Introduction
Forest canopy, understory and topographic factors such as slope aspect interact and shape “safe sites” (Harper, 1977). These microsites play an important role during the plant’s early stages of their life cycle such as germination (Cornett et al., 2000), because they determine the persistence of species and the dynamics of plant populations; they are critical during species regeneration at community level (Grubb, 1977).
Secondary vegetation species are important for forest regeneration (Blanco-García et al., 2011) and in particular for the establishment of some mature forest species, because they can be nurse species for tree species germination and/or establishment (Castro et al., 2002). Nevertheless, there is still some controversy about this, since some understory species are sometimes considered as an obstacle for reforestation with mature forest species, because of competition for light, water and nutrients (Raynor, 1971). This is why in some sites, as part of forest management practices in temperate forests of Central Mexico, an elimination of the understory vegetation (herbs and shrubs) is carried out by local communities and land owners Martínez-Orea et al., unpublished data).
Understory species are important in many ways; they can contain a significant part of the nutrients in forests and they are also part of the forest biodiversity (Tergas and Popenoe, 1971; Warren et al., 1987). Germination of understory species is shaped not only by microsite availability in a forest, but also by germination requirements and seed dormancy.
In the case of species with morphophysiological dormancy, the opportunities for germination in a suitable microsite are restricted to the status of the seeds, either if they are only quiescent or dormant. Symphoricarpos microphyllus Kunth is a shrub and non-timber forest product which inhabits fir forests from New Mexico to Guatemala; it provides forage for herbivores such as deer. Populations of this shrub have been declining in Central Mexico because it is overused in order to make brooms, Christmas crafts and also due to deforestation (Monroy et al., 2007; Mendoza-Bautista et al., 2011). Additionally, in some forests fruit production of this species has only been observed in sites that are not under understory removal management (pers. obs.), resulting in an even more difficult regeneration from seeds.
Reforestation with this species just began few years ago, but through asexual propagation, due to difficulties to germinate its seeds. According to Mendoza-Bautista et al. (2011), the conditions for this species’ establishment are not well known, neither are light requirements for its germination, although several species of Symphoricarpos Duhamel, are known to possess morphophysiological dormancy (Baskin and Baskin, 1998; Hidayati et al., 2001). Since understory removal could be representing another anthropogenic disturbance factor in forests near Mexico City, that are already suffering from constant deforestation and cattle raising (Ávila-Akerberg, 2002), this type of management could have negative effects in some abiotic conditions, and probably decrease the availability of safe microsites for germination of certain species.
Therefore, the aims of this work were: (1) to characterize microsite suitability in terms of slope orientation and forest management for S. microphyllus germination in a fir forest, (2) to measure its germination response under different light qualities in controlled environments and, (3) to know seed viability in a two year period, in order to set some basis for its propagation through seeds.
Materials and Methods
Study site
The Magdalena river basin (MRB) (Fig. 1) is part of the soil conservation area of Mexico City (Ávila-Akerberg, 2002), covering an area of 3100 ha. Two rivers occur in this basin, the Magdalena and the Eslava, providing a large amount of the superficial water in Mexico City (Mazari-Hiriart and Mazari-Menzer, 2008). The first is the main one and has a length of 21 km, 52.5% of it runs in the forest and the rest (47.5%) in the urban area (PUEC-UNAM, 2008). This area is located SW of the valley of Mexico, at an altitude between 2570 and 3870 m a.s.l.
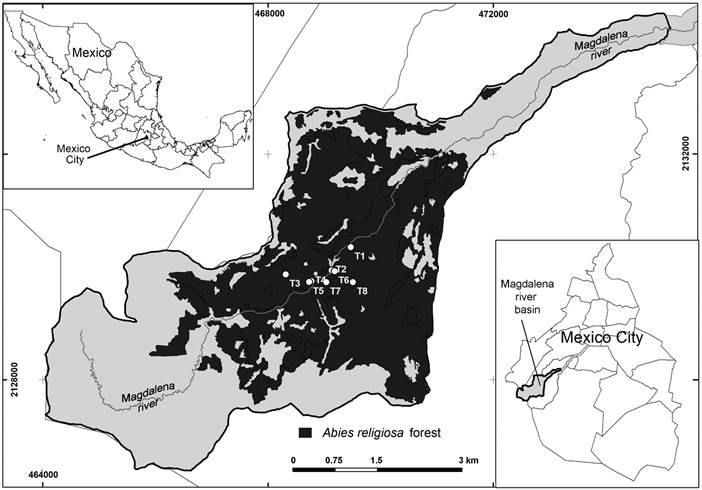
Figure 1: Fir forest (Abies religiosa (Kunth) Schltdl. & Cham.) forest in the Magdalena river basin (MRB), Mexico City. T1-T8 (Study transects).
