Introduction
Soybean (Glycine max (L.) Merr.) is economically the most important legume globally. Besides providing raw materials for the chemical industry, the high lipid and protein content makes it one of the most valuable products for human and animal nutrition (Singh et al., 2008). In Mexico, total soybean production reached 480,000 MT (metric tons) in 2017, while imports were approximately 4,250,000 MT in the same year; national production is forecast to increase slightly in 2018/2019, while consumption and demand for soybean are also expected to increase due to population growth and feed demand from livestock sectors (USDA, 2018).
Genetic transformation of soybean has facilitated the development of new soybean cultivars with higher seed qualities, higher yields and with biotic and abiotic stress tolerances (Tripathi and Khare, 2016). Although soybean genetic transformation and regeneration is routine around the world, the efficiency is often low and the protocols are difficult to reproduce partly because the regeneration capacity and transformation efficiency of soybean are genotype dependent (Zia et al., 2010; Arun et al., 2014). This dependence justifies the screening of soybean varieties that are more suitable for regeneration and Agrobacterium-mediated transformation (Song et al., 2013). It was determined that in China, the most suitable soybean genotype for in vitro regeneration is Hefeng-25 (Ma and Wu, 2008), and in India it is the PK416 cultivar (Arun et al., 2014). However, the seed phenotypic characteristics of the genotypes in question are usually not reported, perhaps because the most common phenotyping techniques are costly, time consuming and destructive to sample (Chen et al., 2014). Consequently, there is a lack of knowledge in terms of desirable phenotypic traits that could contribute to the regeneration and transformation potential. Furthermore, there is a lack of knowledge about Mexican soybean varieties that may be useful for genetic improvement purposes.
Phenotypic characterization of crop seeds is an important tool for plant breeders to identify and improve lineages with better quality (Gupta et al., 2010; Sharma et al., 2016). One tool that has been used, for plant-breeding purposes, to predict crop yield is Near Infrared Reflectance spectroscopy (NIR), which allows a fast, reliable and non-destructive measurement of seed composition (Araus et al., 2001).
We hypothesize that, by using rapid physiology-based screening methods, it is feasible to find a correlation between seed traits and the in vitro performance of soybean. Therefore, the objective of this study is to phenotypically characterize eight Mexican soybean genotypes, and the frequently used soybean line, Jack, as a control, to determine which seed traits are correlated the most with regeneration capability (cotyledonary node approach) and susceptibility to Agrobacterium tumefaciens (Smith & Townsend) Conn infection.
Materials and Methods
Eight Mexican soybean genotypes, described in Table 1, were selected for study based on their commercial availability (Huasteca-100, Huasteca-200, Huasteca-300, Huasteca-400, Huasteca-600, Tamesí, Nainari and Suaqui-86) and were obtained from the INIFAP (Instituto Nacional de Investigaciones Forestales, Agrícolas y Pecuarias, Mexico), while the Jack genotype was kindly provided by the USDA-ARS (United States Department of Agriculture - Agricultural Research Service, USA). The Mexican seeds were harvested in 2013, whereas the Jack cultivar was harvested in 2009. All seeds were stored at 4 ºC until analysis.
Table 1: Description of soybean varieties used in the present study. * (SNICS, 2018) for Mexican genotypes.
