Introduction
Interspecific hybridization has important evolutionary consequences (Whitham et al., 1991), such as introduction of genetic variation into populations, generation of new ecotypes better adapted to novel environments (Pfennig et al., 2016), and development of new lineages (Abbott et al., 2013). Hybridization can also lead to maladaptation or extinction via genetic and demographic swamping (Todesco et al., 2016). Although hybridization is a widespread phenomenon in nature, it is expected that sympatric, related species develop reproductive barriers to avoid hybrid formation and maintain species integrity (Xie et al., 2017). Reproductive barriers to gene flow include prezygotic barriers that act before fertilization, preventing interspecific gene flow, and postzygotic barriers that reduce fertility of hybrids (Lowry et al., 2008; Widmer et al., 2009). For plants, prezygotic barriers include asynchrony between flowering seasons and the specificity of pollinator behavior, while an important formation of postzygotic barriers is the decrease of hybrid pollen viability, which reduces the success of backcrosses with parental species (Campbell et al., 2003; Baack et al., 2015). However, hybrids may overcome postzygotic barriers, and can be more vigorous than parental species, establishing large populations in hybrid zones (Birchler et al., 2006).
Bursera Jacq. ex L. (Burseraceae) is a diverse genus of usually deciduous shrubs and trees that comprises approximately 100 species, whose center of diversification and endemism is in the Pacific drainages of western Mexico (Rzedowski and Kruse, 1979). Bursera species are often dominant or codominant woody elements of the tropical dry forest (TDF), desert scrub and thorn scrub habitats (Miranda and Hernández-X., 1963) in Mexico (Rzedowski and Guevara-Féfer, 1992). Hybridization has been hypothesized an important process in the diversification and adaptation of Bursera (McVaugh and Rzedowski, 1965). Studies showed that natural hybridization is a frequent phenomenon in areas where closely related species co-exist (Toledo-Manzur, 1982; Rzedowski and Ortiz, 1988; Rzedowski and Guevara-Féfer, 1992; Pérez-Navarro, 2001).
Molecular and biochemical evidence has confirmed the hybrid origin of few species (Rzedowski and Ortiz, 1988; Weeks and Simpson, 2004), while field observations have noted that hybrids can be numerous and vigorous with abundant fruit production (Rzedowski and Ortiz, 1988; Rzedowski and Guevara-Féfer, 1992). So far, only one study has evaluated the reproduction of hybrids. Cortes-Palomec (1998) showed that B. medranoana Rzed. & E. Ortiz, a species of hybrid origin from B. morelensis Ramirez and B. schlechtendalii Engl., presents male infertility and thus seed production occurs through apomixis. Other genetically confirmed hybrids in the genus, B. brunea (Urb.) Urb. & Ekman and B. gracilipes Urb. & Ekman, are likely to be sterile as well (Weeks and Simpson, 2004), but no more formal studies exist.
The TDF of the Bajío region in the northeast (NE) of Michoacán and Guanajuato was one of the most widespread native habitats in this region, which at present is disappearing due to the rapid land use change towards agriculture, livestock grazing, and urbanization (Hernández-Oria, 2007). Bursera cuneata (Schltdl.) Engl. and B. bipinnata (Moc. & Sessé ex DC.) Engl. of the section Bullockia (Bridson) Razafim., Lantz & B. Bremer, known as copales, are usually codominant woody elements in the TDF in Michoacán. In rural communities, these species are used for live fences and firewood, while B. cuneata is applied for the elaboration of handicrafts. Both species have a largely allopatric distribution, but occur in sympatry in the NE of Michoacán, where field explorations of Rzedowski and Guevara-Féfer (1992) reported the presence of putative hybrids. These putative hybrids present intermediate leaf characteristics and can show abundant fruit production (Rzedowski and Guevara-Féfer, 1992).
Assessment of pollen viability among parental species and hybrids provides an easier and faster approximation to hybrid fitness relative to common-garden or breeding experiments in the field, particularly for long-lived species, such as trees (Bures et al., 2010). Pollen viability test, such as staining techniques and in vitro germination tests have widely been used to evaluate hybrid viability (Bures et al., 2010; Abdelgadir et al., 2012; García et al., 2015). Thus, the aims of this study were to evaluate whether the putative hybrid between B. cuneata and B. bipinnata is sterile, and to compare pollen viability rates among the parental species and their putative hybrid within the TDF of NE Michoacán. We expected the pollen of putative hybrids to be viable, but with lower viability and germination rates compared to those of the parental species.
