|
Dissolved oxygen varied between 0.79 and 10.9 ml l-1. The highest average concentrations were present in July and September (rainy season) (7.24 and 7.13 mg l-1, respectively; Table 11). The depth of the photic zone varied throughout the study period from 6.6 to 39.7 m, observing that in the rainy season (July to September), the depth decreased considerably due to the increase in water turbidity, with the monthly average minimum of 11.3 m in July 2010 (Table 11).
In relation to nutrients, silicate concentration was low (0.001-8.4 µM, Table 12) throughout the year, showing little variation; however, some slightly higher values occurred in some months (8.2 µM in March 2010, Centro, 10 m depth, and 8.4 µM in August 2010, Sinfonía, 1 m depth). In the case of phosphates, concentrations ranged between 0.02-13.6 µM, with the highest average values in August 2010 (Table 12) in Muelle at 10 m depth and with an annual average of 7.39 µM (Table 12). Nitrites+nitrates showed values between 0.001-18.5 µM, with the highest in the month of November 2010 (Table 12), with a yearly average of 2.67 µM (Table 12). During July 2010 (occurrence of Pbc HAB), low concentrations of nitrogenous forms were registered (1.67-2.67 µM nitrites+nitrates and 1.54-2.1 µM ammonium; Table 12), but high phosphates (1.7-3.3 µM, Table 12), while in November 2010 (occurrence of Gc HAB), there was an increase of nitrites+nitrates (=5.07 µM, Table 12), but ammonium (0.48 µM) and phosphate (1.61 µM) decreased (Table 12). Chl-a in Acapulco Bay was variable throughout the year, ranging between 1.07-46.3 µg l-1, finding the highest concentrations on July/10/2010 (average 10.39 µg l-1; Table 4), coinciding with the HAB of the autotrophic taxon Pbc. With the exception of January 2011, when there was a significant nitrogen loading (nitrates and ammonium mainly, in that order), the stoichiometric relationships Si:N and Si:P showed that in Acapulco there was a potential nitrogen and silicate limitation, as nutrient ratios were below their optimal values (Si:N<1 and Si:P<16; data not shown). These results indicate that the phytoplankton nutrient uptake, as well as low nutrient loading, conditioned the phytoplankton succession in Acapulco Bay on temporal and spatial scales (Rocha et al., 2002).
Table 12: Monthly ranges (minimum to maximum), averages (x) and standard deviations (S) of chemical parameters (nutrients) of the Acapulco Bay, Guerrero, Mexico, in the period of October/2009 to January/2011. Coefficient of variation (CV) for annual data is also determined. SiO2=Silicates, PO43-=Phosphates, NO2-=Nitrites, NO3- =Nitrates, NH4- =Ammonium.
| SiO2 (µM) | PO 3- (µM) 4 | NO - (µM) 2 | NO - (µM) 3 | NO2+NO3 (µM) | NH - (µM) 4 | |
| Date | (Min, Max) x̅, S | (Min, Max) x̅, S | (Min, Max) x̅, S | (Min, Max) x̅, S | (Min, Max) x̅, S | (Min, Max) x̅, S |
| Oct./2009 | (0.33-2.7) 1±0.46 |
(0.53-11.1) 9.11±2.56 |
(0.001-0.42) 0.12±0.10 |
(0.02-6.1) 2.07±1.77 |
(0.001-6.5) 1.53±1.76 |
(2.43-33.41) 8.23±6.59 |
| March/2010 | (0.001-8.2) 1.29±1.60 |
(0.31-2.5) 1.29±0.59 |
(0.11-0.68) 0.40±0.15 |
(0.001-9.1) 1.32±1.86 |
(0.001-9.7) 1.68±1.92 |
(0.26-12.22) 2.09±2.39 |
| May./2020 | (0.35-1.9) 0.85±0.38 |
(0.28-2.4) 1.13±0.43 |
(0.22-1.0) 0.43±0.21 |
(0.001-13.9) 1.60±2.44 |
(0.37-14.3) 1.99±2.43 |
(0.04-5.21) 0.86±0.99 |
| 10/July/2010 | (0.48-4.5) 1.00±0.70 |
(0.17-4.5) 1.70±1.11 |
(0.12-0.92) 0.42±0.20 |
(0.04-13.6) 2.25±3.13 |
(0.28-14.3) 2.67±3.21 |
(0.03-26.41) 2.10±4.50 |
| 17/July/2010 | (0.51-2.4) 1.16±0.43 |
(0.02-5.8) 3.30±1.37 |
(0.08-2.1) 0.30±0.33 |
(0.11-8.1) 1.36±1.35 |
(0.25-9.0) 1.67±1.53 |
(0.08-6.55) 1.54±1.51 |
| Aug./2010 | (0.14-8.4) 0.97±1.31 |
(1.78-13.6) 7.39±2.68 |
(0.15-1.5) 0.54±0.32 |
(0.001-6.3) 1.14±1.40 |
(0.19-7.2) 1.69±1.54 |
(0.05-2.00) 0.54±0.44 |
| Sept./2010 | (0.001-1.3) 0.55±0.26 |
(0.33-3.2) 1.35±0.66 |
(0.23-0.76) 0.40±0.14 |
(0.15-17.8) 3.83±4.23 |
(0.40-18.5) 4.22±4.33 |
(0.03-6.89) 2.10±1.46 |
| Nov./2010 | (0.14-0.9) 0.52±0.18 |
(0.61-3.1) 1.61±0.65 |
(0.10-1.1) 0.34±0.20 |
(0.26-15.5) 4.73±4.01 |
(0.45-16.2) 5.07±4.12 |
(0.26-0.91) 0.48±0.16 |
| Jan/2011 | (0.23-2.5) 0.83±0.63 |
(0.14-3.5) 1.14±0.74 |
(0.03-6.74) 1.8±1.9 |
(0.16-14.7) 2.11±3.08 |
(0.16-15.3) 3.4±3.32 |
(0.04-19.32) 3.76±4.55 |
| Annual data | (0.001-8.4) 0.91±0.66 CV=0.73 |
(0.02-13.6) 3.11±1.17 CV=0.37. |
(0.001-6.74) 0.52±0.39 CV=0.75 |
(0.001-17.8) 2.27±2.59 CV=1.14 |
(0.001-18.5) 2.67 ±2.68 CV=1 |
(0.03-33.41) 2.4 ±2.51 CV=1.04 |
Figure 18 shows the monthly average of atmospheric temperature data for the last 37 years before 2010 in Acapulco Bay and during the year 2010, as well as the precipitation data for the same periods. In the region, the warmest season (>28 °C) occurs from May to October. May and June are the months with the highest environmental temperature, while from November to April, average monthly temperatures were lower. Regarding precipitation, the rainy season in the bay occurs from June to October, with the highest precipitation in August (30 years average of 200 mm), but in 2010 the precipitation in this month was close to 500 mm, more than two times higher than the average (Fig. 18).
