Introduction
Due to the limited known sites where Phanerophlebia C. Presl taxa occur naturally and due to their limited populations, the genus was previously classified as enigmatic by pteridologists (Yatskievych, 1996; Valdez-Avila et al., 2007). To this day, the conservation status of some of its species are still poorly known. It belongs to the family Dryopteridaceae, which comprises 26 genera and an estimated 2115 species (PPGI, 2016). Its relationships with the Asian genus Cyrtomium C. Presl and with Polystichum Roth have consistently been mentioned (Tryon and Tryon, 1982, Barrington, 1985; Yatskievych et al., 1988; Yatskievych, 1989, 1992, 1996; Moran and Riba, 1995; Mickel and Smith, 2004). Together with Arachniodes Blume and Dryopteris Adans., these five genera form the subfamily Dryopteridoideae (Liu et al., 2016; PPGI, 2016), of which Phanerophlebia is the only genus confined to the New World (Yatskievych, 1992, 1996; Moran and Riba, 1995; Mickel and Smith, 2004).
Previous reviews have discussed the need for further studies on the generic distinction and the species of Phanerophlebia (Yatskievych, 1996; Mickel and Smith, 2004). In Honduras, this genus has a limited distribution; Yatskievych (1996) reported only two localities, in the departments of Santa Bárbara and Lempira. So far only two of the eight re- cognized species (PPGI, 2016) have been reported, being Phanerophlebia haitiensis C. Chr. and P. juglandifolia (Humb. & Bonpl. ex Willd.) J. Sm. (Nelson Sutherland et al., 1996). The objective of the present study was to carry out a detailed review of the existing collections of Phanerophlebia and its localities in Honduras, in order to understand the diversity and distribution of the genus in the country.
Material and Methods
We conducted a systematic review of the Phanerophlebia samples deposited in the herbaria Paul C. Standley (EAP) of the Escuela Agrícola Panamericana Zamorano and Tegucigalpa Flora de Honduras (TEFH) of the Universidad Nacional Autónoma de Honduras, as they are the two largest herbaria in the country. Trips were made between March 2018 and February 2019 to three national parks from which the genus has previously been recorded. Ferns were collected randomly and within plots following standard plant collection and preparation protocols, and due to the rarity of the plants, only one individual was collected from each population we encountered. In Celaque National Park we sampled 54 plots of 20 × 20 m2 as part of a wider ecological study that aims to analyze the distribution pattern of ferns along their altitudinal gradient. As a consequence, Phanerophlebia collections were realized opportunistically inside and outside these plots. However, in Montaña de Santa Bárbara National Park and La Tigra National Park only opportunistic collections were made at specific localities from which the genus has previously been known. In addition, the search was extended to cover a wider geographical area inside the parks. This was done to ensure that sampling was adequate.
Subsequently, the material was identified using the keys of Yatskievych (1996) and deposited in EAP. In the future, some specimens will be sent to several herbaria outside the country. A map of the known distributions in Honduras was developed using the sample localities and the QGIS 2.8.4 software (QGIS, 2015). Maps of new records were developed using information from TROPICOS (2019) and Honduran localities.
Results
We revised seven herbarium specimens that were already collected and deposited, by various researchers, in Honduras; six of these belong to P. juglandifolia and one to P. haitiensis. Among these herbarium specimens, collections of P. juglandifolia from La Tigra National Park (PNLT) had not been included in previous revisions, but our visits to the reported locality could not confirm its occurrence (Fig. 1). We collected three Phanerophlebia individuals in the Santa Bárbara Mountain National Park (PANAMOSAB) and five from Celaque Mountain National Park (PNMC). The first records of Phanerophlebia macrosora (Baker) Underw. and Phanerophlebia juglandifolia (Humb. & Bonpl. ex Willd.) J. Sm. × macrosora (Baker) Underw. are added to the Honduran flora.
