Introduction
An alternative for controlling phytopathogens is the use of plant secondary metabolites, which leads to a search for antifungals with faster rates of biodegradation, either because they are rapidly hydrolysed or because they exhibit a higher absorption rate (Eksteen et al., 2001; Leiss et al., 2011).
Species of the Annonaceae family biosynthesise various biologically active secondary metabolites, including benzylisoquinoline alkaloids and annonaceous acetogenins. Together, these compounds comprise approximately one thousand molecules, characterised by potent cytotoxic, insecticidal, neuropharmacological, antibacterial, and antifungal activities (González-Esquinca et al., 2014; Lúcio et al., 2015). The inhibitory activities of extracts and compounds from these species for phytopathogens are less known. A review on this topic has reported the growth inhibition of 15 phytopathogenic fungi (Dang et al., 2011; De-la-Cruz-Chacón et al., 2011).
Among the Annonaceae, Sapranthus Seem. is one of the least-studied genera, consisting of eight species that are mainly distributed in Mexico and Central America (De-la-Cruz-Chacón et al., 2016; Schatz et al., 2018). In particular, there are no reports on the presence of secondary metabolites in Sapranthus microcarpus (Donn. Sm.) R.E. Fr. or their biological activities. The species, known as chufle, and Chac Nich Max in the Mayan language, is native to Mexico, Guatemala, El Salvador and Honduras (Schatz et al., 2018).
This is the first study to examine the biological and phytochemical potential of S. microcarpus. The study was designed to evaluate the in vitro antifungal activities of organic (hexane and methanol) and alkaloid extracts of the leaves and root and stem bark of S. microcarpus against six plant pathogenic fungi (Aspergillus glaucus (L.) Link, Colletotrichum acutatum var. fioriniae Marcelino & Gouli, Colletotrichum gloeosporioides (Penz.) Penz. & Sacc., Curvularia lunata (Wakker) Boedijn, Fusarium oxysporum f. sp. lycopersici (Sacc.) W.C. Snyder & H.N. Hansen and Rhizopus stolonifer (Ehrenb.) Vuill.). The combined activity of the most active extracts for each phytopathogen was also determined.
Materials and Methods
Leaves and the stem and root bark of Sapranthus microcarpus were collected in January 2015 (fruiting stage) in the town of Cintalapa, Chiapas, Mexico. A voucher specimen was deposited under reference #104204 at the Eizi Matuda Herbarium (HEM) of the Universidad de Ciencias y Artes de Chiapas, Mexico.
Fresh plant samples (250 g) were consecutively extracted for maceration, three times for 48 h with hexane and methanol at room temperature in the dark. The solvents were removed through distillation under reduced pressure. To obtain alkaloids, the plant material was extracted via an acid-base technique (De-la-Cruz-Chacón and González-Esquinca, 2012).
The test fungi were Aspergillus glaucus NACF0010, Colletotrichum gloeosporioides NCBI HM562712, Colletotrichum acutatum var. fioriniae ATCC 56897, Fusarium oxysporum f. sp. lycopersici ATCC 9848, Curvularia lunata NACF0030, and Rhizopus stolonifer NACF0011. The NACF and NCBI strains were obtained from the Grupo de Estudios Moleculares aplicados a la Biología (GeMBio), laboratory of the Centro de Investigación Científica de Yucatán (CICY), Mexico.
Antifungal activities of the plant extracts (250 and 500 µg ml-1) were determined based on the inhibition of mycelial growth according to Eksteen et al. (2001). The hexane extracts were dissolved in dimethyl sulfoxide, and the methanol and alkaloid extracts were dissolved in ethanol; the solvents did not exceed 2.5% of the culture medium (potato dextrose agar). Mycelial growth was recorded every 24 h until that in the control (solvent without an extract) completely covered the surface of the culture medium. For each phytopathogen, the extracts that showed the highest antifungal activity were selected, combined, and then evaluated to determine whether their potencies were additive. The experiments were repeated twice with three replicates (n=6). The activity was expressed using the following formula: % Inhibition = ((dc-de)/dc) × 100, where dc=the fungal growth diameter on the control medium, and de=the fungal growth diameter on a medium with the extract.
