Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Acta botánica mexicana
versión On-line ISSN 2448-7589versión impresa ISSN 0187-7151
Act. Bot. Mex no.107 Pátzcuaro abr. 2014
Seasonal changes in epiphytic dinoflagellate assemblages near the northern coast of the Yucatan Peninsula, Gulf of Mexico
Cambios estacionales en conjuntos de dinoflagelados epifícos cerca de la costa norte de la Península de Yucatán, Golfo de México
Yuri B. Okolodkov1,4, Fany del Carmen Merino-Virgilio2, José Antolín Aké-Castillo1, Ana Concepción Aguilar-Trujillo2, Silvia Espinosa-Matías3 & Jorge Alfredo Herrera-Silveira2
1 Universidad Veracruzana, Instituto de Ciencias Marinas y Pesquerías, Laboratorio de Botánica Marina y Planctología, Calle Hidalgo 617, Colonia Río Jamapa, 94290 Boca del Río, Veracruz, México.
2 Instituto Politécnico Nacional, Centro de Investigación y Estudios Avanzados, Unidad Mérida, Carretera antigua a Progreso km 6, 97310 Mérida, Yucatán, México.
3 Universidad Nacional Autónoma de México, Facultad de Ciencias, Laboratorio de Microscopía Electrónica de Barrido, Avenida Universidad 3000, 04510 México, D.F., México.
4 Author for correspondence: yuriokolodkov@yahoo.com.
Recibido en noviembre de 2012.
Aceptado en noviembre de 2013.
Abstract
Epiphytic dinoflagellates were studied in 250 samples from 10 sites in Chelem (a semi-enclosed mangrove lagoon) and Dzilam de Bravo (an exposed coastal locality), on the northern coast of the Yucatan Peninsula, during five surveys in 2008-2009. Temperature, salinity, turbidity, pH, dissolved oxygen, nitrates, nitrites, phosphates, silicates, urea, extractable water column chlorophyll-a, precipitation, and wind speed and direction were measured. The Chelem lagoon system showed minor variability in physical-chemical characteristics compared to the exposed site at Dzilam de Bravo. Dinoflagellates were associated with all the host macrophytes examined including four seagrass species and 33 macroalgal species representing 24 genera. A total of 20 dinoflagellate taxa from 12 genera were recovered from these substrates. The genus Prorocentrum contained the largest number of individual species. The variation in mean epiphytic dinoflagellate abundance over both localities ranged from ~200 to 3500 cells g-1 substrate wet weight. Cell abundances at individual sites, in contrast, ranged from ~100 to >25 000 cells g-1 substrate wet weight. This variation is typical of the patchy distribution of these species in time and space. Overall, Prorocentrum rhathymum (up to 2.41×104 cells g-1) was the most abundant species observed across samples. Other abundant species were Bysmatrum caponii (maximum of 1.19×104 cells g-1) and Amphidinium cf. carterae (maximum of 3.69×103 cells g-1). The highest abundances of Gambierdiscus speciesoccurred in May and November (9.90x103 cells g-1) in Chelem when temperatures ranged from 24.5 to 30.2 oC. The data obtained indicate that the greatest potential for ciguatoxin flux into the food web may occur in protected, low turbulence environments, where salinities are high, nutrients abundant, and water temperatures are between 24 and 31 °C.
Key words: ciguatera, Dinophyceae, epiphytes, Gulf of Mexico, microalgae, microphytobenthos, seasonal changes, Yucatan.