Material and Methods
Study species
Bursera cuneata and B. bipinnata are dioecious and deciduous trees up to 8 m in height, with an aromatic resin, and smooth, grey bare bark (Rzedowski and Guevara-Féfer, 1992). Bursera bipinnata is distributed across the TDF of the Pacific coast from southern Chihuahua to eastern Chiapas with an altitudinal interval of 1650 to 2200 m, while B. cuneata has a smaller distribution from northeastern Michoacán and southern Guanajuato to northeastern Guerrero and south of Puebla with an altitudinal interval of 1850 to 2500 m. These species co-occur in Michoacán and Guanajuato (Rzedowski and Guevara-Féfer, 1992).
For both species, the presence of flowers occurs between May and June, while for the leaves this is between June and November; fruit production occurs from June to November and they can remain attached to the tree for long periods of time (Rzedowski and Guevara-Féfer, 1992). There are no specific studies on pollination and seed dispersal in the study region, but as in other Bursera species, they are expected to be insect-pollinated (Rzedowski and Kruse, 1979) and bird-dispersed (Ramos-Ordoñez and Arizmendi, 2011). The leaves can easily differentiate both species and the putative hybrid: B. bipinnata has bipinnate leaves, B. cuneata has larger, once-pinnate leaves, with oblong to lanceolate leaves of rugose appearance, while their putative hybrid presents intermediate morphological characteristics with partial bipinnate leaves (Figs. 1A-C).
Study site and sample collection
Field explorations during the autumn of 2017 reported a putative hybrid population with the presence of adult trees of B. bipinnata and B. cuneata and their hybrid in the TDF of the municipality Tarímbaro in Michoacán, between La Cañada del Herrero and Cañada de Los Sauces (19°43.962'N, 101°12.043'W) (Fig. 2). Rough estimates of species frequency within two TDF patches suggest that B. bipinnata, B. cuneata and their putative hybrid occur with a proportion of 6:2:1 individuals, respectively. In June 2018, when trees present both flowers and leaves that allow us to distinguish between species and the intermediate individuals, we conducted two field surveys during the mornings (9:00-11:00) to randomly collect inflorescences from male trees of each species and their putative hybrid (B. bipinnata and B. cuneata n=10 and putative hybrid n=8 trees). Inflorescences were stored in paper bags and processed in the laboratory within the same day or the following day.
Staining viability test
We used a staining method to distinguish between living and dead pollen. We prepared a solution of 0.2 g of 2, 3, 5-triphenyltetrazolium chloride (TTC) and 12 g of sucrose in 20 ml of distilled water (Norton, 1966). The TTC 1% indicates the presence of active dehydrogenase enzymes that catalyze mitochondrial respiration. We selected six fresh flowers for each individual tree collected. Pollen grains were spread on a glass slide with one drop of TTC solution, covered with a coverslip, and set aside in the dark at room temperature for 24 hr. The numbers of viable and unviable pollen grains were counted in four random optical 20× fields per slide (Primo Star LED Microscope, Carl Zeiss, Göttingen, Germany). A pollen grain was considered viable if it turned pink or red (Fig. 3A). We measured and plotted the viability of pollen for each individual as percentage, which was determined by dividing the total number of red stained pollen grains by the total number of grains observed per field.
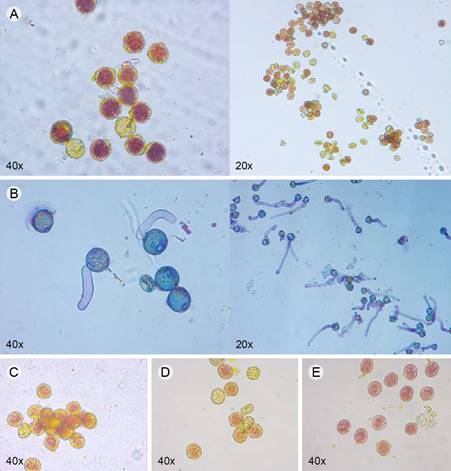
Figure 3: A. pollen of Bursera Jacq. ex L. stained with TTC 1% at 20× and 40× optical fields, red or pink colored grain indicates a viable pollen; B. pollen tube germination colored with Toluidine blue of Bursera for the in vitro germination test at 20× and 40× optical fields, successful germination was counted when the length of the pollen tube was equal to or longer than the diameter of the grain; comparison among pollen grains for C. B. cuneata (Schltdl.) Engl.; D. the putative hybrid; E. B. bipinnata (Moc. & Sessé ex DC.) Engl. at 40× optical fields.