| Genotype | Year of release* | Area of adaptability | Origin | Average yield kg/ha | Height (cm) | Days to maturity | Reference |
| Huasteca-100 | 1995 | Tropical lowland regions (southern Tamaulipas, eastern San Luis Potosí and northern Veracruz) | Cross between Santa Rosa × Jupiter | 2387 | 84 | 118 -144 | (Maldonado Moreno and Ascencio Luciano, 2010a) |
| Huasteca-200 | 1995 | Tropical lowlands regions with humid and subhumid climate | Cross between F81-5344 × Santa Rosa | 2160 | 109 | 111 -118 | (Maldonado Moreno and Ascencio Luciano, 2010b) |
| Huasteca-300 | 2004 | Tropical lowlands regions with humid and subhumid climate | Cross between H82-1930 × H80-2535 | 2657 | 78 | 116 | (Maldonado Moreno et al., 2009) |
| Huasteca-400 | 2004 | Warm humid and subhumid climate | Individual selection of Dois Marcos 301 introduced from Brazil | 3319 | 80 | 111 | (Maldonado Moreno et al., 2010) |
| Huasteca-600 | 2014 | Warm humid and subhumid climate | Hybridization between H88-1880 × H88-3668 | 2988 | 79 | 119 | (Maldonado Moreno et al., 2017) |
| Nainari | 1997 | Northwestern Mexico | Suaqui-86 (seed irradiation) | 2835 | 70 | 120-125 | (Cruz Torres, 2008) |
| Suaqui-86 | 1987 | Northwestern Mexico | RadxCajeme × Tetabiate × Cajeme | 3456 | 90 | 120 | (Cortez et al., 2005) |
| Tamesí | 2011 | Warm humid and subhumid climate (southern Tamaulipas, eastern San Luis Potosí and northern Veracruz) | Cross between Santa Rosa × H80-2535 | 2602 | 66 | 117 | (Maldonado Moreno and Ascencio Luciano, 2012) |
| Jack | 1989 | Athens, GA; Lexington, KY; and Wooster, OH | Fayette × Hardin | 3250 | 100-119 | 112 | (Kaudzu, 2017) |
Seed characterization
Soybean seeds were subjected to near infrared spectroscopy (NIR DA7250, Perten Instruments Inc., Springfield, USA) for proximate seed analysis, which includes moisture, oil, protein, ash, and carbohydrate content using a standard sample dish of 108 cm2. Data were calculated on a dry weight basis in triplicate. Grain dimensions (length and width) were measured using a scanner (Epson perfection V700, Nagano, Japan) and the WinSEEDLE image analyzer (Regent Instruments, Inc., Quebec, Canada). Seed weight was calculated as the mean weight of batches of 100 randomly chosen seeds. The regeneration capability was based on the mean number of shoots per explant obtained on Shoot Induction Medium (SIM, see composition below), while susceptibility to Agrobacterium Conn was measured as the mean percentage of area that stained blue per explant in a transient transformation approach using the Gus gene as a reporter of gene expression.
Regeneration and transient transformation
All culture reagents were obtained from PhytoTechnology Laboratories (Shawnee Mission, KS, USA). The basal medium was Murashige and Skoog salts with B5 vitamins, supplemented with 30 g/l Sucrose, and 8 g/l Plant Agar, pH=5.8 (MSB5). The culture room was under a photoperiod of 16 h, a light intensity of 60 µmol/m2 s1 and a temperature of 27-28 ºC.
Sterilized seeds (Olhoft et al., 2006) were soaked in sterile distilled water overnight (13 h) at 27 ºC and germinated during five days on basal medium supplemented with 0.50 mg/l 6-Benzylaminopurine (BAP). The explants (cotyledonary nodes and a portion of hypocotyl) were prepared from the seedlings as described by Ma and Wu (2008). Explants from 5-day-old seedlings were transferred onto a SIM (basal medium and hormones: 3.0 mg/l BAP, 0.2 mg/l Indole-3-butyric acid (IBA), 0.5 mg/l Kinetin (KT)) and incubated for 15 days. After the shoot induction period, the shoots were counted.