The root bark alkaloid extract was analysed by bioautography (Rios et al., 1998), which included G60-F254 silica gel (200 μm) chromatography (Sigma-Aldrich®, Fluka®, St. Louis, USA), with a mobile phase of CHCl3: methanol (9:1), to obtain the chemical profile of the extract. The activities of the separated constituents were evaluated against Aspergillus glaucus and Curvularia lunata. The active compound was identified based on the area of fungal growth inhibition, followed by its isolation. To this end, preparative thin-layer chromatography (Sigma-Aldrich®, Fluka®, St. Louis, USA) of the root alkaloid extract was conducted on silica gel (1500 µm) under the same conditions. The area of the inhibitory compound was extracted with CHCl3 under constant stirring for 1 h, followed by filtration (Fig. 1).
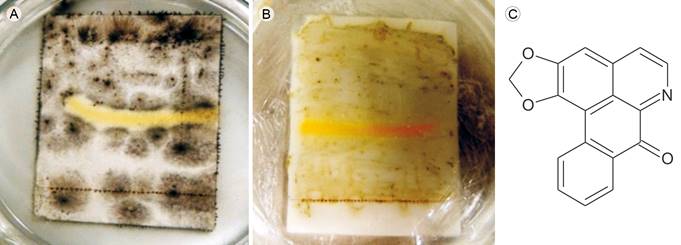
Figure 1: Liriodenine, antifungal alkaloid from Sapranthus microcarpus (Donn. Sm.) R.E. Fr. Bioautography of liriodenine against A. Curvularia lunata (Wakker) Boedijn and B. Aspergillus glaucus (L.) Link; yellow areas indicate inhibition of fungal growth by liriodenine; C. chemical structure of liriodenine.
The compound, isolated as yellow needles, was analysed, identified, and compared with a previously obtained liriodenine reference, based on the melting point (275-276 °C), an ultraviolet absorption spectrum obtained by high-performance liquid chromatography with a diode-array detector (Perkin Elmer Flexar, Norwalk, USA) (De-la-Cruz-Chacón and González-Esquinca, 2012), and mass spectrometry (MS) data. The mass spectral data were obtained by gas chromatography/MS according to Riley-Saldaña et al. (2017), performed on a PerkinElmer Clarus 680 gas (Perkin Elmer, Waltham, USA) chromatograph coupled to a Clarus SQ8T mass spectrometer (Perkin Elmer, Waltham, USA), with a 1:30 split ratio. A Perkin Elmer Elite-1 capillary column (32 m, 0.32 mm, 0.25 mm film thickness) was used as the stationary phase (Perkin Elmer, Waltham, USA). Helium was used as the carrier gas at a flow rate of 1.2 ml/min. The column temperature was programmed as follows: held at 150 °C for one min, raised at a rate of 10 °C/min to 280 °C, and then held at 280 °C for 16 min. The injector temperature was 300 °C. MS was carried out at 70 eV with 2.89 scans per second, fragment detection from 50 to 500 Da, the source temperature of 250 °C, and the quadrupole temperature of 100 °C. The retention time of liriodenine was 17.2±0.1 min, and EM (m/z) (relative abundance) values were 275(100), 247(27), 188(45) and 123(24) (Fig. 2).
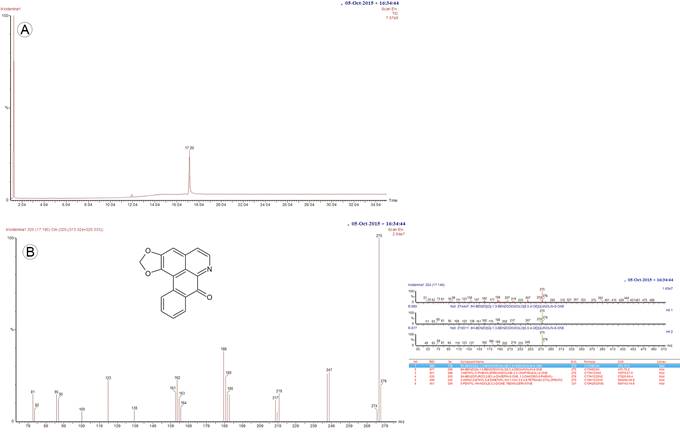
Figure 2: Analytical GC-MS chromatogram of liriodenine alkaloid isolated Sapranthus microcarpus (Donn. Sm.) R.E. Fr. A. the retention time of compound is 17.196 min using the GC-MS method we set; B. chemical structure and EM-MS fragmentation pattern of liriodenine (8H-Benzo(g)-1,3-benzodioxolo(6,5,4-de)quinolin-8-one).