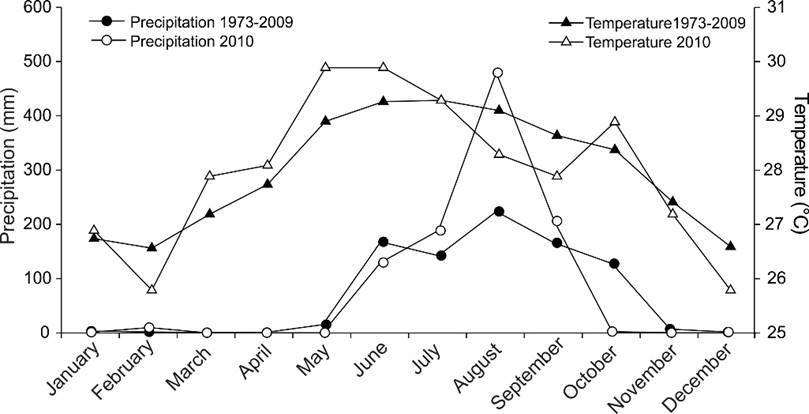
Figure 18: Average monthly values of environmental temperature and precipitation (rain) during the period from January to December 2010, built with data from the weather station 76850 located in Acapulco Bay, Guerrero, Mexico, in relation to the averages of these parameters in the 35 years prior to the year of sampling. Full circles and full triangles=Average monthly data from 1973 to 2009; Empty circles and empty triangles=Average monthly data for the year 2010.
Relation of abundance of toxic dinoflagellates with environmental parameters
Analyses showed that Gc abundances were positively and significantly correlated with total abundance of phytoplankton (r=0.24, p=0.0001, n=315) and inversely with temperature (r=-0.151, p=0.007, n=314). Pbc abundance correlations were significant (p<0.01) and direct with the total abundance of phytoplankton (r=0.146, p=0.009, n=315), oxygen concentration (r=0.268, p=0.0001, n=274), and Chl-a (r=0.765, p=0.0001, n=310) and inversely with salinity (r=-0.156, p=0.006, n=314).
According to interset correlations of the CCA, salinity and temperature were the most important environmental variables affecting the abundance of phytoplankton species (Fig. 19). The CCA also revealed relations between species abundance and environmental variables. Thus, Gc showed an inverse relation with salinity, NO2, and NH4. In contrast, Pbc abundances showed an inverse relation with salinity and a direct relation with temperature (Fig. 19). Moreover, this species showed a direct relationship with the concentration of dissolved oxygen and phosphates. To investigate the relationship of the two taxa with the physicochemical parameters, graphs of the abundances of both species were made against each of the parameters separately. In this way the abundance of both taxa was plotted with respect to the average water temperature per month (Fig. 20A), and the rate of change of temperature between months and the annual average (Fig. 20B) during the annual cycle in 2010, observing that the increase in Pbc coincided with the increment in water temperature (1.05 °C), while Gc HAB coincided with a change in water temperature of 0.947 °C, which means that there was a decrease in temperature.
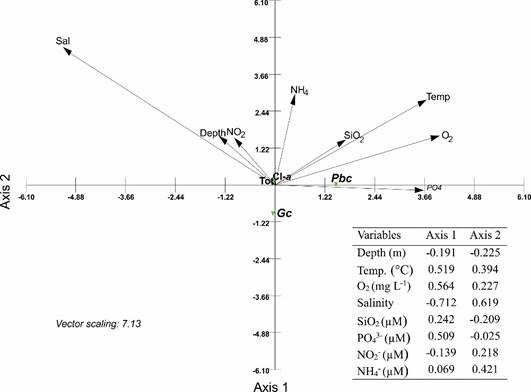
Figure 19: Canonical Correlation Analysis bi-plot of the chlorophyll-a (Cl-a), abundance of total phytoplankton (Tot.), Gymnodinium catenatum Graham (Gc), and Pyrodinium bahamense var. compressum (Böhm) Steidinger, Tester et Taylor (Pbc), in relation to physicochemical parameters. Sal=Water salinity; Temp=Water temperature; O2=Oxygen dissolved in water. Length and direction of arrows indicate the relative importance and direction of change of environmental variables. The table shows the interset correlations derived from the canonical analysis, of the abundance dependent data matrix and the physicochemical data matrix evaluated in Acapulco Bay, Guerrero, Mexico, during October 2009 to January 2011.
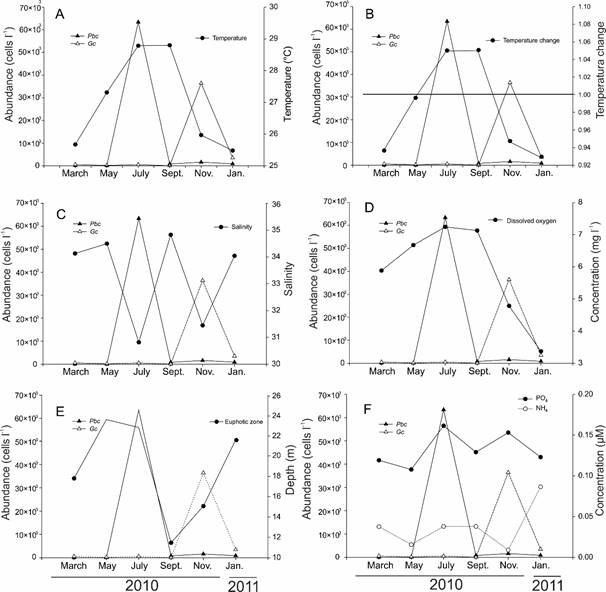
Figure 20: Average abundances per month of the species Gymnodinium catenatum Graham (Gc) and Pyrodinium bahamense var. compressum (Böhm) Steidinger, Tester et Taylor (Pbc) in Acapulco Bay, Guerrero, Mexico, in the months studied during the annual cycle in 2010, in relation to environmental parameters. A. abundance of the species against the average water temperature per month; B. abundance of the species against the change in water temperature. To obtain the values, the temperature main annual value (27.41°C) was subtracted from the average monthly temperature values; thus values >1 indicate temperature increase and values <1 temperature decrease; C. abundance of the species against the average monthly salinity; D. abundance of the species against the average monthly of dissolved oxygen concentration; E. abundance of the species against the average monthly of the depth of euphotic layer (Zeu); F. abundance of the species against the average monthly of concentration of phosphates and ammonium.