The Gus reporter gene, coding for β-glucuronidase , was obtained from the expression vector pBI121 by PCR amplification (primers 5’-CACCATGTTACGTCCTGTAGAAAC-3’ forward and 5’-GATTCATTGTTTGCCTCCCTG-3’ reverse) and subcloned into the pENTR/D-TOPO vector (Invitrogen K240020). The resultant pENTR-GusS vector was recombined into the pB2GW7.0 (VIB-Ghent University, Gent, Belgium) by Gateway cloning (Karimi et al., 2002). The plasmid pB2GW7.0-Gus was transformed into Agrobacterium tumefaciens strain LBA4404. One colony was picked to inoculate 5 ml of liquid LB medium with antibiotics (100 mg/l Spectinomycin and 20 mg/l Rifampicin) and grown overnight (200 rpm, 28 ºC). One day prior to plant transformation, the Agrobacterium overnight culture (one colony in liquid LB with 100 mg/l Spectinomycin and 20 mg/l Rifampicin) was diluted 1/100 in liquid LB medium under the same conditions. When its optical density (OD600) reached 0.8, the culture was centrifuged (5000 G, 5 min) and the pellet re-suspended in liquid co-cultivation medium (SIM without agar, 200 μmol/l acetosyringone, pH 5.5). The explants were prepared from 5-day-old seedlings in the same way as the regeneration protocol described in the preceding section. Explants were inoculated with Agrobacterium for 2 h at room temperature and then transferred to solid co-cultivation medium (SIM supplemented with 1000 mg/l cysteine (Olhoft and Somers, 2001)). After three days of co-cultivation in darkness, the cotyledonary node area was cut and collected for Gus staining. The explants were washed and incubated in Gus histochemical staining solution (GUSS, Sigma-Aldrich) for one day at 37 ºC, after which the explants were maintained in 70% ethanol.
To provide a less subjective measurement of the Gus staining score, the percentage of area that stained blue in each explant was estimated by using a scanner (Epson perfection V700, Nagano, Japan) and the WinSEEDLE image analyzer (Regent Instruments, Inc., Quebec, Canada). Color classifications were created to quantify the blue color area as well as the explant area (mm2) in frontal sections of cotyledonary nodes.
Experimental design and statistics
For regeneration capability and Agrobacterium susceptibility analyses, a completely randomized design was used with nine genotypes and 30 replicates of the entire experiment. The mean number of shoots per explant and the blue staining percentage was recorded. After proving normality assumptions, data was statistically analyzed using ANOVA and the significance of differences among genotypes was contrasted with a Tukey’s test at p<0.05. Analysis of variance and Tukey’s test were also conducted for all the seed phenotypical traits (moisture, protein, oil, ash, total carbohydrate content, as well as length, width, average seed weight) vs. soybean genotypes to determine whether there were significant differences among genotypes. The correlation coefficients (Pearson) between regeneration capability (mean number of shoots per explant), susceptibility to Agrobacterium infection and different seed traits were determined. All statistical analyses were performed using Minitab 17 (Minitab, State College, PA, USA).
Analysis of major and minor nutrients
Weighed soybean flours (0.3 g), and a blank, were digested in a Mars 5 Xtraction CEM microwave oven (Matthews, NC, USA), using 10 ml of concentrated HNO3 with a temperature-time ramp of 18 min from room temperature to 180 ºC, followed by a 10 min hold at this temperature and a maximum power of 800 W. Resulting digests were filtered through Whatman Nº 42 filter paper and diluted to 25 ml in a volumetric flask. Samples were diluted further, 10 and 100 times in 2% HNO3, for minor and major nutrients analysis, respectively. The concentration of elements (Mg, K, Ca, P, Na, Mn, Fe, Cu and Zn) was determined by ICP-MS (Xseries 2 inductively coupled plasma mass spectrometer, Thermo Scientific, Waltham, USA) in the Latin American and Caribbean Water Center facilities. Each sample was measured in triplicate and blank corrected.
Results
Seed Characterization
Table 2 provides protein, oil, ash and total carbohydrate contents (dry basis), as well as length, width and average seed weight, for all Mexican genotypes and the Jack genotype.