Three vegetation types are found in the MRB: oak, fir (Abies religiosa (Kunth) Schltdl. & Cham.) and pine forests (Fig. 1). The fir forest has an extension of 1071 ha (the largest of the three) and is located at an altitude of 2900-3650 m a.s.l. and between 19°13'53"/19°18'12" latitude N and 99°14'50"/99°20'30" longitude W. The dominant tree species is Abies religiosa, but sites with herbaceous and shrub layers are common. Shrub species such as Acaena elongata L., Ageratina glabrata (Kunth) R.M. King & H. Rob., Roldana angulifolia (DC.) H. Rob. & Brettell, and R. barba-johannis (DC.) H. Rob. & Brettell are abundant, whereas others such as Symphoricarpos microphyllus and Ribes ciliatum Humb. & Bompl. ex Roem. & Schult. are less so (Ávila-Akerberg, 2002; Calderón and Rzedowski, 2005). The climate of this forest is temperate subhumid type C(w2)(w)b(i’) (Álvarez-Román, 2000; García, 2004), mean annual temperature is 14 °C (minimum 6 °C, maximum 20 °C), and annual precipitation ranges between 950 and 1300 mm. The coldest period of the year comprises from November to January with a mean temperature of 6 °C and the warmest from March to September with a mean of 18 °C (Dobler-Morales, 2010) (Fig. 2). A high spatial heterogeneity is observed because of the numerous differently oriented slopes (Santibáñez-Andrade et al., 2015).

Figure 2: Climograph of the meteorological stations of Desviación Alta al Pedregal (1967-2000) and Monte Alegre (1967-1987), both in the Magdalena river basin (MRB), Mexico City. Modified from Delgadillo-Durán (2011).
This heterogeneous topography is related to the presence of slopes of 12 to 25%, and others higher than 75%. According to the PUEC-UNAM (2008), 67% of the forests in this area are conserved. Nevertheless, as an urban forest it is constantly affected by deforestation, cattle rising, tourism, induced fires and farming (Ávila-Akerberg, 2002), and recently an increase in tourism has been observed, which has also become a problem since there are no restrictions on the number of visitors and there is a lack of surveillance too. Additionally, the owners of this forest carry out an elimination of the understory vegetation in order to provide light for Abies religiosa seedlings and as part of reforestation programs.
Study species
Symphoricarpos microphyllus (pink snowberry, “perlilla”), belongs to the family Caprifoliaceae and is a branching shrub 2 to 3 m tall, young branches are pubescent, leaves are up to 25 mm long, flowers are pink and bell-shaped and fruits are white or pale white-pink and fleshy. Fruits have 2-4 beige-white seeds that measure 3-5 mm long, their shape is ovate.
This species colonizes different successional sites in fir and oak forests in central Mexico. It is found in mountains in the valley of Mexico and it is distributed from New Mexico to Guatemala (Calderón and Rzedowski, 2005). It is collected for making brooms and art crafts. As a consequence, the overexploitation of this species is causing a decrease in its populations (Monroy et al., 2007). It flowers from July to September, and fructification occurs from October to February in forests of the volcano “El Nevado de Toluca” (Anastacio-Martínez et al., 2015). Martínez-Arévalo (2015) reports that approximately 19 days after sowing the seeds, germination starts, and it continues for 12 more days reaching 70%. Nevertheless, this author does not give information about the light and temperature conditions for germination, neither about the presence of any seed dormancy.