On the other hand, in figure 20C, it is observed that the increase in the abundance of both dinoflagellates coincided with a decrease in salinity. The increase in dissolved oxygen coincided with the higher Pbc densities, whereas when the highest abundances of Gc were present, oxygen levels decreased (Fig. 20D). Moreover, the density of both taxa was plotted with the depth of the photic layer (Fig. 20E) and with the concentration of phosphates and ammonium (Fig. 20F), where it can be distinguished that when the Pbc HAB began in July 2010, the water column was more transparent and therefore, the photic layer reached deeper than when the Gc HAB was present along with Pbc in November 2010. Regarding the most important nutrients (phosphates and ammonium), especially phosphates, concentrations increased as the abundance of Pbc elevated in July 2010. Contrarily, ammonium decreased when the abundance of Gc incremented in November of the same year.
Satellite images
In the satellite images of Figs. 21A-P, corresponding to weekly averages of Chl-a from January to December 2010, the formation of a phytoplankton bloom in the Gulf of Tehuantepec during the month of February is observed in chronological order. At the end of March (Fig. 21F), this bloom moved to the west. In May (Fig. 21G), high Chl-a values are were throughout the southern Pacific coast of Mexico, at which time Pbc cysts with cellular content were first observed in the water column of the Bay of Acapulco, even though there was no record of vegetative cells of this taxon. From June to September (Figs. 21 H-M), no high Chl-a values were registered that could indicate the presence of phytoplankton blooms in the Mexican Pacific. However, quantification of phytoplankton in Acapulco Bay verified the existence of a Pbc HAB during the month of July, ending on August/5 (320 cells l-1). In mid-October (Fig. 21N), the formation of a phytoplankton bloom in the Gulf of Tehuantepec is observed, which later (November, Fig. 21O) again moved westward. This second event matched with the occurrence of the second Pbc HAB recorded in Acapulco Bay during the present study in the November/20 collection. This second HAB was also registered by other authors in coastal areas of Guerrero and Michoacán (Gárate-Lizárraga et al., 2012) and even on the southern coast of the peninsula of Baja California, at the entrance of the Gulf of California (Gárate-Lizárraga and González- Armas, 2011).
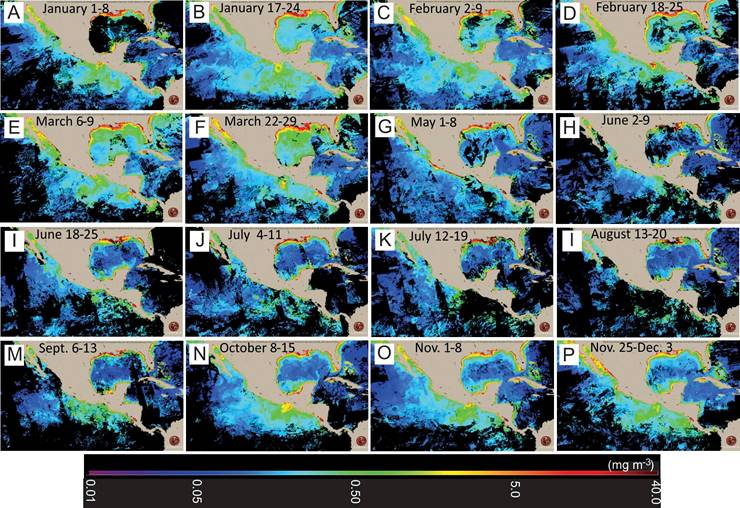
Figure 21: Chlorophyll-a satellite images corresponding to weekly averages of different months of the year 2010. A. 1-8/January; B. 17-24/January; C. 2-9/February; D. 18-25/February; E. 6-9/March; F. 22-29/March; G. 1-8/May; H. 2-9/June; I. 18-25/ June; J. 4-11/July; K. 12-19/July; L. 13-20/August; M. 6-13/September; N. 8-15/October; O. 1-8/November; P. 25/November to 25/December. The images were obtained from .
HABs and global climatic factors
The location and dates of HABs during the study period (October 2009 to March 2011) were recorded on a graph of temperature anomalies elaborated with standardized values of the multivariate index of the ENSO (MEI; Meave del Castillo and Zamudio-Resendiz, 2018) Figure 18 indicates that the end of 2009 and the first months of 2010 corresponded to “El Niño” conditions, and from June to December “La Niña”.
The monthly averages of environmental temperature and precipitation in Acapulco were plotted for 2010 and contrasted to the monthly averages from the previous 30 years. The comparison clearly showed that 2010 was a year with higher temperatures from January to June, and the rest of the year a little colder. However, the year 2010 had more rainfall until September; it reached a peak of ~500 mm of rain in August. Pbc is a tropical eurythermic (20-30 °C) and euryhaline species, whitstanding brackish (20) to marine (36) salinities in tropical regions (Usup et al., 2012). Such conditons exist in Acapulco Bay making it a suitable habitat for Pbc. A Pbc HAB occurred in the Bay in July, after intense rains delivered terrigenous elements, necessary for the suitable growth of this dinoflagellate. The Pbc inoculum responsible for the bloom could have been present in the Bay as cysts, or cysts and high concentrations of phosphorus could have been transported from the east. In November 2010 (“La Niña” conditions) the decrease in temperature along with the increase in nitrogen concentrations (especially ammonium) offered the opportunity for Gc, a common inhabitant in Acapulco Bay, to flourish and produce a HAB. However, since the conditions were still favorable for the development of Pbc, these two toxic dinoflagellates coexisted.