Table 2: Phenotypic seed traits of Jack and Mexican soybean seeds. Protein, oil, ash and carbohydrate content are expressed on a dry weight basis. Values represent the mean ± SE of n replicates. a Significance level from ANOVA test (variety vs. trait). H1: Huasteca-100, H2: Huasteca-200, H3: Huasteca-300, H4: Huasteca-400, H6: Huasteca-600, Na: Nainari, Su: Suaqui-86, Ta: Tamesí and Jack. Means that are not connected by the same letters are significantly different according to the Tukey’s test (p<0.05)
| Variety | Protein % | Oil % | Ash % | Total Carbohydrate % | Length mm | Width mm | Average 100-seed weight g |
| Jack | 42.6±0.8d | 23.2±0.1f | 6.09±0.06a | 28.1±0.8b | 6.77±0.06e | 6.07±0.04e | 14.4±0.3de |
| H1 | 45.8±0.1b | 24.2±0.1d | 5.98±0.03cd | 24.0±0.1c | 7.12±0.06cd | 6.34±0.05cd | 14.9±0.2cd |
| H2 | 47.6±0.05a | 24.6±0.02bc | 6.05±0.01abc | 21.76±0.01d | 7.61±0.06b | 6.68±0.05b | 16.3±0.3bc |
| H3 | 45.8±0.04b | 25.6±0.04a | 6.08±0.03ab | 22.5±0.1d | 8.00±0.06a | 7.20±0.05a | 18.3±0.5a |
| H4 | 45.6±0.1b | 24.3±0.16cd | 6.03±0.02abc | 24.1±0.1c | 6.88±0.06de | 6.05±0.04e | 14.3±0.2de |
| H6 | 44.9±0.2bc | 25.4±0.14a | 5.99±0.01bc | 23.6±0.1c | 7.22±0.07c | 6.42±0.05cd | 17.0±0.3ab |
| Na | 40.4±0.04e | 23.9±0.13e | 5.89±0.02de | 29.7±0.1a | 6.84±0.07de | 6.22±0.06de | 13.3±0.1ef |
| Su | 42.5±0.2d | 22.3±0.02g | 5.81±0.01e | 29.3±0.2a | 6.82±0.07e | 6.08±0.07e | 12.8±0.3f |
| Ta | 44.6±0.04c | 24.9±0.03b | 6.04±0.03abc | 24.40±0.02c | 7.21± 0.07c | 6.51±0.06bc | 16.7±0.3b |
| n | 3 | 3 | 3 | 3 | 56 | 56 | 3 |
| p valuea | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
A significant variation was found for all of the traits among the tested genotypes (p<0.001). The protein content ranged from 40.4-47.6%, the lowest and highest protein contents were registered for Nainari and Huasteca-200, respectively; the oil content ranged from 22.3% (Suaqui-86) to 25.6% (Huasteca-300). The ash content varied from 5.81-6.09%, the minimum ash content were determined for genotypes Suaqui-86 (5.81%) and Nainari (5.89%), while the maximum was shown for Jack (6.09%). The total carbohydrate content ranged from 21.76-29.7%, with lower values of carbohydrate content being registered by Huasteca-200 and Huasteca-300, while higher values were found for Nainari and Suaqui-86. The seed length varied from 6.77-8.00 mm, while the seed width varied from 6.05-7.20 mm, the minimum seed length was registered by the genotype Jack and the maximum by Huasteca-300; the minimum seed width was recorded for Huasteca-400 and the maximum for Huasteca-300. Finally, the 100-seed weight ranged from 12.8-18.3 g, with lower values registered for Suaqui-86 and Nainari and higher values for Huasteca-300 and Huasteca-600.
Regeneration and transient transformation
All evaluated Mexican genotypes produced shoots. According to ANOVA analyses, shoot induction was affected by soybean genotype (p<0.05). Figure 1A shows that higher multiple shoot formation was obtained using varieties Huasteca-100, Nainari and Suaqui-86. Figure 1B illustrates that elongated shoots were able to regenerate directly from the cotyledonary node without an intervening callus phase.