Microsites characterization
Eight 50 m transects were set on south (S) oriented (N=4) and in north (N) oriented slopes (N=4). Half of them were located in areas where secondary species (shrubs and herbs) have been eliminated by the management that local people carry out in order to provide light for Abies religiosa seedlings. This results in a design of two transects oriented to the S with shrubs and herbs (undisturbed transects t5 and t6, US), two transects with the same orientation without shrubs and herbs (perturbed transects t2 and t3, PS), and other four transects N oriented, two with shrubs and herbs (t1 and t4, undisturbed, UN) and two without them (t7 and t8, perturbed, PN). Three microsites were set in each transect: at the lowest part of the slope (0 m), the middle (25 m) and in the highest part (50 m), resulting in a total of 24 microsites (three microsites for each transect). The size of microsites was 1 m2.
Microsites were characterized through their values of light, soil temperature and moisture. Light was quantified through hemispherical photographs (nine per microsite) analysis, they were taken in April 2016 at 1 m above the ground with the camera oriented towards the magnetic N, using a NIKON camera D80 (Nikon, New York, USA) with a fish eye lens EX SIGMA (Sigma, New York, USA) 4.5, 1.28 DC DSM. Photographs were taken under overcast sky conditions at 8 am, following Messier and Puttonen (1995). They were analyzed with the Hemiview canopy analysis software ver. 2.1 (Delta-T, 1998) in order to estimate the global site factor (GSF, MJ/m-2/yr-1). Red:Far red light ratios (R:FR) were also registered in five aleatory spots of each microsite with a radiometer at the soil level (Skye Instruments, SKR 100, Wales, UK). These data were registered in April 2016, between 10 am and 5 pm. In order to register soil moisture and temperature in each microsite we set one hobo data logger (easy LogUSB-ONSET, Massachusetts, USA) from January to June. Three soil samples were collected at each microsite in January and another three in June. Consequently, soil pH (Model w-22xd, HORIBA, Kyoto, Japan) and nitrogen (N-Kjeldal) contents were analyzed (Model S040, HORIBA, Kyoto, Japan).
Germination and viability tests
Fruits of Symphoricarpos microphyllus were collected from 30 individuals in January 2016, from a site under no management of secondary vegetation removal. Seeds were extracted and cleaned from the fleshy fruit containing them, and they were sown in microsites two weeks after seed collection in February 2016, in three nylon-cloth bags filled with 50 seeds each (150 seeds per microsite). The dimensions of the bags were 8 × 8 cm with a 0.2 mm2 mesh. Each bag was sown in a plastic pot (20 cm diameter × 20 cm depth) according to the methodology of Hidayati et al. (2001), sowing each bag 4 cm deep in each pot. Five extra bags with ten seeds each were sown in each microsite; one was exhumed every two weeks to monitor germination. Once germination was registered, all bags were exhumed and germinated seeds (emergence of radicle) were counted. With the rest of the seeds, germination tests were carried out in chambers in February 2016 to register the species response to light quality, but no seeds germinated.
A tetrazolium test for viability was carried out with 30 extra seeds right after seed collection and again following one year and two years (2017, 2018). The rest of the seeds were stored in dark and dry conditions in paper bags in a laboratory (Dinámica de Comunidades, Departamento de Ecología y Recursos Naturales, Facultad de Ciencias, UNAM, Mexico) for two years. In 2017 and 2018 tests for germination in different light qualities were again carried out. Six hundred of the stored seeds were disinfected in sodium hypochlorite (1%) during three minutes, they were sown in different light qualities (darkness, white, far red, and red light) in Petri dishes in a germination chamber (NuAire model I-36LL, Massachusetts, USA) at 22°C/20°C, 16/8 photoperiod. Dishes were sealed with a plastic film (Egapack, Mexico) in order to maintain humidity. Three Petri dishes (9 cm diameter) with humid absorbent paper (previously sterilized, as a substrate) per light treatment and with 50 seeds each were set in a germination chamber under: (1) white light (WL; photon flux density (PFD)=33.21 µmoles m-2s-1, R:FR=1.73; (2), red light (RL; PFF=5.18 µmoles m-2s-1, R:FR=3.39), (3) far red light (FRL; PFF=1.2 µmoles m-2s-1, R:FL=0.05), and (4) darkness (D). PFD between 400 and 700 nm was measured with a quantometer (Apogee, model MQ-200, Apogee Instruments, Inc., Logan, Utah, USA) and the R/FR ratio (R=640-670 y RL=690-748 nm) was measured with a radiometer SKR-100 (Skye Instruments, Wales, UK). For the treatment with RL, Petri dishes were set inside a red plexiglass box (3 mm thick, 48 × 32 × 8 cm, Series 2424 Rohm and Hass, Mexico). For FRL, treatment Petri dishes were set in a red plexiglass box with a cover of blue plexiglass (same dimensions of the red box, Series 2423). For the treatment in darkness, Petri dishes were covered with aluminum foil. All Petri dishes and plexiglass boxes were set in the mentioned germination chamber equipped with fluorescent lamps (OSRAM of 17 watts and 60% relative humidity). During 30 days, every third day we registered germination in WL boxes and one month after being sown, we registered germination in the other light treatments.