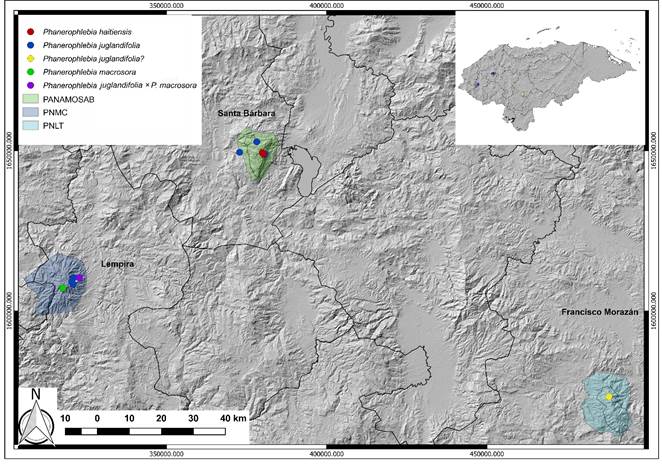
Figure 1: Known distribution of the genus Phanerophlebia C. Presl in Honduras: Phanerophlebia haitiensis C. Chr., P. juglandifolia (Humb. & Bonpl. Ex Willd.) J. Sm., P. macrosora (Baker) Underw., P. juglandifolia (Baker) Underw. × P. macrosora (Humb. & Bonpl. ex Willd.) J. Sm.
Key to Honduran Phanerophlebia species and their hybrids
1a. Veins free; fresh leaves with strong and unpleasant odor .………...........…... P. macrosora (Baker) Underw.
1b. Veins regularly or at least commonly anastomosing towards the pinna margins; leaves without strong and unpleasant odor ……..…………………………...…..……..…… 2
2a. Sori with some black and collapsed sporangia; indusia and rhizome scales bicolorous …......... Phanerophlebia juglandifolia (Humb. & Bonpl. ex Willd.) J. Sm. × P. macrosora (Baker) Underw.
2b. Sori without black and collapsed sporangia; indusia, if present, concolorous …...…… 3
3a. Sori indusiate; rhizome scales brownish or reddish, concolorous …….. P. haitiensis C. Chr.
3b.Sori exindusiate; rhizome scales bicolorous, with dark centers and lighter margins …...............................…. P. juglandifolia (Humb. & Bonpl. ex Willd.) J. Sm.
1. Phanerophlebia haitiensis C. Chr., Kongl. Svenska Vetensk. Acad. Handl., Ny Följd 16: 42. 1937. TYPE: HAITI. Ekman 3119 (S).
≡ Cyrtomium haitiense (C. Chr.) C.V. Morton, Am. J. Bot. 47: 55. 1957.
Plants not strongly scented; rhizomes up to 10 mm diameter, apparently deeply seated in substrate, short-repent to ascending, not branched at maturity; rhizome scales 4.5-7 mm long, 2-4 mm wide, ovate to elliptic-lanceolate, erose-denticulate, with few, short cilia at base, concolorous, brown (rarely light color with age); leaves up to 50 cm long; petioles slightly shorter or longer than the laminae; petiolar scales usually deciduous, loosely overlapping, much like rhizome scales, the broadest up to 3 mm wide, tapering into reduced, hair-like structures above; pinnae 1-4 pairs, up to 9 cm long, lanceolate to lance-ovate, usually falcate, apex acute to attenuate, base unevenly cuneate, lacking an acroscopic auricle, margins sometimes slightly undulate, spinulose-serrulate in distal half; buds absent from axils of distal pinnae; veins with irregular marginal anastomoses, 2-3 branched; sori in 1-2(-3) series between costa and margins; indusia 0.7-1.1 mm diameter, membra- nous, flat or concave centrally, not umbonate, shriveled at maturity; spores 35-56 µm long, often lacking a well-developed perispore.
Ecology: in Honduras this species had been reported only from an area 7 km north of the “Mochito” in the department of Santa Bárbara. However, we located a population of around 40 individuals, growing in clusters on shaded limestone rocks, near the community of “Cedral” at 2500 m. It is likely to occur in multiple zones within the PANAMOSAB above 2200 m, due to the large number of shaded limestone surfaces were the species previously has been recorded.
Distribution: Guatemala, Haiti, and Honduras (Yats- kievych, 1996).
Additional material examined: HONDURAS. Department Santa Bárbara, municipality Las Vegas, El Mochito, 2200-2500 m, 14°55'00''N, 88°07'00''W, 23.X.1991, R. Moran 5706 (EAP, TEFH); El Cedral, 2500 m, 14°54'48.7''N, 88°06'48.8''W, 7.II.2019, J. Reyes et al. 331 (EAP).