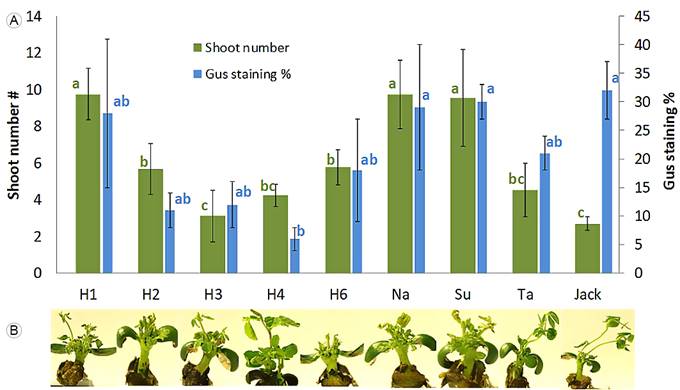
Figure 1: Regeneration capability and Agrobacterium tumefaciens (Smith & Townsend) Conn infection susceptibility in nine soybean genotypes. A. mean number of shoots per explant and blue staining percentage (n=30), error bar with 95% confidence intervals; B. explants with multiple shoots after 15 days in SIM. From left to right: H1: Huasteca-100, H2: Huasteca-200, H3: Huasteca-300, H4: Huasteca-400, H6: Huasteca-600, Na: Nainari, Su: Suaqui-86, Ta: Tamesí and Jack. Means that are not connected by the same letters are significantly different according to the Tukey’s test (p<0.05).
The percentage area of blue stained tissue (Gus assay), as an indication of infection susceptibility (Figs. 1A, 2), also demonstrated significant variation, ranging from 6% in Huasteca-400 to 32% in Jack.
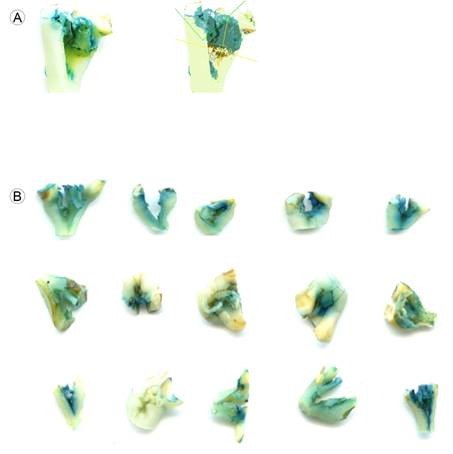
Figure 2: Gus staining assay: A. color classification for the estimation of the blue staining area using image analysis (Winseedle software); B. image of 15 explants (Nainari) showing on average 29% of blue staining.
According to the ANOVA test, the genotype significantly affected infection susceptibility (p<0.001). Mexican varieties such as Huasteca-100, Nainari and Suaqui-86 presented a high staining percentage, comparable to Jack, indicating efficient Agrobacterium early stage infection. With varying degrees, all of the nine tested varieties were susceptible to Agrobacterium-mediated transformation.
Correlations
According to Table 3, the ash content was the only significant factor (p<0.01) that correlated negatively with the number of shoots per explant; in other words, the higher the ash content the less the regeneration capability.
Table 3: Pearson correlation coefficients between regeneration capability, Agrobacterium tumefaciens (Smith & Townsend) Conn susceptibility and phenotypic traits of Jack and Mexican soybean. *,** Significant at p<0.05 and p<0.01 respectively, n=9.
| Length mm | Width mm | Average 100-seed weight g | Protein % | Oil % | Ash % | Total Carbohydrate % | Shoots/ explant # | |
| Width mm | 0.980** | |||||||
| Average 100-seed weight (g) | 0.870** | 0.863** | ||||||
| Protein % | 0.672 | 0.529 | 0.651 | |||||
| Oil % | 0.747* | 0.744* | 0.894** | 0.585 | ||||
| Ash % | 0.471 | 0.434 | 0.675 | 0.562 | 0.573 | |||
| Total Carbohydrate % | -0.769* | -0.662 | -0.812** | -0.955** | -0.798* | -0.644 | ||
| Regeneration shoots/explant # | -0.340 | -0.305 | -0.593 | -0.394 | -0.511 | -0.896** | 0.496 | |
| Agrobacterium tumefaciens (Smith & Townsend) Conn infection Blue % | -0.576 | -0.467 | -0.557 | -0.730* | -0.648 | -0.494 | 0.778* | 0.587 |
The susceptibility of soybean to A. tumefaciens infection correlated directly with carbohydrate content and negatively with protein content.