Data analysis
In order to characterize whether microsites are affected by the slope orientation and forest management, we measured different environmental variables. The orientation factor data were analyzed in two levels: S and N. The management factor data were also analyzed in two levels: U and P. Generalized Linear mixed models (GLMM), packages glmmTMB and Ime4 were used to analyze data. Our random effects were microsite and transect. The types of distributions were applied as follows: GSF, R/FR ratios, soil moisture and soil nitrogen content (error: beta, link function: logit), soil temperature (error: normal, link function: identity), soil pH (error: normal, link function: identity), seed germination (error: binomial, link function: logit) (Crawley, 2012; R Core Team, 2015). A Kruskal-Wallis test (Stat Soft, 2007), and variation coefficients (VC) were calculated with the R/FR ratio values in order to find differences depending on the day time. Statistical differences in the (VC) values were searched through a Z test (Zar, 1974). A PCA (Principal Component Analysis) was carried out in order to find a relationship between the germination percentages of S. microphyllus and the environmental variables of microsites and to identify which of the factors influenced the distribution of microsites according to their environmental variables (PC-ORD software, ver 5.10, McCune and Mefford, 2006).
Results
Light in microsites
Microsites showed differences in light, with the highest values of the Global Site Factor (GSF) in the US, while the smallest values were found in the P microsites. No significant differences were observed in the R/FR ratios between microsites. The GSF values showed a significant effect of slope orientation and of the interaction between disturbance and slope orientation (Table 1, supplementary material).
Table 1: Values (means±S.E.) of light (GSF and R/FR ratios), soil temperature (°C), moisture (%), pH nitrogen (%), and germination (%). Statistical analysis was carried out using Generalized Linear mixed models (GLMM). Different letters indicate significant differences at p≤0.05 between microsite types.
| Variables | Undisturbed South (US) | Undisturbed North (UN) | Perturbed South (PS) | Perturbed North (PN) |
| GSF (MJ/m-2/yr-1) | 450±12a | 236±9.6 b | 134±9.1a | 143±4 b |
| R/FR | 1.72±0.14 | 1.28±0.06 | 1.3±0.14 | 0.72±0.05 |
| Soil temperature (°C) | 11.41±0.08 | 9.99±0.08 | 12.24±0.06 | 11.46±0.06 |
| Soil moisture (%) | 66.51±0.32 | 68.07±0.34 | 57.52±0.3 | 64.64±0.32 |
| Soil pH | 6.06±0.11 | 5.9±0.03 | 5.9±0.01 | 5.8±0.08 |
| Soil nitrogen (%) | 0.54±0.09 | 0.51±0.07 | 0.61±0.03 | 0.63±0.07 |
| Germination | 43.3±2.01 | 34.1±2.02 | 25.3±2.01 | 31.1±2.02 |
Light quality in microsites R/FR showed the highest peak in PS microsites. The lowest values were observed in the PN microsites. US, PS and UN microsites showed the highest values at 13:30 hrs (Fig. 3). In all microsites these values varied statistically along day (H=26.8, p<0.0001).

Figure 3: Mean values of R/FR ratios in four microsite types: US (undisturbed facing south), UN (undisturbed facing north), PS (perturbed facing south) and PN (perturbed facing north), SE bars are shown.