The antifungal activity of liriodenine (100 and 200 nmol ml-1) was evaluated based on mycelial growth inhibition under the same conditions as those used for the extracts. The fungicide Captan® was used as a positive control at 200 and 1000 nmol ml-1.
The mycelial growth inhibition data were analysed for the extracts, mixtures, and liriodenine using the Kruskal-Wallis non-parametric analysis, followed by the Mann-Whitney multiple comparison test at p<0.5. To detect a general pattern of inhibition as a result of the evaluated factors, a principal component analysis (PCA) was performed, and the groups were confirmed by permutational multivariate analysis of variance (PERMANOVA). The Past statistical software (Hammer et al., 2001) was used (Natural History Museum, University of Oslo, Oslo, Norway).
Minimum inhibitory concentrations (MICs) of liriodenine were determined by a macrobroth (Sabouraud dextrose) dilution technique (Moussa et al., 2013). Liriodenine was dissolved in ethanol at a final concentration of 1% (v/v) and then evaluated in serial dilutions (500 to 1.0 nmol ml-1). All experiments were performed in triplicate.
Results
The growth of all six phytopathogens tested was inhibited by at least one type of Sapranthus microcarpus extract, although not with the same potency (Table 1).
Table 1: Antifungal activity of extracts from Sapranthus microcarpus (Donn. Sm.) R.E. Fr. The values are means ± DE (n=6). The separation of groups was performed with the Mann-Whitney test (p<0.05). Capital letters represent comparisons in columns, and lowercase letters represent comparisons in rows.
| Tissue | Extract: ug ml-1 | % Inhibition Mycelial Growth | |||||
| Aspergillus glaucus (L.) Link NACF0010 | Colletotrichum gloeosporoides (Penz.) Penz. & Sacc NCBI HM562712 | Colletotrichum acutatum var. fioriniae Marcelino & Gouli ATCC 58697 | Fusarium oxysporum f. sp. lycopersici (Sacc.) W.C. Snyder & H.N. Hansen ATCC 9848 | Curvularia lunata (Wakker) Boedijn NACF0030 | Rhizopus stolonifer (Ehrenb.) Vuill. NACF0011 | ||
| Roots | Hexanic 250 | 10±5 Ac | 5±3 Ad | 0±0 Cd | 6±3 Ae | 20±5 Ae | 0 Bd |
| 500 | 5±3 Ccd | 28±7 Bc | 8±2 Bc | 28±7 Bd | 37±5 Ad | 41±6 Ab | |
| Metanolic 250 | 1±2 Ad | 36±10 Bb | 6±1 Cc | 40±10 Bc | 37±6 Ad | 0 Ac | |
| 500 | 38±6 Ab | 65±2 Aa | 6±3 Bc | 65±2 Ab | 62±5 Ab | 60±1 Aa | |
| Alkaloidal 250 | 47±3 Aab | 44±3 Ab | 60±1 Ab | 55±3 Ac | 47±1 Ac | 36±2 Ac | |
| 500 | 48±2 Aa | 60±2 Aa | 73±2 Aa | 90±3 Aa | 76±5 Aa | 56±2 Aa | |
| Stem | Hexanic 250 | 8±2 Ac | 26±5 Ab | 7±1 Bc | 15±5 Ad | 12±3 Bc | 0 Bc |
| 500 | 30±10 Ab | 24±4 Bb | 15±4 Ab | 28±4 Bc | 16±3 Cc | 25±5 Bb | |
| Metanolic 250 | 3±2 Ad | 18±3 Cc | 10±6 Ac | 20±5 Cd | 0±0 Cd | 0 Ac | |
| 500 | 13±2 Bc | 26±2 Bb | 17±3 Ab | 58±2 Bb | 23±6 Cb | 24±4 Cb | |
| Alkaloidal 250 | 21±2 Bb | 26±1 Bb | 3±1 Cd | 32±2 Bc | 37±5 Ba | 0±0 Ac | |
| 500 | 43±1 Ba | 47±4 Ba | 32±5 Ca | 70±4 Ba | 38±5 Ba | 55±1 Aa | |
| Leaves | Hexanic 250 | 10±6 Ac | 25±5 Ac | 15±2 Ac | 25±5 Ac | 23±2 Ad | 25±3 Ab |
| 500 | 14±2 Bc | 45±2 Ab | 24±6 Ab | 35±2 Ab | 27±1 Bd | 41±14 Aa | |
| Metanolic 250 | 0 Ad | 50±9 Aa | 0 Cd | 30±5 Abc | 7±2 Be | 0 Ad | |
| 500 | 18±1 Bb | 59±8 Aa | 13±3 Bc | 62±8 Aa | 42±3 Bb | 29±8 Bb | |
| Alkaloidal 250 | 20±1 Bb | 26±2 Bc | 20±5 Bb | 30±2 Bbc | 34±1 Bc | 1±1 Bc | |
| 500 | 46±2 Aa | 41±2 Cb | 47±1 Ba | 68±2 Ca | 64±5 Aa | 42±3 Ba | |
The PCA (Fig. 3) and PERMANOVA confirmed that the alkaloid extracts were the most potent, whereas the hexane extracts showed less activity (F=25.85, p=0.0001); the root and leaf tissues showed higher activity than the stems (F=5.84, p=0.003), and the highest concentration was the most effective (F=48.5, p=0.001).