Taxonomic notes: this species is the smallest Phanerophlebia in Honduras (Fig. 2) and is similar to P. pumila (M. Martens & Galeotti) Fée, differing in not having persistent petiolar scales, veins frequently anastomosing to- ward the pinnae margins (vs. free or unfrequently anastomosing) and in the size of its rhizome scales (4.5-7 mm vs. 3-5 mm long). The specimen Moran 5706 (EAP) was misidentified as P. pumila (duplicates at TEFH and MO later corrected to P. haitiensis by other botanists) showing how similar these species can be.
2. Phanerophlebia juglandifolia (Humb. & Bonpl. ex Willd.) J. Sm., J. Bot. (Hooker) 4: 187. 1841.
≡ Polypodium juglandifolium Humb. & Bonpl. ex Willd., Sp. Pl. 5: 195. 1810. TYPE: VENEZUELA. Humboldt y Bonpland s.n. (B-W-19688).
≡ Amblia juglandifolia (Humb. & Bonpl. ex Willd.) C. Presl., Tent. Pterid. 185: t. 7, f. 2. 1836.
≡ Aspidium juglandifolium (Humb. & Bonpl. ex Willd.) Kun- ze ex Klotzsch, Linnaea 20: 363. 1847.
= Amblia latifolia Fée. Mém. Foug. 8: 101. 1857.
≡ Cyrtomium juglandifolium (Humb. & Bonpl. ex Willd.) T. Moore, Index Filicum. 83. 1857.
≡ Dryopteris juglandifolia (Humb. & Bonpl. ex Willd.) Kuntze, Rev. Gen. Pl. 2: 813. 1891.
≡ Polystichum juglandifolium (Humb. & Bonpl. ex Willd.) Diels., Nat. Pflanzenfam. 1(4): 193. 1899.
Plants not strongly scented; rhizomes up to 7 mm diameter, generally deeply penetrating in substrate (sometimes superficial), short-repent to nearly erect, not branched at maturity; rhizome scales 6-10.5 mm long, 3-5 mm wide, lanceolate, ciliate, bicolorous with broad or sometimes narrow, dark brown, sometime with sclerotic center or narrower; leaves up to 60(-85) cm long; petioles shorter to slightly longer than the laminae; petiolar scales subpersistent, overlapping and often dense, much like rhizome scales, the broadest up to 4 mm wide, tapering into reduced, hair-like structures; pinnae 2-4(-6) pairs, up to 17.5 cm long, ovate to lance-ovate, usually somewhat falcate, apex attenuate, base obliquely cuneate to rounded, margins often slightly undulate proximally, spinulose-serrulate in distal half or more commonly in distal two-thirds; buds present on at least some leaves in each population; gemmae in axils of distal pinnae (rarely in axils of more proximal pinnae); veins with 1-3 series of regular marginal anastomoses, 3-5 branched; sori in 2-4(-5) series between costa and margins; sori exindusiate; spores 41-60 µm long.
Ecology: this species is the most common Phanerophlebia in Honduras (Fig. 3). We observed it in PANAMOSAB and PNMC but could not confirm it in PNLT. It has both terrestrial and epipetric habits; however, the species has fewer pinnae pairs when growing on rocks (2-4 vs 3-7 pinna pairs).

Figure 3: Phanerophlebia juglandifolia (Humb. & Bonpl. ex Willd.). J. Sm. A. habitat and fronds; B. pinnae and exindusiate sori.
Distribution: Colombia, Costa Rica, El Salvador, Guatemala, Honduras, Mexico, Nicaragua, Panama and Venezuela (Yatskievych, 1996).
Additional material examined: HONDURAS. Department Francisco Morazán, municipality Tegucigalpa, La Tigra Mountain, 1776 m, C. Ayllón 18 (TEFH); loc. cit., 1700 m, 11.XI.1989, M. Bueso 87 (TEFH). Department Lempira, municipality Gracias, Celaque Mountain National Park, Naranjo River, 24.V.1991, P. House et al. 961 (EAP, TEFH); trail from Río Naranjo to camp one, 2000 m, 14°33'00''N, 88°40'00''W, 14.XI.1991, R. Moran 5565 (EAP, TEFH); loc. cit., 1800 m, 14°30'55.2''N, 91°20'57.6''W, 5.IX.2018, J. Reyes 218 (EAP); loc. cit., 2045 m, 14°33'39.4''N, 88°39'40.4''W, 10.XI.2018, J. Reyes and E. Segura 332 (EAP). Department Santa Bárbara; municipality Las Vegas, El Mochito, 2200-2500 m, 14°55'00''N, 88°70'00''W, 23.XI.1991, R. Moran 5667 (EAP, TEFH); municipality Santa Bárbara, Mountain of Santa Bárbara, 2275 m, 14°55'03''N, 88°10'57''W, 28.IV.1973, A. F. Clewell and D. Hazlett 3875 (EAP); loc. cit., 1800 m, 14°56'50.2''N, 88°07'58.4''W, 4.II.2019, J. Reyes et al. 278 (EAP); loc. cit., 1800 m, 14°54'46.3''N, 88°06'28.7''W, 7.II.2019, J. Reyes et al. 334 (EAP).