Discussion
Total abundance of phytoplankton in Acapulco Bay
The results showed that diatoms are the most abundant group in Acapulco, with the exception of May (warm dry season). The diversity of the phytoplankton community of Acapulco Bay recorded in this study (H'=1.9-4.7, annual mean=3.8) is high compared to other bays in the Mexican Pacific (La Paz, BCS Bay, H'=0.1-4.7, Muciño-Márquez et al., 2018; Bahía Concepción, H'=3.43-3.67, Estradas-Romero et al., 2009). An extensive study covering several oceanic locations of the Mexican Pacific reports H'=1.89-3.83, being H'=2.24 the value for the region near Acapulco (Gaxiola Castro et al., 1987). The data of H'=1.4-3.92, presented by Rojas-Herrera et al. (2012), for an annual cycle carried out in 2009 in Acapulco Bay, are probably an underestimation due to lack of proper identification of all species, especially small and rare ones. Only the HAB of Pbc occurred during July 2010, especially on the 10th, when a maximum abundance of 773×103 cells l-1 was registered, affecting both the H' of the phytoplankton community and the average values of the phytoplankton biomass in Acapulco.
The low depth of the photic zone (i.e. a high turbidity of the water column; 11.5±3.1), which occurred in September, could be a consequence of the high total density of phytoplankton in the water column (577.57×103 cells l-1). However, Chl-a concentration was low (monthly average of 4.07 μg l-1) and the predominant diatom species in that month were very small (Pseudo-nitzschia spp., Thalassionema nitzschioides (Grunow) Mereschkowsky, T. frauenfeldii (Grunow) Tempère et Peragallo, Skeletonema pseudocostatum Medlin and Rhizosolenia setigera f. pungens (Cleve-Euler) Brunel. It is assumed that the turbidity of the water column was due to the suspended solids that entered the bay through the small creeks in the rainy season or the turbulence generated by storms rather than by phytoplankton. It has been pointed out that Acapulco Bay receives a heavy discharge of household waste (4086.58 m3 year-1) during the rainy season, via 78 streams that flow into its margins and of some hotels that surround it, increasing the presence of suspended solids, nitrogen, phoshphorus, as well as biological oxygen demand (BOD) which exceed the limits allowed by the Official Mexican Regulations (Sampedro-Rosas et al., 2014).
On the other hand, the depth of the photic layer (15.1±1.5 m) in November 2010 was due to the high concentration of total phytoplankton (662.5×103 cells l-1), which was corroborated by the high Chl-a (6.54 μg l-1). This may explain why Gc and Pbc had low relative abundances in this month even though both species occurred jointly forming a HAB. This could mean that competition with several other species present at that moment prevented this toxic species to dominate the community during this month.
Morphometry of Pyrodinium bahamense var. compressum
The Pbc cells collected in Acapulco from July/7/2010 to January/14/2011, had cell length (Lc), transdiameter (Td), ratio (Lc/Td), length of the apical horn, and length of the left sulcal list quite similar to what has been reported in the literature for var. compressum and with highly similar morphometry with the specimens with the Pbc morphotype of New Guinea (Balech, 1985, Table 10). Although Mertens et al. (2015), based on their data, questioned all the differences pointed out by Steidinger et al. (1980) for the two varieties of Pb, in most of the references consulted, var. compressum always has values of Td above those of Lc, so the ratio Lc/Td is below 0.9 (Table 10). Contrarily, the Pbb cells have a length generally greater than Td and therefore the Lc/Td ratio is higher than 1. It was also interesting that in the morphometrical comparison of Pbc specimens that formed the HAB in Acapulco, with those of Pbb studied by Martínez-López et al. (2007) and Morquecho (2008) in shallow, slightly turbulent and mangrove areas, near the San José Island in the Gulf of California, Mexican Pacific, morphological differences of the varieties were maintained although both locations are located in the Mexican Pacific. Thus, Pbc cells of Acapulco are shorter and wider than Pbb cells of Isla San José. In addition, the apical horn and the left antapical spine are shorter in Pbc and, on the contrary, the diameter of the cysts is greater in Pbc. The literature shows that cysts in the water column or recent sediments of Pbc have a larger diameter and can surpass 70 μm (Matsuoka, 1989; Matsuoka et al., 1989). This happened in the current study (Table 10), while cysts of var. bahamense have a diameter that generally does not exceed 55 μm (Wall and Dale, 1969; Morquecho, 2008).
Although it has been mentioned in the literature that Pbc can have five apical plates, all the organisms observed in Acapulco constantly had only four apical plates. In this study not all sulca plates were analyzed in detail. In literature (Steidinger et al., 1980; Balech, 1985; Mertens et al., 2015), it has been established that, regardless of of the variety, Pb presents a constant number of six cingular plates (C1-C6), but the authors differ in the number of sulcal and postcingular plates. Balech (1985) and Mertens et al. (2015) indicate the existence of nine sulcal plates: Sa, Sdacc, Sda, Ssa, Sma, Smp, Sdp, Ssp and Sp, being evident Sa, and especially Sp, that is perfectly visualized in the antapical view. Half of the sulcal plates were very difficult to visualize in ventral view, because they are hidden under plates 1p and 5’’’ of the hypotheca. Steidinger et al. (1980) and Fensome et al. (1993) consider that the plate 1’’’ (first postcingular plate) is tiny and located in the sulcus. However, Taylor and Fukuyo (1989) point out that this small plate is considered as the Ssa plate by Balech (1985), whereas Usup et al. (2012) and Mertens et al. (2015) consider that this small plate actually corresponds to a growth band. For that reason, all the recent authors account for nine sulcal plates and only five postcingular plates, instead of six as indicated by Steidinger et al. (1980).
HAB of the species
Gymnodinium catenatum
Comparing the HAB of Gc reported in the present study for November 2010 with other events reported in Acapulco from 2005 to 2009 by Gárate-Lizárraga et al. (2009, 2016), with an abundance of 1.6×106 to 10×106 cells l-1, or in other locations in the Mexican Pacific such as Mazatlán Bay, Sinaloa (1.0-1.15×106 cells l-1) (Mee et al., 1986; Cortés-Altamirano et al., 1995), Manzanillo Bay or Puerto Interior, Colima (2.5-3.8×106 cells l-1)(Morales-Blake et al., 2000; Figueroa-Torres and Zepeda-Esquivel, 2001; Zepeda-Esquivel and Meave del Castillo, 2007; Quijano-Sheggia et al., 2012), the present HAB was moderate, although with significant toxicity (392-739 μg SXT eq.100 g-1). Due to the high H' (4.27 bits) value of the phytoplankton community and the low relative abundance value of Gc (0.11-27.17%) during this HAB, we can conclude that Gc did not dominate the community. This could have been due to limiting environmental conditions restricting their maximum growth rate, or because of the competition of diatoms in Acapulco Bay that are abundant in the cold dry season (November to February).