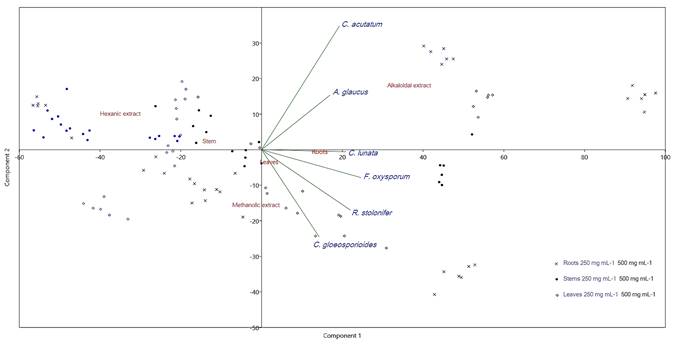
Figure 3: Principal component analysis of antifungal activity of Sapranthus microcarpus (Donn. Sm.) R.E. Fr. PCA bi-plot based on fungal inhibition percent of three types of extracts in two concentrations from three plant tissues. PC1 and PC2 accounted for over 80% of the total variance. The arrows indicate the direction of maximum correlation by fungus species.
For the extracts that showed the highest antifungal activity, the growth inhibition rate was calculated (Table 2).
Table 2: Effect of extracts from Sapranthus microcarpus (Donn. Sm.) R.E. Fr. on the growth rate of fungi. For extracts that showed the highest antifungal activity, the growth rate was calculated from the slope of the linear regression (y=a + bx, where “a” is the intercept and “b” is slope). E=Evaluated extracts (E). A=Alkaloidal, M=Methanolic; H=Hexanic; L=Leaf; S=Stem; R=Root.
| Fungus | Extract at 500 µg ml-1 | Slope | Intercept | Correlation | |
| Control | 0.367 | 6.713 | r=0.9902; r2=0.9804; P=8.92E-30 | ||
| Aspergillus glaucus (L.) Link NACF0010 | EAR | 0.186 | 4.226 | r=0.9569; r2=0.9157; P=2.70E-19 | |
| EAS | 0.203 | 5.600 | r=0.9699; r2=0.9408; P=7.88E-22 | ||
| EAL | 0.181 | 3.257 | r=0.9766; r2=0.9537; P=4.21E-12 | ||
| Control | 0.131 | 4.254 | r=0.9916; r2; 0.9833; P=4.45E-63 | ||
| Colletotrichum acutatum var. fioriniae Marcelino &Gouli ATCC 58697 | EAR | 0.032 | 4.526 | r=0.9661; r2; 0.9333; P=2.63E-42 | |
| EAS | 0.106 | 5.265 | r=0.9889; r2; 0.9779; P=6.72E-59 | ||
| EAL | 0.079 | 6.001 | r=0.9881; r2; 0.9764; P=7.20E-58 | ||
| Control | 0.415 | 3.530 | r=0.9829; r2; 0.9661; P=7.76E-26 | ||
| EAR | 0.131 | 3.287 | r=0.9566; r2; 0.9149; P=3.12E-19 | ||
| Colletotrichum gloeosporoides (Penz.) Penz. & Sacc NCBI HM562712 | EMR | 0.085 | 4.435 | r=0.9648; r2; 0.9309; P=1.01E-20 | |
| EAS | 0.204 | 2.956 | r=0.9740; r2; 0.9487; P=7.35E-23 | ||
| EML | 0.169 | 3.348 | r=0.9766; r2; 0.9538; P=1.31E-23 | ||
| Control | 0.269 | 4.582 | r=0.9929; r2; 0.9859; P=2.76E-43 | ||
| Curvularia lunata (Wakker) Boedijn NACF0030 | EAR | 0.039 | 5.029 | r=0.9003; r2; 0.8105; P=7.25E-18 | |
| EAS | 0.085 | 4.435 | r=0.9648; r2; 0.9309; P=1.03E-20 | ||
| EAL | 0.086 | 5.164 | r=0.9718; r2; 0.9441; P=7.01E-30 | ||
| Control | 0.240 | 9.296 | r=0.9575; r2; 0.9167; P=3.42E-29 | ||