Taxonomic notes: specimens in Honduras are highly variable, as mentioned by other authors (Moran and Riba, 1995; Mickel and Smith, 2004). None appear to have axillary buds, even in the densest populations found, and this feature diverges from populations in Mexico and from the description of the species (Yatskievych, 1996).
Some specimens from PANAMOSAB overlap in the size of the scales and spores between P. juglandifolia and P. gastonyi Yatsk., and this had been previously mentioned by Mickel and Smith (2004). Molecular or cytological studies may be necessary to differentiate them, because P. juglandifolia is a tetraploid species and P. gastonyi is a diploid (Yatskievych, 1996).
3. Phanerophlebia juglandifolia (Humb. & Bonpl. ex Willd.) J. Sm. × P. macrosora (Baker) Underw.
Plant not strongly scented; rhizomes up to 24 mm in diameter, deeply penetrating in substrate, erect or ascending, rhizome scales 8-13.2 mm long, 4.8-6.5 mm wide, lanceolate, erose to shortly ciliate, bicolorous with broad dark brown centers; leaves 16-24 cm long, 3.2-4.2 cm wide; petioles longer than the laminae; petiolar scales subpersistent, overlapping and often dense, much like rhizome scales, mixed and with tapering into reduced, hair- like structures; pinnae 5-9 pairs, up to 24 cm long, ovate to lance-ovate, slightly falcate, apex attenuate, base obliquely cuneate to unevenly rounded, margins undulate and often slightly spinulose towards the apex, buds absent; veins with 1-4 series or regular anastomoses, 3-4 branched; sori 3-4 series between costa and margins, often two lumped together; indusia not consistently present, somewhat bicolorous, 4-7 mm in diameter, flat, not umbonate, shriveled; spores blackened and mishaped.
Ecology: We found three individuals at 1500 m in the PNMC with distinctive characteristics mentioned by Yatskievych and Gastony (1987) as a hybrid between Phanerophlebia juglandifolia and P. macrosora (Fig. 4). It occurs in an area without any of the putative parental species. Phanerophlebia juglandifolia can be found within this elevational range; however, P. macrosora has only been found at 2700 m on the opposite side of the PNMC. Because its distribution is very limited, as mentioned below in notes about P. macrosora, there is little likelihood of crossing with P. juglandifolia in that specific location. We propose three hypotheses to explain this hybrid:

Figure 4: Phanerophlebia juglandifolia (Humb. & Bonpl. ex Willd.) J. Sm. × P. macrosora (Baker) Underw. A. habitat and fronds; B. pinnae and indusiate sori.
1) In the past, the putative parental species shared a niche at this elevation. In this sense, the discovery of the rare hybrid corresponds to a remnant of this interaction in the area. However, this contrasts to the situation in Costa Rica mentioned by Yatskievych and Gastony (1987), where the hybrid is common and occurs in the same niche as the putative parental species.
2) There exists a population of P. macrosora that has not yet been found and considering the potential dispersal range of the spores, these could converge on the site to generate the hybridization event. This is unlikely as a perimeter of 300 m2 was intensively sampled without finding the putative parental species.
3) The P. macrosora population found at the other side of the mountain, at 2700 m, has a long dispersion range that could converge on this specific place as part of the “spore rain”. Considering that extensive surveys were conducted at the PNMC and that no other occurrence, even on similar elevations and conditions along the altitudinal gradient, was detected, it appears impossible to explain why this hybrid population is so local and small. The discovery of this hybrid represents a new record for the Honduran flora (Fig. 5).

Figure 5: Herbarium specimen of Phanerophlebia juglandifolia (Humb. & Bonpl. ex Willd.) J. Sm. × P. macrosora (Baker) Underw.