The widest VC value of this variable along day was found in the PS microsites (VC=1.02), while for the other microsites variation it was smaller, UN (VC=0.85), US (VC=0.82), PN (VC=0.67). Statistical differences in the VC values were found for all comparisons: UN and PN (Z=2.1>0.73, p<0.05), UN and PS (Z=1.3>0.95, p<0.05, PS and US (Z=2.4>0.95, p=0.03), PS and PN (Z=1.17>0.48, p<0.05), US and PN (1.30>0.48, p=0.01), except for the comparison between UN and US microsites (Z=0.85<1.24, p=0.31).
Soil temperature and moisture in microsites
Microsites with the highest values of temperature (mean values from January to June) were those PS (x̄=12.24±S.E.=0.06), lowest values were registered in UN (x̄=9.99±S.E.=0.08). Lowest values of soil moisture were registered in PS (x̄=57.52±S.E.=0.3) (Table 1). Even though these tendencies point out to higher temperature and smaller moisture values in P microsites, no significant differences were found.
Soil pH and nitrogen in microsites
Soil pH and % N (means obtained from soil collected in January and June) are shown in table 1. US microsites showed the highest soil pH (x̄=6.06±S.E.=0.11), the highest soil % N mean values corresponded to PN (=0.63±S.E.=0.07%). No significant differences were found between S and N facing microsites, neither between P and U microsites (Table 1).
Response of seeds to different light qualities and viability
Viability tests showed that 65% of the seeds were viable right after seed collection from the field. One year later, in 2017, viability measurement resulted in almost the same value (60%) and 60% in 2018 (two years after seed collection) as well. Germination percentages in chambers resulted as follows: no seeds germinated in the year of seed collection (2016). One year later in 2017: 10% in white light (WL), 20% in red light (RL), 25% in darkness (D) and the highest value was reached in far red light (FRL) (28%). In 2018 seeds germinated in 8% in WL, 26% in RL, 28% in D and the highest value was reached in FRL (32%).
Seed germination in microsites
The highest germination percentages of S. microphyllus in the forest were registered in US microsites, while the lowest were found in PS microsites. Even though these results point to a tendency of smaller germination in P microsites, no statistical differences in germination percentages resulted from the disturbance, orientation or interactions between factors according to the GLMM analysis (Table 1, supplementary material).
In the PCA analysis (Fig. 4) we see that microsites associated to the highest germination percentages are U microsites, where there are also higher moisture and lower temperature values.
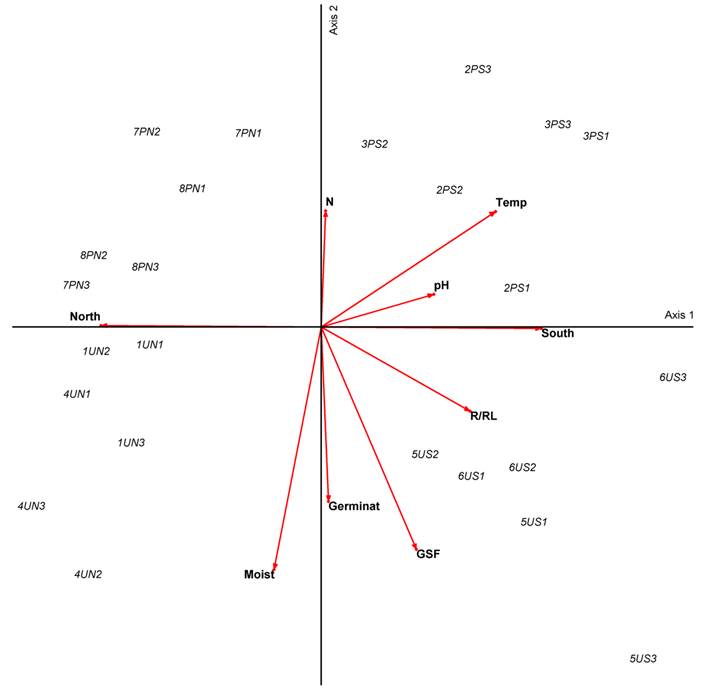
Figure 4: Principal component analysis (PCA) for 24 microsites in the Magdalena river basin Abies religiosa (Kunth) Schltdl. & Cham. forest, Mexico City. Numbers from 1 to 8 indicate the transects. U and P indicate undisturbed and perturbed microsites, S and N indicate south and north facing slopes respectively. Numbers 1, 2, and 3 indicate microsite locations at the lowest, middle, and highest parts of the slope. Germinat indicates highest germination values of Symphoricarpos microphyllus Kunth. X axis (34.86% variance), Y axis (58.29% variance).