| Fusarium oxysporum f. sp. lycopersici (Sacc.) W.C. Snyder & H.N. Hansen ATCC 9848 | EAR | 0.025 | 4.047 | r=0.8444; r2; 0.7129; P=1.97E-15 | |
| EAS | 0.171 | 2.375 | r=0.9789; r2; 0.9583; P=7.34E-37 | ||
| EAL | 0.077 | 4.359 | r=0.9691; r2; 0.9392; P=1.10E-32 | ||
| Control | 0.915 | 11.516 | r=0.9089; r2; 0.8261; P=442E-07 | ||
| EAR | 0.395 | 5.387 | r=0.9655; r2; 0.9322; P=3.59E-10 | ||
| Rhizopus stolonifer (Ehrenb.) Vuill. NACF0011 | EMR | 0.354 | 5.774 | r=0.9676; r2; 0.9363; P=2.25E-10 | |
| EAS | 0.358 | 5.903 | r=0.9805; r2; 0.9614; P=5.15E-12 | ||
| EHL | 0.512 | 9.452 | r=0.8686; r2; 0.7545; P=6.15E-06 | ||
| EAL | 0.493 | 7.000 | r=0.9583; r2; 0.9184; P=1.45E-09 |
The growth rate of all fungi was attenuated by the treatments (60-95% of control); the alkaloid root extract was the most potent and fast acting, except for Colletotrichum gloeosporioides.
The combined extracts displayed similar or lower activities (25-59% inhibition) compared with those of the individual extracts (Table 3).
Table 3: Antifungal activity of the combined extracts of Sapranthus microcarpus (Donn. Sm.) R.E. Fr. Combined extracts. E=Extract; A=Alkaloidal, M=Methanolic; L=Leaf; S=Stem; R=Root. The values are means ± DE (n=6). The separation of groups was performed with the Mann-Whitney test (p<0.05). Capital letters represent comparisons in columns, and lowercase letters represent comparisons in rows.
| Phytopathogens | Combined activity | Combined extracts | |
| 250 µg ml-1 | 500 µg ml-1 | ||
| Aspergillus glaucus (L.) Link NACF0010 | 14±1 Cb | 30±2 Ca | EAL, EAS, EAR |
| Colletotrichum gloeosporoides (Penz.) Penz. & Sacc NCBI HM562712 | 0±3 Db | 25±2 Da | EAL, EAS, EAR |
| Colletotrichum acutatum var. fioriniae Marcelino & Gouli ATCC 58697 | 19±1 Bb | 35±5 Ba | EAL, EAS, EAR, EMR, EML |
| Fusarium oxysporum f. sp. lycopersici (Sacc.) W.C. Snyder & H.N. Hansen ATCC 9848 | 50±5 Aa | 63±2 Ab | EAL, EAS, EAR, EMR, EML |
| Curvularia lunata (Wakker) Boedijn NACF0030 | 45±5 Aa | 59±1 Ab | EAL, EAS, EAR, EMR, EML |
| Rhizopus stolonifer (Ehrenb.) Vuill. NACF0011 | 0±0 Eb | 2±1 Ea | EAL, EAS, EAR, EMR, EML |
Liriodenine was more potent than the most active extracts were for all of the phytopathogens, except Rhizopus stolonifer (Table 4), although it was superior to Captan® against all tested species.