Resumen
Se estudió a los dinoflagelados epífitos en 250 muestras de 10 sitios en Chelem (una laguna rodeada parcialmente por manglar) y Dzilam de Bravo (una localidad costera expuesta), en la costa norte de la Península de Yucatán, obtenidas durante cinco exploraciones en 2008-2009. Se midió la temperatura, salinidad, turbidez, pH, oxígeno disuelto, nitratos, nitritos, fosfatos, silicatos, urea, clorofila-a extraída de la columna de agua, precipitación, así como velocidad y dirección del viento. El sistema de la laguna de Chelem reveló menor variabilidad de las características físico-químicas comparado con la localidad expuesta de Dzilam de Bravo. Los dinoflagelados se encontraron asociados con todos los macrófitos hospederos incluyendo cuatro especies de pastos marinos y 33 de macroalgas representantes de 24 géneros. Un total de 20 taxa de dinoflagelados correspondientes a 12 géneros se obtuvieron de estos sustratos. El género Prorocentrum incluyó la mayor cantidad de especies. La variación en la abundancia promedio de dinoflagelados epifíticos en ambas localidades osciló entre ~200 y 3500 células g-1 de peso húmedo de sustrato. La abundancia de células en los sitios, en contraste, varió entre ~100 y >25 000 células g-1. Esta variación es típica de la distribución en parches de estas especies en el tiempo y el espacio. En general,Prorocentrum rhathymum (máximo 2.41×104 células g-1) fue la especie más abundante en las muestras. Otras especies abundantes fueron Bysmatrum caponii (máximo 1.19×104 células g-1) y Amphidinium cf. carterae (máximo 3.69×103 células g-1). La abundancia máxima de Gambierdiscus spp. se presentó en mayo y noviembre (9.90x103 células g-1) en Chelem cuando la temperatura varió de 24.5 a 30.2 °C. Los datos obtenidos muestran que el mayor potencial para el flujo de ciguatoxinas a través de la red trófica puede encontrarse en ambientes protegidos de turbulencia baja, donde la salinidad es alta, los nutrientes abundantes y la temperatura de agua entre 24 y 31 °C.
Palabras clave: cambios estacionales, ciguatera, Dinophyceae, epífitos, Golfo de México, microalgas, microfitobentos, Yucatán.
INTRODUCTION
Tropical epiphytic/epibenthic dinoflagellates are abundant in shallow marine habitats throughout the world. Some of these species produce potent toxins that can concentrate in the food web, adversely affecting ecosystem and human health. The most important of these are the ciguatera toxins produced by species in the genus Gambierdiscus. These toxins accumulate in finfish causing ciguatera fish poisoning, globally the most frequent cause of non-bacterial food poisoning. It is possible that toxins recovered from the flesh of fish match those produced by Gambierdiscus species. In the Caribbean, it is the top predator fish living in coral reef environments that have the greatest potential for becoming ciguateric. Other epiphytic/epibenthic dinoflagellate species can produce a range of toxins that cause gastrointestinal and respiratory distress. While seasonality and annual cycles of phytoplankton communities, including mainly diatoms and dinoflagellates, have been repeatedly investigated throughout the oceans, studies of the annual dynamics of epiphytic/epibenthic dinoflagellates are still scarce. The major exception is studies that have followed the seasonality of individual species that were thought to cause ciguatera (Bagnis et al., 1985; Carlton & Tindall, 1985; Gillespie et al., 1985; Mitchell, 1985; Ballantine et al., 1988; Bomber et al., 1988; Tindall & Morton, 1998; Chinain et al., 1999; Vila et al., 2001; Levasseur et al., 2003). Habitat preference, the influence of environmental factors and population dynamics of the epiphytic dinoflagellates are still poorly known (Richlen & Lobel, 2011). In the southern Gulf of Mexico, the only study on seasonality (covering eight months) of epiphytic dinoflagellates was conducted in the state of Veracruz by Okolodkov et al. (2007). The principal objectives of the present work were to follow the seasonal dynamics of epiphytic dinoflagellates in two representative habitats on the northern coast of the Yucatan Peninsula. The first habitat was the semi-enclosed Chelem lagoon and the other one was a coastal locality open to the ocean at Dzilam de Bravo. The dinoflagellate species assemblages with host seaweeds and seagrasses were specifically determined to examine the correlations between selected dinoflagellate species and the total abundances of dinoflagellate cells and physical and chemical characteristics at each site. Of particular interest was identifying the conditions that promoted the growth of Gambierdiscus species, thereby increasing the potential risk of ciguatera.
MATERIAL AND METHODS
The study sites are characterized by three distinct seasons: a dry season from March to early June, a rainy season from June to October, and the ''nortes'' (northerly winds) season, a period of brief storms and strong northerly winds, from November to February (Herrera-Silveira, 1993). The first study site was the semi-enclosed elongated Chelem lagoon that is 14.7 km long, and 0.925 km wide, with a total surface area of 13.6 km2. The lagoon is orientated parallel to the coastline of the Gulf of Mexico and is surrounded by mangroves (Fig. 1). Both runoff and underground freshwater or brackish water discharges of karstic origin, which enter from the bottom of the lagoon, help maintain the water level. Water circulation is slow due to its almost complete isolation from the sea and a relatively low tide height that usually does not exceed 50-60 cm and never exceeds 1 m. The second study site was located near the village of Dzilam de Bravo (Dzilam, for short). This site is characterized by open exposure to the ocean where the seashore is partly fringed with mangroves.