Distribution: Costa Rica and Honduras (Fig. 6).
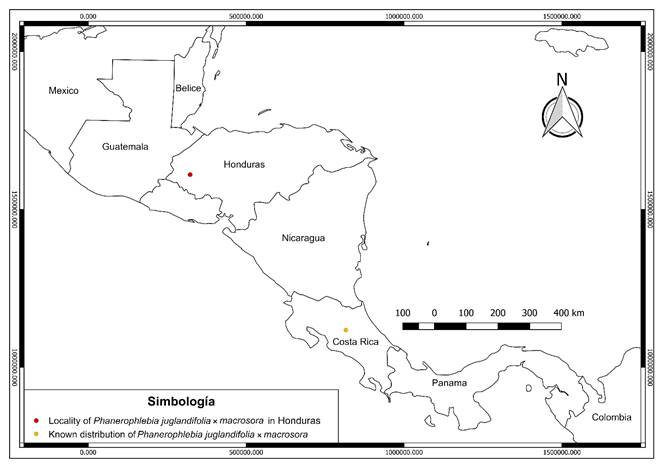
Figure 6: Known distribution of Phanerophlebia juglandifolia (Humb. & Bonpl. ex Willd.) J. Sm. × P. macrosora (Baker) Underw.
Additional material examined: COSTA RICA. Province Heredia, 2 km east of Sacramento on highway 114 from San José de la Montaña, south slope of Volcan Barva, 10°06'00''N, 84°07'00''W, 1980 m, 18.III.1986, G. Yatskievych and K. Mc- Crary 86-31A (MO). HONDURAS. Department Lempira, municipality Gracias, Celaque Mountain National Park, 1400 m, 14°33'38.5''N, 88°38'40.4''W, VIII.2018, J. Reyes et al., 201 (EAP).
Taxonomic notes: we observed that the individual collected of this hybrid, besides having misshaped and aborted spores, shares morphological characteristics such as the presence of indusia and the size and shape of the pinnae and the lamina with P. macrosora, and the anastomosing venation pattern and color of the scales with P. juglandifolia (Fig. 7). Nevertheless, it is notable that its general appearance resembles P. macrosora. Because P. juglandifolia is tetraploid and P. macrosora is diploid (Yatskievych, 1992), the putative hybrid theoretically should be morphologically more similar to P. juglandifolia, which is not the case of this hybrid either in Honduran or Costa Rican territory.

Figure 7: Comparison of Phanerophlebia juglandifolia (Humb. & Bonpl. ex Willd.) J. Sm. × P. macrosora (Baker) Underw. and its putative parental species. A-C. bicolorous rhizome scale, exindusiate sori, and anastomosing venation of Phanerophlebia juglandifolia (Humb. & Bonpl. ex Willd.) J. Sm., D-F. bicolorous rhizome scale, indusiate sori, and anastomosing venation of Phanerophlebia juglandifolia (Humb. & Bonpl. ex Willd.) J. Sm. × P. macrosora (Baker) Underw., G-I. concolorous rhizome scale, indusiate sori, and free venation of Phanerophlebia macrosora (Baker) Underw.
4. Phanerophlebia macrosora (Baker) Underw., Bull. Torrey Bot. Club 26: 213. 1899.
≡ Aspidium juglandifolium (Humb. & Bonpl. ex Willd.) Kunze ex Klotzsch var. macrosorum Baker, J. Bot. 25: 25. 1887. TYPE: COSTA RICA. Cooper s.n. (K).
= Phanerophlebia guatemalensis Underw., Bull. Torrey Bot. Club 26: 214. 1899.
≡ Aspidium macrosorum Christ., Bull. Herb. Boissier ser. 2, 4: 963. 1904.
≡ Cyrtomium macrosorum (Baker) C.V. Morton., Amer. Fern J. 47: 55. 1957.
= Cyrtomium guatemalense (Baker) C.V. Morton., Amer. Fern J. 47: 55. 1957.