Aside from the correlations between phenotypic traits and in vitro performance, a direct correlation was observed between oil content and seed weight, a similar correlation has been reported previously (Anwar Malik et al., 2006). We also found a negative correlation between carbohydrate and protein, as well as between carbohydrate and oil content. Other studies have also demonstrated that protein content increases at the expense of total carbohydrates (Wilcox and Shibles, 2001), but contradictory results have been reported by other researchers, who found a positive correlation between total carbohydrates and oil (Li et al., 2012).
Analysis of major and minor nutrients
We performed an analysis of major and minor nutrients (Table 4) that could possibly correlate with ash content and regeneration capability (number of shoots per explant).
Table 4: Major (Mg, K, Ca and P) and minor (Na, Mn, Fe, Cu and Zn) elements in soybean seeds determined by ICP-MS. Values represent the mean ± SE of three replicates. aSignificance level from ANOVA test (variety vs. element). Means that are not connected by the same letters are significantly different according to the Tukey’s test (p<0.05).
| Soybean Variety | 24Mg mg/g | 39K mg/g | 44Ca mg/g | 31P mg/g | 23Na mg/kg | 55Mn mg/kg | 56Fe mg/kg | 65Cu mg/kg | 66Zn mg/kg |
| Jack | 6.19±0.09a | 44.3±0.7ab | 3.81±0.06a | 5.28±0.18b | 432.1±10.1a | 65.7±0.3d | 167±2b | 23.5±0.1d | 97.3±1.0c |
| H1 | 4.27±0.12d | 36.8±0.9d | 2.27±0.09e | 3.97±0.16c | 46.1±4.1f | 48.4±1.0e | 88±2e | 22.7±0.4d | 86.3±2.1de |
| H2 | 4.93±0.05c | 41.3±0.2c | 2.23±0.02e | 3.21±0.10d | 45.7±1.4f | 47.8±0.4e | 107±5de | 26.5±0.7c | 82.3±1.5e |
| H3 | 5.09±0.02c | 41.1±0.2c | 2.82±0.03c | 3.43±0.02cd | 68.2±1.9de | 69.3±1.0c | 98±4e | 19.5±0.4e | 82.8±1.5de |
| H4 | 4.62±0.07d | 42.4±0.2bc | 2.26±0.04e | 3.66±0.07cd | 113.2±4.5b | 74.4±1.0b | 141±4bc | 23.1±0.4d | 87.2±2.0de |
| H6 | 5.08±0.02c | 43.9±0.2ab | 2.85±0.01c | 3.71±0.03cd | 96.8±1.0b | 65.9±0.3cd | 127±2cd | 22.2±0.2de | 90.6±0.3cd |
| Na | 5.57±0.03b | 44.6±0.2ab | 3.09±0.02b | 5.93±0.15a | 58.8±0.5ef | 78.0±0.4a | 211±13a | 29.9±1.3b | 116.7±1.1b |
| Su | 5.83±0.10b | 45.8±0.5a | 2.58±0.04d | 6.03±0.25ab | 104.9±4.0b | 75.2±0.7ab | 225±5a | 43.1±0.4a | 136.2±2.7a |
| Ta | 4.95±0.06c | 41.1±0.4c | 2.73±0.03cd | 3.30±0.06d | 80.9±3.7c | 67.1± 0.9cd | 108±4de | 22.4±0.3d | 82.6±1.3de |
| p valuea | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
In the same manner, the elements were tested for correlation with susceptibility to A. tumefaciens infection. Table 4 shows that mineral content variation was significant among soybean genotypes. From lowest to highest, the following was determined: Mg content ranged from 4.27 mg/g in Huasteca-100 to 6.19 mg/g in Jack; K from 36.8 mg/g in Huasteca-100 to 45.8 mg/g in Suaqui-86; Ca from 2.23 mg/g in Huasteca-200 to 3.81 mg/g in Jack; and P from 3.21 mg/g in Huasteca-200 to 6.03 mg/g in Suaqui-86. In a similar manner, with respect to concentration of micronutrients, the following was determined: Na ranged from 46.1 mg/kg in Huasteca-100 to 432.1 mg/kg in Jack; Mn ranged from 48.4 mg/kg in Huasteca-100 to 78 mg/kg in Nainari; Fe from 88 mg/kg in Huasteca-100 to 225 mg/kg in Suaqui-86; Cu from 19.5 mg/kg in Huasteca-300 to 43.1 mg/kg in Suaqui-86; and Zn ranged from 82.3 mg/kg in Huasteca-200 to 136.2 mg/kg in Suaqui-86.