Rojas-Herrera et al. (2012) also reported the increase in density of Gc in October and November of the previous year (2009), although with lower maximum density (12×103-17×103 cells l-1, Fig. 5). In this study, on October/24/2009 density values close to 6.2×103 cells l-1 were recorded for Gc. These abundances above background levels for the Bay suggest its occurrence as a HAB. It is striking that most of the Gc HAB reports in locations further north in the Mexican Pacific (including the bays of Mazatlán and Manzanillo) occur from March to April (eg. Mee et al., 1986), with density up to 3.8×106 cells. l-1. In contrast, in Acapulco the Gc HAB seems to occur rather towards the end of the year (October-December) or the beginning of the next one (January to February), as found in this study (November 2010) similar to previous reports for Acapulco (Gárate-Lizárraga et al., 2007, 2009, 2012; Bustamante-Gil, 2011; Rojas-Herrera et al., 2012; Pérez-Cruz et al., 2016). The relationship of this species with the evaluated physicochemical conditions indicates that Gc increased its abundance when temperature, salinity and ammonium decreased.
The difference of dates for the presence of Gc HAB in this tropical portion of the Mexican Pacific could be explained partially by the availability of nutrients, since in Acapulco the concentrations of ammonium and nitrites+nitrates are generally low throughout the year, reaching the maximum just at the end of a year and in the beginning of the next one (Table 12). The Gc bloom coincides with the decrease in ammonium in November and probably is responsible for the decrease, since it is the preferred nitrogenous form (Hallegraeff et al., 2012). However, another factor that may favor Gc HABs at the end of the year in Acapulco is the decrease in water temperature during this cold dry season. Meave del Castillo and Zamudio-Resendiz (2018) showed that most of the Gc HABs reported in Acapulco Bay coincided with a decrease in temperature in the water column, which can be catalogued as “La Niña” events. The results of the analysis of the average water temperature throughout the annual cycle (January 2010 to February 2011) also showed a clear tendency to decrease from September to November by 2.8°C, being January 2011 the coldest month. It has been pointed out that different strains of Gc in the Mexican Pacific tolerate a wide temperature range (12-31 °C), with the maximum growth rates between 19-30 °C in the Gulf of California (Band-Schmidt et al., 2014), which is why the Mexican ecotype is classified as tropical (Hallegraeff et al., 2012). However, it has also been mentioned that in the North Pacific, HABs of Gc generally occur at temperatures between 16-25 °C (Cortés-Altamirano et al., 1999; Gárate-Lizárraga et al., 2004; Band-Schmidt et al., 2010). In Acapulco the water temperature interval in November 2010, when the greatest abundance of Gc occurred in the Bay, was 24-27.1 °C, and in November 2009 it was 29 °C. It can be assumed that it is not the temperature that favors the formation of the Gc HABs, but an abrupt decrease in temperatures causing water column changes in nutrient concentrations. Since it has been pointed out that Gc requires high concentrations of nutrients, mainly ammonium, to proliferate (Hallegraeff et al., 2012), surely the concentrations of ammonium along with those of nitrites+nitrates registered at the end of the year, open the window of opportunity for the occurrence of HAB of this species in November 2010, similar to those HABs registered by other authors on a comparable date for several years (Gárate-Lizárraga et al., 2007, 2012, Bustamante-Gil, 2011; Rojas Herrera et al., 2012; Pérez-Cruz et al., 2016). In the present study, phosphates increased significantly in the rainy season and ammonium increased significantly especially in October 2009 and October 2010 (data not presented in this study), but had a drastic fall in November 2010, which can be explained by the increase in consumption, not only by the two toxic species (Gc and Pbc), which were abundant in that month, but also by the increase in the total phytoplankton density (from 576 cells l-1 in September 2010 to 662.5×103 cells l-1 in November 2010). This behavior has also been recorded by Rojas-Herrera et al. (2012), who observed a decrease in ammonium during October and November 2009, that coincided with an increase in phytoplankton density, from 248 to 709×103 cells l-1. Specifically, the moderate cell density of Gc registered in November 2010 could be due to the combination of several elements, among which the still high temperature (25.97 oC) and the abrupt fall of ammonium x=0.48 µM, S=0.16 µM) seem important. This may also be due to the competition with Pbc, which in this month reached a mean abundance of 1717 cells l-1 and a maximum density of 16,560 cells l-1. Considering the relatively low density of Gc cells observed during this event (188×103 cells l-1), the high toxicity can be explained by its coexistence with Pbc, also a producer of saxitoxins, especially knowing that during July 2010, when that species occurred as HAB in Acapulco Bay, the concentrations of saxitoxins reported in molluscs were very high, up to 2092 μg SXT eq.100 g-1 (COFEPRIS, 2010; Gárate-Lizárraga et al., 2012).
Pyrodinium bahamense var. compressum
The significant and direct relation of Pbc abundance with the total abundance of phytoplankton, oxygen concentration and biomass (Chl-a), suggests that the increase of phytoplankton on July 10 was due to the density of this species, which contributed more than 64% of the biomass of Chl-a. Consequently, there was an increase in dissolved oxygen concentration. Since February 2010, but with greater abundance in May 2010, live Pbc cysts (with cellular content) were found in the water column, indicating the potential to produce planktonic populations. However, it was not until July 2010 (during the rainy season) that adequate temperature, salinity and nutrient conditions within the bay were conducive for a Pbc bloom (Usup and Azanza, 1998). The timing of the presence of viable cysts and environmental conditions suitable for germination is important, although it has been pointed out that the P. bahamense cysts have a short period of forced dormancy from 2.5 to 3 months (Usup and Azanza, 1998; Hallegraeff, 1998), which can be explained by being a tropical species, since in the tropical zones the temperature is fairly stable throughout the year. The increase in the density of this species in July (Figs. 5, 7A) coincided with events reported from El Salvador to Los Cabos in Baja California Sur, Mexico, during the same year (Table 2, Licea at al., 2008; Gárate-Lizárraga and González-Armas, 2011). The HAB that was present in Acapulco (July 2010) can be considered moderate compared to the one that occurred along the coasts of El Salvador from November 2009 to May 2010, when Pbc reached densities of up to 15.3×106 cells l-1 (Table 2; Licea et al., 2008). Certainly the Pbc cysts found in the water column in the interior of Acapulco Bay since February 2010 and especially in May (day 15) of that same year, arrived by horizontal transport, hauled from that portion of the Central American Pacific to the Bay of Acapulco by the Mexican coastal current, which dominates the region during this time of year. Only five weeks after having observed the more abundant cysts of Pbc inside Acapulco Bay, on July/7/2010, the main HAB of this taxon in the bay was already occurring. In relation to the morphology of the cells of P. bahamense, something worth noticing during the HAB of the month of July was the presence of solitary cells, or cells interspersed within a chain with higher dimensions, which we assume are planozygotes or gametangia, which may indicate the existence of adverse environmental conditions that led to the species reproducing sexually and thus contributed to the formation of new hypnozygotes. It was also important to find in the month of January 2011, at 10 m depth, a little before the vegetative cells completely disappeared from the water column of Acapulco Bay, masses of agglutinated cells, possibly corresponding to temporary pellicle cysts (Onda et al., 2014).