Plants with a strong, unpleasant, skunk odor when fresh; rhizomes up to 20 mm diameter, deeply penetrating in substrate, erect or ascending, unbranched at maturity; rhizome scales 10-15 mm long, 5-7 mm wide, ovate to lance-ovate, erose-ciliate, concolorous (rarely with a slightly darkened central area); leaves up to 0.7-2.7 m long; petiolar scales persistent, dense and overlapping, much like rhizome scales, the broadest 7 mm wide, mixed and reduced, hair-like structures above; pinnae (4-)6-17 pairs, up to 27 cm long, narrowly oblong-lanceolate, occasionally slightly falcate, apex attenuate, base obliquely cuneate to rounded, lacking an acroscopic auricle, margins spinulose-serrulate nearly to base; buds absent from axils of distal pinnae; veins free, 3-4 branched; sori in 2-4 series between costa and margin; indusia 0.6-1.1 mm in diameter, membranous, flat or concave centrally, not umbonate, shriveled at maturity; spores 41-60 µm long.
Ecology: we found a population of around 40-50 individuals in the PNMC. This population was limited to a relatively small, humid, flat site at 2700 m in an area of approximately 800 m2. This population is dispersed and has the skunk scent mentioned by other authors (noted as unpleasant). We confirm this odor in the juvenile leaves; however, it is not perceptible in old leaves found in the field. This represents a new record for the flora of Honduras (Fig. 8).

Figure 8: Herbarium specimen of Phanerophlebia macrosora (Baker) Underw. A. blade; B. medial pinnae; C. proximal pinnae and stipe; D. indusiate sori; E. stipe base scales.
Distribution: Costa Rica, El Salvador, Guatemala, Honduras (Fig. 9), Mexico and Panama (Yatskievych, 1996).
Additional material examined: HONDURAS. Department Lempira, municipality Gracias, Celaque Mountain National Park, 0.5 km below camp “El Quetzal”, 30.III.2018, J. Reyes et al. 93 (EAP).
Taxonomic notes: this species is the largest of the genus in Honduras and is characterized in the field by the presence of its persistent, pale orange, scales on the stipe bases and obvious indusia (Fig. 10).
Discussion
As mentioned by Reyes-Chávez et al. (2019), Phanerophlebia remotispora E. Fourn, now Phanerophlebia nobilis var. remotispora (E. Fourn.) Yatsk., is not known from Honduras, even though it was mentioned in the management plan of the Río Plátano Biosphere Reserve (RPBR) by Martínez (2014). This might correspond to a misidentified specimen, but this cannot be verified because the lists of Martínez (2014) do not contain information about voucher specimens.
Ayllón 18 and Bueso 87 (TEFH) were collected in the PNLT in 1983 and 1989 respectively and were mistakenly identified as Thelypteris ghiesbreghtii (Hook.) C.V. Morton and the previous circumscription of the genus Cyrtomium C. Presl, later corrected as P. juglandifolia. Both specimen labels mentioned approximately the same location and due to the time difference and circumstances of their verification, this seem to indicate that this was an area where the species occurred. It is probable that this is not longer the case, as it has not been found by recent expeditions to the area by other authors (Hernández-Cibrián et al., 2005, 2017) or by us.
As indicated by Ward (2012), PANAMOSAB is the largest limestone mountain in Central America. This formation has caves and holes in an irregular terrain that drains almost all the available surface water and conducts it in underground channels. Despite this topography, agriculture is generating pressures on the ecosystem and large patches of fields can be observed at elevations above 1800 m, which is the legal limit for anthropogenic activities adjacent to the protected area. Considering that in Haiti Phanerophlebia haitiensis is considered extinct and that in Guatemala it has been collected only once (Yatskievych, 1996), there is an urgent need for an effective protection and conservation of this area in Honduras.
The PNMC is the protected area of Honduras with the greatest richness of lycophytes and pteridophytes (Rojas-Alvarado, 2012). The presence of the rare new records seems to indicate that a more systematic and exhaustive study is necessary, especially to understand the ecological relationships, richness, and diversity of this group. Unfortunately, this ecosystem is threatened by anthropogenic factors, and there is an urgent need for more exhaustive floristic inventories (Vega et al., 2016).
To better delimit the species in the country, molecular and cytological studies on Honduran species are needed, like the case of P. juglandifolia and P. gastonyi. The number of lycophytes and pteridophytes reported for Honduras is un- certain and the need for more baseline research and a new catalogue has been mentioned (Batke et al., 2016; Rojas-Alvarado, 2017; Reyes-Chávez, 2018). A molecular approach for difficult to delimit genera might help clarify this situation.











 text new page (beta)
text new page (beta)