Table 4: Antifungal activity of liriodenine. The values are means ± DE (n=6). The separation of groups was performed with the Mann-Whitney test (p<0.05). Capital letters represent comparisons in columns, and lowercase letters represent comparisons in rows.
| Liriodenine | Captan® | |||||
|---|---|---|---|---|---|---|
| Phytopathogens | % Inhibition Mycelial Growth (nmol ml-1/µg ml-1) | CMI in broth | % Inhibtion Mycelial Growth (nmol ml-1/µg ml-1) | |||
| 100/27.5 | 200/55.1 | nmol ml-1 | 200/60.1 | 1000/300.6 | ||
| ml | ||||||
| Aspergillus glaucus (L.) Link NACF0010 | 38±2 Cb | 50±2 Ca | 250 | 15±1 Bd | 29±1 Cc | |
| Colletotrichum acutatum var. fioriniae Marcelino & Gouli ATCC 58697 | 43±2 Bb | 52±2 Ca | 250 | 43±4 Ab | 50±7 Ba | |
| Colletotrichum gloeosporoides (Penz.) Penz. & Sacc NCBI HM562712 | 35±2 Cb | 30±3 Db | 500 | 5±3 Cc | 59±2 Aa | |
| Fusarium oxysporum f. sp. lycopersici (Sacc.) W.C. Snyder & H.N. Hansen ATCC 9848 | 70±2 Ab | 100±2 Aa | 125 | 43±4 Ad | 50±7 Bc | |
| Curvularia lunata (Wakker) Boedijn NACF0030 | 46±2 Bc | 85±2 Ba | 250 | 12±7 Bd | 65±4 Ab | |
| Rhizopus stolonifer (Ehrenb.) Vuill. NACF0011 | 0±0 Dc | 10±0 Eb | 500 | 3±0 Cc | 47±7 Ba | |
The most sensitive fungus was Fusarium oxysporum f. sp. lycopersici, whose mycelial growth on agar was completely inhibited at 200 nmol ml-1 (MIC: 125 nmol ml-1).
Discussion
The results indicated that the tissues of Sapranthus microcarpus biosynthesise and accumulate substances with antifungal activities against phytopathogens. This property has rarely been reported for other Annonaceae, which have been mostly studied against fungi of medical importance (Rios et al., 2003; Lago et al., 2007; Akendengué et al., 2009). The fractionation of plant secondary metabolites into two groups, nonpolar (hexane extracts) and polar (alkaloid and methanolic extracts), yielded information on the presence of active substances of a different nature in each tissue. Species of the Annonaceae family produce nonpolar and polar metabolites (annonaceous acetogenins, essential oils, benzylisoquinoline alkaloids, etc.) with antimicrobial activity (Rios et al., 2003; Lago et al., 2007; Dang et al., 2011; González-Esquinca et al., 2014).
One assessed hypothesis was that it might be possible to increase the antifungal activity by combining extracts. The combined extracts showed good activity, although it was never greater than that of the individual extracts, reflecting the average activity of the combined extracts. Nevertheless, combined extracts are an alternative that offers the advantage of phytochemical diversity and that, according to Reuveni and Sheglov (2002), is suitable for fungicides, since different modes of action can minimise the risk of the development of resistance by target microorganisms, improving disease control.
The presence of liriodenine as an active substance in alkaloid and even methanol extracts is a recurring phenomenon in the Annonaceae (Feitosa et al., 2009; Alias et al., 2010) since it is one of the most widely distributed benzylisoquinoline alkaloids in plants (González-Esquinca et al., 2014; Lúcio et al., 2015). This is the second report on the genus Sapranthus, as Etse and Waterman (1986) reported the presence of liriodenine in the stem bark of Sapranthus palanga R.E. Fr.
Resistance of phytopathogens to fungicides is a frequently occurring biological phenomenon. One strategy to find new effective fungicides is to start with in vitro tests of secondary metabolites. Spore germination and mycelial growth are central to the life cycle of a fungus. Therefore, a plant metabolite that inhibits either of these two processes can reduce the ability of phytopathogens to cause the disease in host plants (Morrisey and Osbourn, 1999; Balkis et al., 2002). Our results suggest that S. microcarpus tissues and its bioactive alkaloids can be promising alternative fungicides for the control of various plant diseases.











 nueva página del texto (beta)
nueva página del texto (beta)