Discussion
Microsite characteristics are variable due to several factors such as slope orientation, forest structure and even topographic position (Méndez-Toribio et al., 2016). For example, light in microsites is modeled by canopy structure and gaps (Martínez-Camacho et al., 2018). In the northern hemisphere south (S) facing slopes show higher sunlight conditions than north (N) facing ones (Matlack, 1993; Méndez-Toribio et al., 2016). As a consequence, in our study microsites on S facing slopes showed significantly higher values of light than microsites on N facing slopes. These results match those of Ackerly et al. (2002), who reported higher values of diurnal insolation in S than in N sites in a Californian chaparral as well as Gallardo-Cruz et al. (2009) and Méndez-Toribio et al. (2016) for a seasonally dry forest in Mexico. Méndez Toribio et al. (2016) reported 16% more solar radiation on S facing slopes than on N facing ones. In our study site the same pattern was detected but with a higher value of difference between both slopes (22%). This difference can vary according to the onset of the rainy season. Contrary to what we expected, in perturbed microsites (P) light values (of GSF and R/FR ratios) were smaller than in undisturbed (U) microsites, because canopy gaps were larger in these microsites. Even though gaps and slope orientation determine most of the light environment of microsites as some authors have reported (Jiao-Jun et al., 2003), our undisturbed microsites were not completely under perturbation because light entrance was increased due to the presence of gaps (caused by branch or tree fall and deforestation) which also is an important factor that has effects in light at the microsite level. R/FR ratios were higher in PS microsites indicating that secondary vegetation probably has a function of filtering light, like forest litter or soil (Vázquez-Yanes et al., 1990) and in its absence due to this management practice, RL is higher than FR light at the microsite level. Therefore, understory species have important effects on light dynamics at the soil level like Messier and Puttonen (1995) report. Similar values of R/FR (close to 1.15) were reported by Endler (1993) which correspond to small gaps which match our results for P microsites, and are also close to those reported by Martínez-Camacho (2015) for this study site under small gaps (0.99-1.13). Values for large gaps according to these authors are 1.43 and between the intervals of 1.21-1.37 respectively, which are similar to 1.5 for our U microsites under larger gaps.
It is interesting to note that in the PS microsites light heterogeneity was higher than in the other microsites. This might be due to the absence of herbs and shrubs; therefore, light is only filtered by the tree canopy, and values of RL are higher than FR ones. Observing the variations of light in microsites, their highest R/FR ratio values occurred at 13:30 hours in most microsites, especially in the S microsites. For light availability at soil level, canopy gaps and slope orientation are the main factors, but for light quality the absence of understory vegetation may play an important role because it filters light and may buffer drastic light changes. However, light as a signal for seed germination (Pons, 2000) is a highly variable environmental factor in forests (Messier and Puttonen, 1995) as we registered in our microsites and in previous research by Bonilla-Valencia et al. (2017a) in this study site.
According to Mendoza-Bautista et al. (2011), the study of the particular light requirements of Symphoricarpos microphyllus has been limited, even though it is known to grow under different canopy conditions. These authors studied the effect of solar radiation on the survival and growth of this species and worked with different sites: agricultural land without canopy with radiation levels of 8201 MJ/m-2/y-1, a pine tree plantation (7886 MJ/m-2/y-1), and at an oak-alder plantation (2794 MJ/m-2/y-1). In pine plantations (intermediate levels of radiation) this species growth was the highest; survival was also high in the agricultural land and under pine trees, while the lowest survival occurred under the lowest levels of light, results that differ to those reported by Hernández and Rodríguez (2008), who found that in the first year of establishment, this species reaches the highest growth and survival rates in high levels of light. Our light levels in microsites were lower and were between 140 MJ/m-2/y-1 in PS microsites and 450 MJ/m-2/y-1 in US microsites, because our study site is a forest with different structure and the canopy is composed by fir trees. However, Hernández and Rodríguez (2008) point out that even at high radiation levels S. microphyllus survival tends to decrease when soil moisture decreases. Even though microsite characteristics for seed germination are sometimes similar to those for seedling establishment (Fowler, 1988), the lack of soil moisture is an environmental factor that can limit not only growth and survival, but also germination, since the entrance of water to the seed triggers this response once seeds have overcome dormancy (Baskin and Baskin, 1998).