Considering that the cellular dimension (Lc/Tr ratio) of Pbc had a significant inverse correlation with temperature and dissolved O2, and since this variety is completely tropical (Usup et al., 1994), certainly when the organisms are actively dividing most of the cells are shorter than long (giving the compressed appearance of the entire population), instead, when the cell division is relaxed (eg. when the HAB starts to decrease), the cells lengthen further, having an Lc/Td ratio greater than 1.0 and resembling the morphotype of var. bahamense. In addition, the cells of the ends of the chains, or solitary cells of Pbc, have greater similarity with the var. bahamense, potentially explaining the conclusions of Vargas-Montero and Freer (2003) and Gárate-Lizárraga and González-Armas (2011) that we consider to be erroneous, about the occurrence of both varieties in the Central American and Mexican Pbc HABs, respectively. In addition, the following questions arise: why is the Pbb morphotype always scarce during the Pbc HABs of the Mexican Pacific?; as well as, why do these morphotypes show differences in both the intervals and the average values of the measurements indicated for Pbb in the literature? The analysis carried out in this study showed that in the population of Pyrodinium bahamense in Acapulco Bay the highest number of cells coincided with the var. compressum. The longest cells appeared just when the bloom was already declining, reinforcing the assumption that the growth rate is the cause of the existence of cells with variable morphology within the same population of Pbc, and not the coexistence of both varieties. We believe that the different morphotypes found in a Pbc HAB are part of the life cycle, but to verify this it is necessary to carry out experiments with laboratory cultures and population studies in the field, in addition to molecular genetics. It is also necessary to assess the type of environment, since in the literature it is indicated that the varieties of Pyrodinium bahamense develop in very different environments: mangroves, coastal lagoons and enclosed estuaries for the var. bahamense (Pbb) and open ocean or dynamic coastal areas for var. compressum (Pbc) (Phlips et al., 2006). Occasionally, Pbc HABs remain unnoticed in the coastal area and only its effects are noticed e.g., the mass mortality of hundreds of turtles Chelonia mydas Linnaeus (1758) and Lepidochelys olivacea Eschscholtz (1829), that occurred in the coast from El Salvador in October-November 2013 and November 2017. Saxitoxins were recorded in different organs and gastric contents of the turtles up to 1616 µg SXT eq.100 g-1 of tissue, as well as Pyrodinium cells in their digestive tract. However, Pbc was not registered in the water column or it was extremely scarce (20 cells l-1) (Amaya et al., 2018).
Although the Pbc HAB for July probably started to decline shortly after the 7th, it still lasted a little over a month because the negative growth rates recorded in the study (-0.082 to -0.575) were lower or similar to the value of 0.3 divisions/day recorded in the field for this species variety by Usup and Azanza (1998).
The termination of the HAB in January could be due to multiple causes, but temperature is suspected to have played an important role as was the increase in turbulence. In July 2010, the water column was stratified, with a thermocline at 10 m, but in January 2011 the column was more homogeneous. The organisms with morphology similar to the temporal pellicle cyst stage, found at the end of the period of occurrence of Pbc in Acapulco Bay, as masses of agglutinated cells, at 10 m depth, could be produced by multiple causes, even the lack of adequate light (Onda et al., 2014).
It has been pointed out that Pbc is a poor competitor of inorganic nutrients, i.e. it has low affinity for inorganic nutrients and therefore, it produces blooms as long as it is supported by organic nutrients (Usup and Azanza, 1998), which suggests that it uses chelating agents to improve its nutrition (Bruland et al., 1991). Pbc also requires soil extracts that are not essential for an adequate growth rate, but for maintaining exponential growth for longer periods (Usup and Azanza, 1998). The soil extract among other things could contain humic substances and trace elements as Fe and Se (Boyer and Brand, 1998). There is evidence that trace elements may influence phytoplankton population dynamics, because they affected several physiological parameters and species composition (Boyer and Brand, 1998). Ingle and Martin (1971) reported the use of a Fe index to predict he occurrence of Karenia brevis (C.C. Davis) Gert Hansen & Moestrupin in Florida. Moreover, Fe limited blooms of Alexandrium sp. in the Gulf of Maine (Boyer and Brand, 1998).
Nevertheless, Se is the trace element considered to be among the most important for the growth of red tide-forming organisms (Hallegraeff, 1998; Boyer and Brand, 1998; Hatano and Imai, 2010). In coastal areas the concentration of Se is generally from 0.6 to 4.5 nM. However, this essential trace element becomes frequently limiting, since concentrations even as low as 0.02 nM are common in some places (Koike et al., 1993). Selenium is incorporated into the sea through rivers, the atmosphere (rain), sediments or sources of pollution such as refineries (Cutter, 1989). Hallegraeff (1998) points out that Se is the most important trace element for the formation of blooms, and along with mangrove runoff, are considered as niche-defining factor of Pb and Gc blooms. In the case of Gc, its absence reduces the exponential phase of growth in culture, as well as cell division developing only as short chains (Doblin et al., 1999). This species seems to tolerate high concentrations of Se (10-9 or 10- 11 M) without showing negative signs, although the best results have been obtained by adding a concentration of 10-7 M (Doblin et al., 1999). It has also been found that strains of Gc from different geographical regions present a variable requirement of selenium, as well as strains obtained from different dates of the same locality, which shows that the populations of Gc exhibit a wide phenotypic diversity regarding the use of traces of this element, partially explaining the differences of behavior of the taxon in different places (Doblin et al., 2000). For Pb, Usup and Azanza (1998) pointed out that the soil extract supplement (mainly as a source of Se) was important for its growth, regardless of the medium employed. Certainly, based on this, Mertens et al. (2015) used the ES-DK culture medium developed by Kokinos and Anderson (1995) with the addition of 10-7 M selenium (as sodium selenite) to cultivate Florida isolates of Pbb and Morquecho et al. (2014) successfully used the GSe medium to germinate Pbb cysts collected in Isla San José, BCS, Mexico. However, isolates of Pbc from Malaysia were unable to grow in an artificial sea-water based medium, even when supplemented with soil extract, showing the importance of other land-derived nutrientes in promoting bloom in coastal waters (Usup and Azanza, 1998). Dissolved organic matter (DOM) in form of humic substances increased both yield and growth rates of marine dinoflagellates and diatoms, suggesting that these substances acted as chelators and made the essential metals available for algae (Carisson and Granéli, 1998).