Table 5 illustrates that the ash content (determined by NIR) had a significant correlation with Na and Ca; both minerals negatively affected regeneration under the present protocol conditions.
Table 5: Pearson correlation coefficients between ash content (NIR), regeneration capability, Agrobacterium tumefaciens (Smith & Townsend) Conn susceptibility and elements measured by ICP-MS. *,** Significant at p<0.05 and p<0.01 respectively. N=9.
| Mg | K | Ca | P | Na | Mn | Fe | Cu | Zn | |
| Ash content % | 0.544 | 0.220 | 0.770* | -0.010 | 0.871** | 0.029 | -0.052 | -0.354 | -0.238 |
| Regeneration Shoots/ explant # | -0.037 | -0.048 | -0.272 | 0.534 | -0.435 | -0.075 | 0.399 | 0.597 | 0.590 |
| Agrobacterium tumefaciens (Smith & Townsend) Conn infection Blue % | 0.596 | 0.241 | 0.558 | 0.760* | 0.389 | 0.111 | 0.535 | 0.442 | 0.616 |
However, this interaction was not significant. Additionally, we did not find any significant correlation between specific minerals and regeneration capability. Regarding susceptibility to Agrobacterium infection, P was the only element that correlated significantly with the percentage area of blue staining.
Discussion
Under these protocol conditions, Mexican varieties such as Huasteca-100, Nainari and Suaqui-86 had a better regeneration capacity than Jack in terms of the number of shoots produced from one explant. In previous studies, Jack has been found to induce highly embryogenic responses (Lee et al., 2013), which makes it optimal for somatic embryogenesis regeneration approaches. However, our results have shown that the Jack genotype was not highly proliferative in a direct regeneration approach using the cotyledonary node as explant, which is in agreement with recently reported results (Raza et al., 2017).
Three Mexican varieties were the most regenerative. With regard to Huasteca-100, it is a genotype developed from the hybridization of a Brazilian genotype (Santa Rosa) and an American genotype (Jupiter); it is adapted to tropical Mexican areas (Maldonado Moreno and Ascencio Luciano, 2010a). Nainari (also known as Hector) was developed by seed irradiation of Suaqui-86 (Cruz Torres, 2008), and hence both are closely related and both are adapted to northwestern Mexico. The eight Mexican genotypes analyzed in this study were adapted for different agroclimatic zones and, as such, it was expected to observe phenotypic differences among them (Vasconcelos et al., 2006). We showed that different genotypes exhibited significant differences in composition within already reported ranges (Bellaloui et al., 2011), as well as significant differences in size and dimensions. Among all observed genotypic differences between seed traits, regeneration capability and susceptibility to infection, ash content had a significant negative correlation with regeneration capability, whereas carbohydrate content presented the most significant correlation with susceptibility to Agrobacterium infection. These results suggest that ash content may be an indicator of soybean regeneration capability, with carbohydrate content being indicative of infection susceptibility using the current protocol.