Our results indicate that light could be acting on germination at a fine level. In our study in germination chambers, a preference for FR light by the seeds after one and two years of being stored was found, as in the field. In U microsites, where the highest germination percentages were registered, presumably the presence of secondary vegetation is filtering light, therefore causing more FR light than R light. Similar results have been found by Hidayati et al. (2001), who found high percentages of germination in seeds that were buried under Quercus L. leaf litter and soil, which is related to a higher supply of FR light.
Light affects other microsite variables for example soil moisture and temperature. Microsites facing north do not receive the input of energy that south-facing microsites do, hence, they are generally cooler and moister than those facing southwards (Harris, 1984). As a consequence, in a six-month period we registered different tendencies of these variables between S facing and N facing microsites. Méndez-Toribio et al. (2016) found that S facing sites are 2 °C warmer than N facing sites in a seasonally dry forest in Mexico. In our study, the differences in these variables were also associated to disturbance, because PS microsites were the highest in temperature and the lowest in soil moisture, while UN were the highest in soil moisture and the lowest in soil temperature. Even though gaps are brighter and warmer due to an increased irradiance and their surface soils contain more water due to the reduction in plant transpiration (Denslow, 1987), our study site was reported by Bonilla-Valencia et al. (2017a, b) as highly heterogeneous in terms of soil moisture conditions, showing 15% more soil moisture in sites under small gaps than in sites under larger gaps. In our study, since P microsites were 8% less humid than U microsites, our results could indicate that secondary vegetation exerts a protective effect against the loss of soil moisture, matching the results of Chaneton et al. (2010). A cumulative effect of the orientation of slope facing S and the absence of secondary vegetation could be acting in PS microsites making them less humid and warmer.
In temperate environments temperature is frequently a primary factor and germination could be mediated by its seasonal changes. For some species with morphophysiological dormancy a light requirement can be fulfilled after stratification with low temperatures. Seeds with this type of dormancy must be exposed to certain temperature changes that break it, so that they germinate afterwards under the required light and soil moisture conditions (Baskin and Baskin, 1998). Species of Symphoricarpos are known to have underdeveloped linear embryos that must grow before they can germinate. Additionally, its physiological dormancy is not yet fully known, which, according to Hidayati et al. (2001) and Baskin and Baskin (1998), corresponds to a non-deep complex morphophysiological dormancy, where temperature plays a critical role (Nikolaeva et al., 1977). Still a lot of knowledge on Symphoricarpos species seed biology is lacking, but some authors report that the embryos of these species grow when exposed to fluctuations in temperature between 0 and 10 °C (Hidayati et al., 2001). According to Pelton (1953), alternating temperatures of 5 and 15 °C resulted in higher germination percentages (45.7%) of Symphoricarpos occidentalis Hook., than constant temperatures of 10 °C, which resulted in 40% germination. In our study site there was unusual snow March 2016, and the low temperatures registered in this month could have caused the growth of embryos and the loss of dormancy. Also, during this time there were temperature fluctuations from -2.5 °C at 6 am to 20.5 °C at 4 pm in U microsites. Pelton (1953) also reported that the embryo of S. occidentalis is dormant at maturity and that both a warm stratification followed by a cold one are needed to overcome dormancy. These conditions of variations in temperatures up to 22 °C could have favored seeds in the field of our study site to overcome dormancy, resulting in higher percentages in US and UN microsites where soil moisture values were greater, compared to P microsites. Germination of S. occidentalis showed larger values in saturated (more than aerated) soils according to Pelton (1953). In the first year our seeds only germinated in field conditions, where they were subjected to the mentioned variations in temperature and to the variation of other factors such as light and soil moisture. Adams (1972) reports that Symphoricarpos racemosus Michx. reached germination values of 50% after seeds were stored outdoors under exposure to cold winter temperatures for one year and a 10% viability for four years. These findings match our results, since we observed the maintenance of viability in values higher than 50% after two years of storing in laboratory conditions. However, other species such as Symphoricarpos orbiculatus Moench do not appear to remain viable for more than two years in the soil (Hidayati et al., 2001). The higher germination percentages in U microsites indicate that low temperatures and high soil moisture conditions could have played an important role in the germination of this species by helping embryos mature, and even though its survival and growth occur in a wide interval of light intensity, the presence of secondary vegetation could be affecting germination positively, by providing higher soil moisture and lower temperatures conditions.