From May 2008 through May 2009, samples were taken every three months (19 and 21 May, 20 and 25 August, 25 and 28 November 2008, 3 and 6 March, 19 and 22 May 2009), at the Chelem lagoon and offshore of Dzilam. Samples were collected from four mid-lagoon stations in Chelem and two stations near the channel connecting the lagoon with the ocean, and from four stations at Dzilam (Fig. 1). A total of ~250 samples of macroalgae and seagrasses with associated microalgae (together here termed macrophytes) were collected over the course of the study between 10:00 and 15:00 h (Fig. 1, Table 1). Only two stations (St. 2 and 4) in the Chelem lagoon were consistently influenced by freshwater discharge. Station 2 was situated near a freshwater discharge. Station 4 was located approximately 20-30 m from a brackish water discharge. Rich algal vegetation dominated by soft masses of mainly filamentous species was observed around the spring. At each sampling time, two to ten samples from various, usually monospecific macrophyte substrates, were taken at each station. The seafloor was finely sandy (at Dzilam) to muddy (in the Chelem lagoon), sometimes forming a very loose superficial layer up to 30 cm thick. Sampling site depth was almost uniform in the lagoon (about 1 m) and varied from 0.5 m (St. 4) to 4.0 m (St. 2) at Dzilam. Macrophytes (50 to 200 g) were gently detached from the bottom during snorkeling, manually or using a knife, and were immediately placed into 0.5 or 1.0 liter plastic jars in situ underwater, sorted according to species. All the samples were taken together with the surrounding sea water and transported to the boat where 37% stock formalin was added to the jars to a final concentration of 4%.
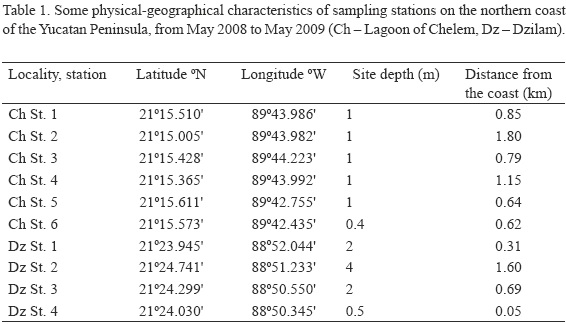
The techniques used for counting cells have been described previously (Okolodkov et al., 2007). The only difference was the use of an Olympus CKX41 inverted microscope in the present study. Abundances are given as the number of dinoflagellate cells per gram of substrate wet weight (WW), and they were averaged among the various macrophyte substrates taken at the same station.
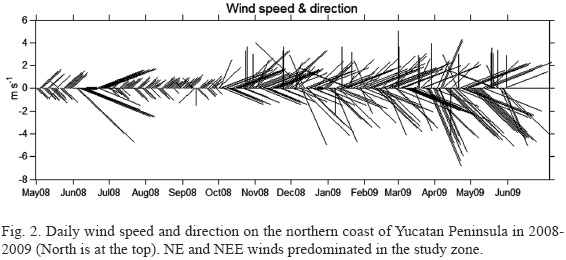
Temperature, salinity, pH and dissolved oxygen (DO) of the surface layer of water (~0.5 m) were measured in situ and recorded using a multiparameter YSI-Professional Plus meter (Yellow Springs, Ohio, USA). Turbidity was measured in the field with an Aguafluor handheld fluorometer and turbidimeter (Turner Designs, model 8000-001, Sunnyvale, CA, USA). Sampling site depth was measured with a portable depth-meter. Samples for analysis of DO, dissolved inorganic nitrogen (DIN: NO3-N + NO2-N + NH4-N), urea – (NH2)2CO, soluble reactive phosphorus (SRP) and soluble reactive silicon (SRSi) were collected in 8-liter plastic tanks from the surface layer (during a pilot survey in May 2008, the nutrient contents were not measured). Urea was measured by the direct diacetylmonoxime method modified by Mulvenna and Savidge (1992). Determinations of DO and nutrients were made in the laboratory using a portable Hach DR/2010 data logging spectrophotometer (Hach Company, Loveland, Colorado, USA) following Strickland and Parsons (1972). Chl-a was determined spectrophotometrically in the laboratory using a trichromatic technique (Richards & Thompson, 1952). Precipitation, wind speed and direction (Fig. 2) were averaged from the daily data obtained by the Meteorological Station of CINVESTAV at Chelem, Yucatan, Mexico (21°15'15" N, 89°44'30" W).