Specific phenotypic seed traits have been reported and associated with field performance (Walter et al., 2015). A negative correlation between ash content and grain yield exists in several cereals under specific environmental conditions (Misra et al., 2006). Although the underlying mechanism remains unclear, the accumulation of minerals in mature seeds has been considered a complimentary criterion to evaluate water use efficiency and to predict yield in C3 and C4 plants (Cabrera-Bosquet et al., 2011). In a similar way, seed ash content could be an indicator of water-use efficiency in a regeneration process; however, there is scarce information in the literature on this relationship in in vitro settings.
Considering that ash content was an important factor that could affect regeneration, we performed an analysis of major and minor nutrients. Our findings show that two specific elements: Ca (a major element) and Na (a trace element) correlated the most with ash content values (NIR). However, we did not find any significant correlation between regeneration and any specific element, suggesting that regeneration was perhaps affected either by the combination of two or more elements present in the ash or by additional elements that were not quantified in this study. Although the correlation between regeneration capability and these two elements (Ca and Na) was not significant, it was an inverse relationship, meaning that higher amounts of calcium and sodium accumulated in the seeds could have a detrimental effect on soybean in vitro performance. In contrast, the macroelement P and the microelements Fe, Zn and Cu showed a positive (but not significant) correlation with regeneration capacity. Phosphorus (P) plays essential roles in metabolic pathways and is a key component of molecules such as ATP, nucleic acids and phospholipids (Schachtman et al., 1998); Fe is essential mainly in the metabolism of chlorophylls and its deficiency induces chlorosis, while Zn is also essential because it is a co-factor in a large number of enzymes and its deficiency inhibits cell growth (Ghasemian et al., 2010). Cu is also important for plant growth and development since it can act as co-factor in many enzymes and has a role in transcription-signaling pathways and protein trafficking (Yruela, 2005).
The susceptibility to Agrobacterium infection correlated with phosphorus, carbohydrate content and, to a lesser degree, inversely with protein content. Phosphorus is an essential nutrient which plays a well-documented role in synthetic, developmental and signaling pathways important to plant function (Raboy, 2009), but such effects in the Agrobacterium transient transformation of soybean have not been reported. Carbohydrates may correlate with susceptibility to Agrobacterium infection due to a higher concentration of monosaccharides being released from plant wounds; monosaccharides, in combination with phenolic compounds, are required for transcriptional activation of the Agrobacterium transformation system (Tzfira and Citovsky, 2002). Genotypes with higher carbohydrate content have naturally less protein content, hence the negative correlation found between protein and Agrobacterium infection.
In most cases, seed phenotypic traits have been overlooked, at least in relation to regeneration and transformation potential. However, we present here evidence of associations between specific seed traits with soybean regeneration and transient transformation. More research in this area would lead to a better understanding of important factors that affect these two processes under determined conditions. The seed characterization can be easily performed with non-invasive and reliable methods such as those presented in this work (Ferreira et al., 2014).
In conclusion, three Mexican soybean varieties, Huasteca-100, Nainari and Suaqui-86, were the most suitable for in vitro regeneration through a cotyledonary node explant; all three varieties were also found to be highly susceptible to gene transfer by Agrobacterium in a transient transformation approach. After characterizing eight Mexican varieties, and the Jack genotype as a control, it was possible to determine an inverse correlation between ash content and regeneration capability and a direct correlation between carbohydrate and phosphorus content and susceptibility to Agrobacterium infection. According to the reviewed literature in that regard, this is the first reported study performed on these Mexican genotypes and the first to use NIR as a screening tool to correlate phenotypic traits with soybean in vitro performance. This study will be advantageous for future soybean genetic improvement and transformation research.











 text new page (beta)
text new page (beta)