Other factors that have been identified for affecting seed germination are soil pH and nitrogen. After the removal of secondary vegetation in our study site, it is not deposited elsewhere but it is piled up in the sites. According to Raulund-Rasmussen et al. (2008), after a sudden deposition of plant material in the soil, increases of nitrogen and more acid values of pH can be registered. This matches our results because it was in the PN microsites where more acid values of pH and higher values of nitrogen in soil were found. However, no significant differences between microsites were registered, maybe due to the fact that our study had a short period of observations after the removal of secondary vegetation was carried out. Nevertheless, the values of both variables were similar to those reported by Bonilla-Valencia et al. (2017a) for this study site. They reported there are micro-environmental variations in soil nitrogen, with the highest values (ca. 0.62) in sites with the lowest pH values (around 5.5). It is important to mention that nitrogen is not a limiting factor in this forest, because there is a constant atmospheric nitrogen deposition from the city and from the practices of cattle raising as well. However, we did not find any relation between germination and these two variables, because germination was the highest in US microsites. Other studies have reported that high concentration of nitrates in acid solutions in soil do increase germination of some species in a Californian chaparral (Keeley and Fotheringham, 1998), for some species in temperate sites (Weber and Lee, 1979; Fan and Wang, 2000) and for species of the Chenopodiaceae family (Williams and Harper, 1965). Maybe this management practice does not clearly induce germination of Symphoricarpos species as Pelton (1953) mentioned that pH values of 5.9 and 7.3 showed no appreciable differences in germination of after-ripened seeds.
The lack of knowledge on Symphoricarpos microphyllus germination and viability of its seeds is probably related to the fact that at least in central Mexico this species is propagated mostly through vegetative parts instead of seeds. With this there is a risk of losing the genetic variability of populations, which are already decreasing because of the intense collection of individuals to fabricate rustic brooms and Christmas crafts. Additionally, there is a continuous deterioration of temperate forests where it is distributed (Mendoza-Bautista et al., 2011). As a consequence, studies that provide information on seed biology of S. microphyllus are needed for restoration and reforestation programs because of its economic and ecological importance, since it is also part of the diet for white deer and birds (Quintero et al., 2008).
Conclusions
The removal of secondary vegetation is not recommended by us because it negatively affects the microenvironment where seeds germinate. This causes important decreases and increases in soil moisture and soil temperature, respectively. Secondary vegetation modulates light quality at the microsite level, and species that require not only RL for germination, but also FRL like our study species, will be affected negatively in its germination percentages. Therefore, an adequate forest management should be provided in order to maintain microsite availability for germination of S. microphyllus, with enough soil moisture, alternating high and low temperatures (due to its complex morphophysiological dormancy) and adequate light conditions (higher availability of FRL) that can be provided by secondary vegetation. We consider secondary vegetation important in terms of maintaining soil moisture and filtering light. Future studies could prove the effect of this forest management in germination of S. microphyllus in microsites by sowing non-dormant seeds (after six months and/or one year of being stored in high/low temperatures). This would show a clearer effect of germination in response to environmental variables and how they are affected by disturbances or forest management, without the dormancy factor. Nevertheless, an experimental exclusion of this type of forest management in sites with different gap sizes is necessary to find more direct effects of secondary vegetation removal on micro-environmental conditions and biotic responses such as germination, because disturbances can be different in the understory and at the canopy level. This is important according to Méndez-Toribio et al. (2016) because some biotic responses can be closely related to the environmental heterogeneity caused by natural conditions (such as slope orientation) and others are more affected by disturbances.











 text new page (beta)
text new page (beta)