We can hypothesize that the presence of cysts in the water column of Acapulco Bay, whose dormancy was about to end, in conjunction with the high rainfall of 2010 (El Niño year) during the rainy period, generated abundant runoff from the temporary streams in July that incorporated Se and humic substances into the water column. Moreover, the rise in water temperature to 28.5 °C, the decrease in salinity from 34.5 to 30.8, and the increase in phosphates (from 0.62 to 1.7-7.4 µM), could have allowed Pbc to bloom during July 2010, and again in November 2010, although to a lesser extent because the conditions were not as favorable. We can assume that several of the conditions that led to the HAB in Acapulco Bay are not local but rather regional, because a Pbc HAB occurred from November 2009 in Central America, then in February 2010 in the Gulf of Tehuantepec, and finally from July to December 2010 in our study area. This HAB of the second half of 2010 was important in this oceanic zone, and even its distribution reached latitudes farther north than it usually does, i.e. entering into the Gulf of California (Gárate-Lizárraga and González-Armas, 2011).
Something that certainly facilitates the fact that Pbc HABs are durable is the ability of Pbc to take nutrients from the different layers of the water column, due to their vertical migrations. There are data indicating that a similar species of Alexandrium Halim can move at a speed of 1.5 m/hour in the laboratory (Fraga et al., 1989). These vertical migrations are more important, especially when the chains are long, being able to move up to 10 m/day (Fraga et al., 1989). In addition, it is important to consider its ability to produce temporary pellicle cysts that allow it to survive adverse physicochemical conditions (lack of light, turbulence, nutrient depletion; Onda et al., 2014).
Predators of Pyrodinium bahamense var. compressum
It has been reported that Pbc can be phagocytized by calanoid copepods and tintinid ciliates such as Favella sp. (Usup et al., 1989; Ramírez-Camarena et al., 1996), which occur in Acapulco Bay. However, an interesting finding of the present study was that vegetative cells were significantly preyed upon by the heterotrophic dinoflagellate Gyrodinium fusus (Meave del Castillo et al., 2012; Meave del Castillo and Zamudio-Resendiz, 2014) and their cysts parasitized by the dinoflagellate Chytriodinium affine, which up to now had been reported only as a parasite of crustacean eggs (Cachón and Cachón, 1968), including copepods (Gómez et al., 2009).
HABs toxicity
Regarding toxicity, the reported maximum levels of toxins of 739 μg STX eq.100 g-1 in November 2010 (COFEPRIS, 2010) could be due to a small extent to Gc, mainly because although chains of up to 32 cells were found, the average was six cells per chain, and it has been reported that chain length directly influences toxicity (Band-Schmidt et al., 2006; Hallegraeff et al., 2012). In addition, in relation to the toxicity of the Gc population, the toxicity is greater when environmental ammonium dominates rather than nitrates. In Acapulco Bay, during November 2010, the nitrogenous forms predominant were nitrites+nitrates, with ammonium being very low (0.48 μM as average value). Therefore, we can assume that the HAB toxicity of November 2010 in Acapulco Bay, where Gc and Pbc co-existed, was probably due mainly to Pbc, a taxon that in July 2010 proved to be quite toxic (up to 2092 μg SXT eq.100 g-1; COFEPRIS, 2010; Gárate-Lizárraga et al., 2012). Previously, it was believed that the toxic dinoflagellates Gc and Pbc did not compete, since the former has a rather warm distribution, while Pbc is distinctly tropical (Usup et al., 2012). However, the presence of both species in the same locality and with high densities is currently common (Gárate-Lizárraga et al., 2011). Certainly, Gc’s HAB production in November could also be related to the heavy rains of 2010 that increased the concentrations of Se, and probably also Fe and humic compounds as chelators in the water column (Carisson and Granéli, 1998).
Dinoflagellate HAB and climatic factors
The temperature anomaly data through the MEI index (Wolter, 2012) showed that the second half of 2010 was clearly a “La Niña” year, with an anomaly of almost 2 °C lower temperature (Fig. 6, in Meave del Castillo and Zamudio-Resendiz, 2018). In the quoted graph it can be clearly seen that the HABs of both toxic species involved in the present study (Gc and Pbc) during 2010, occurred in “La Niña” conditions. Meave del Castillo and Zamudio-Resendiz (2018) found that Gc HABs in Acapulco Bay frequently, but not necessarily, occur in “La Niña” conditions and they point out that the most important factor for Gc HAB production is the abrupt drop in water temperature in conjunction with the elevation of ammonium in the water.
In the case of Pbc this study corroborates what had already been mentioned through studies of Pb cysts in recent sediments from 1938 to 2010 in the Gulf of Tehuantepec in the Mexican Pacific (Sánchez-Cabeza et al., 2012) and also by Meave del Castillo and Zamudio-Resendiz (2018) for Acapulco Bay, all of which find that Pbc in the Mexican Pacific is related to “La Niña” and not to “El Niño” conditions, as Maclean (1989a) and Siringan et al. (2008) suggested for the western Pacific. Nevertheless, there is a point of agreement in the presence of HAB of Pbc for both the West and East Pacific, i.e. the torrential rains with monthly average values greater than 400 mm that seem important for the occurrence of the HAB. This shows that more than the phenomenons “El Niño” or “La Niña” per se, the important factor is the transition between “El Nino-La Niña”, which presents adequate conditions of both temperature and precipitation for the development of Pbc as a bloom. Again, the last Pbc HAB reported at the end of January 2016 along the coast of Oaxaca had a high mortality (118 organisms) of three turtle species Chelonia mydas Linnaeus (1758) (green), Lepidochelys olivacea Eschscholtz (1829) (olive ridley) and Eretmochelys imbricata Linnaeus (1776) (hawksbill) who presumably died due to poisoning related to a large number of salps (Pegea confoederata Forskål) that they consumed which in turn contained a large number of Pyrodinium cells. Maximum abundance of Pbc in the water was low (56×103 cells l-1) but the oysters (Striostrea prismatica Gray; as Crassostrea iridescens Hanley) had a toxicity of 380 μg SXT eq.100 g-1, which caused a shellfish fisheries closure in the region from February 25 to June 2016 (Herrera-Galindo et al., 2015). This HAB occurred just at the end of a long “El Niño” period that lasted from December 2015 to March 2016 and torrential rains were reported in the region of the coasts of Oaxaca at the beginning of January 2016, which again reinforces the importance of a combination of high temperatures with heavy rainfall for Pbc to develop as a bloom.