In vitro germination test
Previous tests evaluated different sucrose concentrations (5, 10, 15, and 30%) on 1% agarose medium with 0.01% boric acid (H3B03) to find the most optimal pollen tube germination conditions. We found that the agarose-sucrose medium of 15% yields the highest success of pollen germination. Pollen grains from four fresh flowers per tree were evenly distributed on a glass slide with the 15% agarose-medium (0.2 cm tick) and kept in the dark at room temperature for 24 hr. To better visualize the formation of pollen tubes, a drop of Toluidine blue with 30% of glycerin solution was placed on the glass slide with a coverslip. Pollen germination was counted in four random optical 40× fields per slide (Primo Star LED Microscope, Carl Zeiss, Göttingen, Germany). A pollen grain was considered germinated when the length of the pollen tube was equal to or longer than the diameter of the grain (Fig. 3B). We estimated and plotted germination percentages for each group, which was determined by dividing the total number of germinated pollen tubes by the total number of grains observed per field.
Statistical analyses
Because data did not conform to normality assumptions even after transformation, we conducted General Linear Models (GLM) with a quasibinomial distribution to test for significant differences in the proportion of viable pollen grains among the three groups, using the glm function in R (R Core Team, 2018). The response variables were the number of viable/germination pollen grains and the number of unstained/no germinated grains per observed field. Post hoc test for pairwise comparisons were carried out by Tukey contrast with the lsmeans function in R (Lenth, 2016; R Core Team, 2018).
Results
From our pollen count observations, we did not notice apparent differences in the size of pollen grains between the putative hybrids and the parent species (Figs. 3C-E). Percentages of pollen viability for the TTC method showed that B. bipinnata presented the highest pollen viability values (46.2%, n=190 observed fields, n=10,467 observed grains), followed by B. cuneata (38.5%, n=124 observed fields, n=7625 observed grains), and the putative hybrid (36.7%, n=186 observed fields, n=10,050 observed grains) (Fig. 4A). Differences in the proportion of pollen viability through TTC were statistically significant (df=2, deviance=1015, p=0.005). Tukey post hoc comparisons revealed that B. bipinnata showed significantly higher pollen viability than the putative hybrid (p= 0.0001) and B. cuneata (p= 0.001).
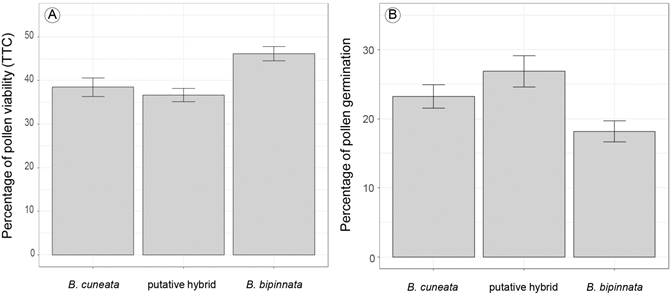
Figure 4: A. total percentage of pollen viability with TTC 1%; B. in vitro germination tests, for Bursera cuneata (Schltdl.) Engl., B. bipinnata (Moc. & Sessé ex DC.) Engl., and the putative hybrid. Bars denote ± standard errors.
For the germination test, the putative hybrid showed the highest percentage of pollen tube formation (26.9%, n=88 observed fields, n=2022 observed grains), followed by B. cuneata (23.3%, n=86 observed fields, n=2174 observed grains), and then B. bipinnata (18.2%, n=98 observed fields, n=2465 observed grains) (Fig. 4B). Differences in the proportion of pollen germination were statistically significant (df=2, deviance=35.3, p=0.0001). Post hoc comparisons revealed that the putative hybrid showed significantly higher rates of pollen tube formation than B. bipinnata (p=0.03).
Discussion
Our study represents a first test of hybridity between B. cuneata and B. bipinnata, since no genetic data are available to confirm the hybrid status of individuals with intermediate morphological characteristics. Yet, it contributes with relevant information to understand the role and consequences of hybridization in Mexican Bursera species.
The staining and in vitro germination techniques yielded good results in assessing pollen viability of Bursera species examined in this study. Both tests demonstrated that the male gametophyte in adult trees of the putative hybrid between B. cuneata and B. bipinnata is not sterile. This result contrasts with the study of Cortes-Palomec (1998), who found that the hybrid species B. medranoana is sterile. Our findings thus confirm previous field observations that suspected that Bursera hybrids can be fertile (Rzedowski and Guevara-Féfer, 1992). While some hybrids can break postzygotic barriers, it is usually expected that they present lower fertility relative to the parental species (Marques et al., 2011). We found for the staining test that the putative hybrid showed the lowest rates of pollen viability relative to both Bursera species, but the opposite was found for the in vitro germination test. Specifically, significant differences occurred between B. bipinnata and the putative hybrid, and not with respect to B. cuneata. Remarkably, the putative hybrid is morphologically more similar to B. cuneata (Fig. 1), which may imply a different degree of parental genetic background in putative hybrids.