A principal component analysis based on physical-chemical characteristics (temperature, salinity, DO, site depth, nitrates, nitrites, ammonium, SRP, SRSi and urea), Chl-a and population density of epiphytic dinoflagellates was used to establish correlations between the dinoflagellate abundances and the environmental factors including seasonality. A cluster analysis was applied to evaluate the degree of heterogeneity of sampling sites and seasonal differences in species composition between surveys. A canonical correspondence analysis was used to reveal relationships between species of epiphytic dinoflagellates and environmental parameters. All the data were log10 (data+1) transformed prior to analysis. Canoco for Windows 4.5 was used for this analysis. First, a canonical correspondence analysis including 10 environmental variables (DO, SRP, nitrates, urea, ammonium, SRSi, salinity, temperature, depth and turbidity) was run in automatic mode selection with 1000 permutations to test the significance of each variable. After this, nitrates and urea were eliminated, which resulted in an F-ratio less than 1 (P>0.6) and could have influenced negatively in the complete model significance of all canonical axes. The second run included the eight remaining environmental variables, and Montecarlo tests of the first and all canonical axes were performed using 1000 permutations under a reduced model to maintain type I error in small data sets (Braak & Šmilauer, 2002).
RESULTS
Physical-chemical conditions
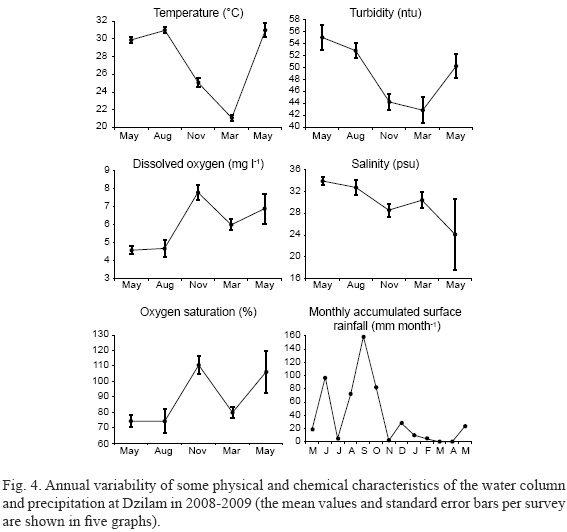
The mean and standard deviations for the physical parameters measured at the Chelem and Dzilam sites are shown in Figs. 3 and 4. Water temperatures ranged between 20.5 and 32.7 oC at Dzilam and from 22.7 to 30.9 oC at Chelem. Both locations showed the same annual temperature cycle with highest temperatures in May and August and lowest in March. Changes in the turbidity measurements followed the annual temperature cycle: at the Dzilam open water site it ranged between 36.7 and 57.8 ntu and in the Chelem lagoon between 43.8 and 63.8 ntu. The variability among sampling stations was greater in the Chelem lagoon than at Dzilam. In general, monthly precipitation was higher from June through October, with the maximum in July at Chelem (115 mm month-1, with the mean value 4 mm day-1) and in June and September at Dzilam (almost reaching 160 mm month-1, with the highest mean value of 5 mm day-1 in September). Runoff and/or groundwater inputs consequently caused the overall salinity to drop by 5 from May to August during sampling period. The overall variation in salinity ranged between 25.8 and 35.1 at Dzilam and between 28.9 and 38.6 at Chelem. The salinity drop of 2 in Chelem from March to May 2009 was not associated with increased precipitation (Fig. 3). Rainfall had a less direct effect on ambient salinities at Dzilam where significant rainfall in June and August-September 2008 only caused a salinity drop of 3. As was the case in the Chelem lagoon, there was a large decrease in salinity from 34 to 24 not associated with significant rainfall inputs in June-July (Fig. 4). In both sampling areas, groundwater was likely responsible for this decrease in salinity. DO concentrations and oxygen saturation were greatest in November 2008 at Chelem (Fig. 3) and in November 2008 and May 2009 at Dzilam (Fig. 4). The DO maximum in November at Chelem coincided with the peak Chl-a concentration.