Considerations on the varieties of Pyrodinium
It has been questioned whether two varieties of Pyrodinium bahamense should be recognized. However, in the coastal and oceanic zone of the Mexican tropical Pacific, the HABs of Pb have always had the characteristic morphotype of the var. compresssum, as highly compressed cells, with short apical horn, united in long chains that provide them with rapid mobility and high toxicity. It is also interesting that on the coasts of the Mexican tropical Pacific (excluding the bays) when there is no Pbc HAB, no planktonic vegetative cells are found in the phytoplanktonic community. Moreover, in this region, HABs are quite sporadic, with time periods of 4-7 years between two episodes. The question then arises: where are the organisms when they do not produce HABs? The answer certainly lies in the nature of its hypnozygotic cysts, regardless of whether or not it has a short mandatory period of dormancy, as is common in tropical environments, in which there are no sharp changes in temperature throughout the year. Its quiescence in conditions of anoxia and darkness allows it to survive and germinate to the Pyrodinium cysts until after a lapse of approximately 97 years from sediment cores obtained from Laguna San José, BCS, Mexico (Cuéllar-Martínez, 2018). It has also been suggested that spiny cysts, such as Pb, can become entangled with each other, thus increasing the speed of their descent to the bottom once they have formed, to prevent them from germinating in conditions unsuitable for their growth development (Bravo and Figueroa, 2014). On the other hand, its ability to produce temporary pellicle cysts (similar to the agglutinated cells that were found in Acapulco in January 2011 at 10 m depth), in which the cell undergoes deep physiological changes in its organelles and concentration of chlorophyll, and this state is easily reversible, allowing organisms to survive unfavorable conditions for short times (Onda et al., 2014). In addition, its ability to swim swiftly to form long chains allows them to make important vertical migrations, and thus exploit the nutrients in different layers of the water column. The above mentioned means that the HABs of this species continue to be important in marine environments of the Pacific and one of the most dangerous microalgae for human health. That is why we encourage an in-depth study, not only of the morphology, but of the behavior and physiology of the varieties, taking into account the molecular differences found by Mertens et al. (2015) in the ribotypes of the strains coming from the Indo-Pacific in comparison with those of the Atlantic. Are there only two varieties of the species worldwide or are there more? Have the varieties been crossed and viable F1 or F2 generations produced? Do they still maintain genetic flow? Is the differential geographic distribution valid? The fact that var. compressum has the ability to form long chains allows it to exploit environments other than those inhabited by the var. bahamense? Answering such questions will make it possible to understand the ecological differences between the varieties that were pointed out by Phlips et al. (2006), where Pbb inhabits shallow environments, with mixed salinities, and long residence times of the water, while Pbc forms blooms in open littorals, where certainly the residence times of the water are short and the salinity more stable. In an era in which cryptic and pseudo-cryptic species are well-understood, why ignore the behavioral, physiological, ecological and molecular differences that the two taxa show? Continue studying this species is imperative because there are still many issues to elucidate with respect to its sexuality. Mertens et al. (2015) point out that the species is heterothallic, and Morquecho et al. (2014) indicate that the cysts are homothallic, suggesting that cultured populations of strains derived from the germination of cysts cannot undergo sexual reproduction or form hypnozygotes unless they are crossed. There is also no certainty about its dormancy time (regulated by an internal clock) and quiescence (regulated by environmental conditions), and the morphology of its different states of life in the sexual cycle (gametangia, gametes, planozyygotes and planomeiocytes).
This study makes important contributions as it demonstrates that in Acapulco Bay 1) the cysts are transported horizontally from distant places and were present in the water column to initiate the HAB and 2) the possible morphology of the planocygotes is shown. It also gives relevant information of its natural predators, which undoubtedly function as biological control within the same phytoplankton community, especially for the voracious phagotroph Gyrodinium fusus and the ability to parasitize its cysts by Chytriodinium affine.
In this study we conclude the following: 1) Pbc and Gc co-occurred in Acapulco during 2010 due to the fact that during the first half of the year “El Niño” conditions were present and in the second half “La Niña”. 2) Heavy rainfall, the rise in temperature and phosphates in the water column, as well as the presence of full cysts since May, allowed Pbc to bloom from the beginning of July maintaining the HAB throughout the month and rebounded in November. 3) The Pbc HABs in the Mexican Pacific and in the eastern Pacific are related to “La Niña”, or rather to the transition from an intense “El Niño” to the arrival of “La Niña”, as it is necessary that there is a rise in water temperature and heavy rains that increase the phosphates, and possibly Selenium, in the water column, so Pbc flourishes. 4) The decrease in water temperature, linked with the increase in nitrogen nutrients formed at the end of the year, allowed Gc, a species at low density in Acapulco Bay throughout the year, to bloom in November. 5) The Pbc HABs that occur along the Guerrero and Michoacán coasts, and specifically in Acapulco Bay, originated in the tropical Central American Pacific and Gulf of Tehuantepec, Mexico, and arrived at these coasts by the horizontal transport of hypnozygotes by oceanic currents. 6) Pbc can represent up to 17% of cells with morphology similar to Pbb, and this percentage decreases when the taxon density increases, so it seems to be related to the growth rate. 7) The vegetative cells of Pbc are voraciously phagocytosed by the athecate dinoflagellate Gyrodinium fusus, while the cysts are parasitized by the dinoflagellate Chytriodinium affine.











 nueva página del texto (beta)
nueva página del texto (beta)