The TTC staining technique only differentiates between living and dead pollen and is an easier technique than the in vitro germination test, as successful germination depends on finding the right medium conditions (Ilgin et al., 2007). Contrasting results between both type of techniques, as found in this study, have previously been reported in several species, and in general, it is expected that staining methods overestimate pollen viability (Nybom, 1985; Scorza and Sherman, 1995; Leila-Soares et al., 2013; Sulusoglu and Cavusoglu, 2014). Instead, in vitro germination test can yield results that better approximate the actual pollen viability, as the sucrose agar medium emulates the stigma exudate where pollen is deposited (La Porta and Roselli, 1991).
Consequences of hybridization highly depend on hybrid fitness (Todesco et al., 2016). It is usually expected that spontaneous hybrids present low fertility, but polyploid hybrid plants may have increased fertility and local adaptation respective to their diploid parental species (Alix et al., 2017). Allopolyploids are typically expected since it occurs via hybridization from the combination of divergent species of diploid genomes (Osabe et al., 2012), although autopolyploid hybrids may also occur (Barker et al., 2016). The observed higher pollen germination rate for the putative hybrid relative to at least one parent (B. bipinnata) did not follow our initial expectations. This result might be explained by the occurrence of hybrids with different genetic backgrounds, such as the formation of polyploids. Some authors argue that polyploid hybrids are characterized by a larger pollen size relative to their progenitors (Hossain et al., 1990; Wrońska-Pilarek et al., 2013, 2016), while others have found no such relationship (Franssen et al., 2001; Karlsdóttir et al., 2008; Lazarević et al., 2013). For example, Wrońska-Pilarek et al. (2013) conducted a pollen morphological comparison among three Crataegus Tourn. ex L. species, and they found that natural hybrids had larger pollen grains than the parental species, except for one species whose pollen size was similar to that of hybrids. In contrast, Franssen et al. (2001) for ten Amaranthus L. species and their interspecific hybrids, observed no differences in pollen size between hybrids and parental species, but marked differences in the number of pollen apertures. We did not observe differences in pollen size between the two Bursera species and the putative hybrids (Figs. 3C-E); however, we cannot rule out such morphological differences as we did not systematically evaluate the morphology of pollen grains. Another possibility is that the putative hybrids are not true hybrids, but only genetic data can confirm their identity.
Moreover, putative hybrids in our study site can develop vigorous adult trees with abundant number of fruits. Marques et al. (2011) found for Narcissus cavanillesii (Cav.) Barra & G. López, that F1 hybrids show high vigor at early stages of development, such as high level of growth and bulb propagation, which was explained to compensate for the low fertility of mature hybrids. In our case, it is possible that a low proportion of living pollen in the putative hybrid is compensated by a higher amount of pollen grains able to germinate. The ability of a pollen grain to grow a pollen tube is a necessary condition for fertilization. During our field collection, we observed that flowers of both Bursera species and the putative hybrid were equally visited by prospective pollinators, such as bees and wasps. This suggests that backcrosses between the parental species and the putative hybrid are likely to occur.
Pollen viability studies for TDF tree species are still scarce. Reproductive biology data present highly relevant information with consequences for species conservation and restoration. For instance, for Pachira quinata (Jacq.) W.S. Alverson fruit production was related to pollen load size, while half of the pollen grains were able to germinate and develop pollen tubes on the flower stigma (Quesada et al., 2001). Another study in Protium spruceanum (Benth.) Engl., a tree species of the Burseraceae family, showed the occurrence of high percentage (90%) of viable pollen (De Almeida-Viera et al., 2010). We found pollen viability rates below 50% for both Bursera species and the putative hybrid, which may have consequences for seed production and viability. Germination studies in Bursera species highlight low germination rates, likely due to high occurrence of empty seeds (Bonfil-Sanders et al., 2008; Hernández-Téllez, 2015). As pollen viability is only one component of fitness, future studies should contrast seed production and germination rates of parent species and their putative hybrids.
Given the frequent anecdotical observation of hybridization among Mexican Bursera species, more studies are needed to better understand the mechanisms and consequences of natural hybridization within this group. Specifically, genetic studies are required to confirm the genetic identity of putative hybrids, the maternal and paternal contribution of hybrid origin, the occurrence of diploid or polyploid hybrids, and whether several generations of hybrids coexist and if genetic introgression has occurred.











 nueva página del texto (beta)
nueva página del texto (beta)