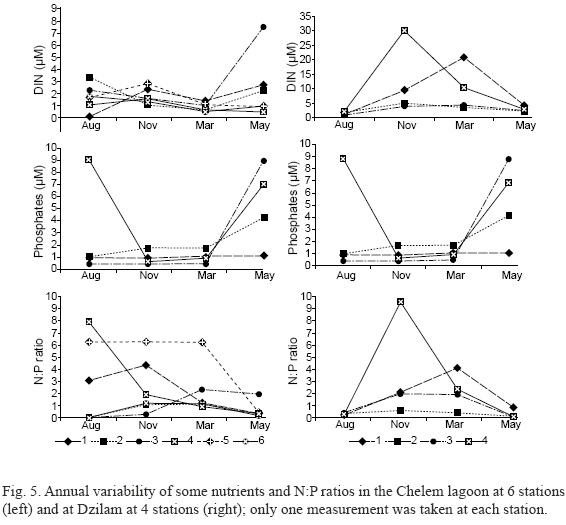
Among the three forms of inorganic nitrogen, NH4-N was usually the major form: up to 5.36 µM in Chelem, and 3.67 µM in Dzilam in November. In three cases, in Chelem (St. 3 in May 2009) and in Dzilam (St. 4 in November and St. 1 in March) very high values of NO3-N were measured, 18.31 µM, 26.56 µM and 18.18 µM, respectively. SRP concentrations remained at rather low levels, less than 1.1 µM, in both Chelem and Dzilam from late summer through early spring (Fig. 5) with only a few exceptions (up to 1.76 µM at St. 5 in Chelem in March). The N:P (DIN to SRP) ratio varied markedly at St. 4 and 5 in Chelem (with the highest ratio of 18 to 24 at St. 5 and 4 in August) and at two inshore St. 1 and 4 (with the highest ratio of 40 at St. 4 in November). Urea concentrations were always high: from 2.76 to 10.12 µM in Chelem, and from 3.61 to 7.21 µM in Dzilam.
Macrophyte substrates
In the Chelem lagoon, the dominant seagrass was Halodule wrightii that occurred with various macroalgal species, especially Caulerpa spp. Among red algae, only Heterosiphonia gibbesii was occasionally very abundant in Chelem, forming large irregularly shaped aggregates several meters in diameter or in length and not attached to the sea bottom. A list of macrophyte species at various Chelem stations is given in Table 2. In Chelem, vast areas covered with sand lacking macrophytes were common at St. 1-4. The macrophyte beds in Dzilam, in contrast, were dominated by the seagrasses Thalassia testudinum and Syringodium filiforme to a lesser extent. The dominant macroalgal species at Dzilam stations included mainly the green algae Caulerpa ashmeadii, C. paspaloides, C. prolifera, Halimeda incrassata and Udotea flabellum. Macrophyte vegetation was usually much more abundant and continuous than in Chelem, forming a multi-species canopy. Dzilam is also characterized by very scarce young solitary corals closer to the coast (St. 4) and large sponges further from it (St. 2), reflecting typical marine conditions. In total, four seagrass and 33 macroalgal species from 24 genera were identified in both localities (Table 2). Green algae were the most diverse in both species and generic richness and abundance. Seagrass beds, although sometimes monospecific, were frequently composed of two or three species mentioned in Table 2, but never Ruppia maritima. The macrophytes were usually covered with abundant fine sediment or detrital particles that were easily detached during sampling. A thick layer of detritus, up to 30 cm, was observed at St. 5 and 6 in Chelem and at St. 4 in Dzilam. Both localities are influenced by fringe mangrove forests characteristic of low energy shorelines in the tropical zone.

Species composition of dinoflagellates
Twenty dinoflagellate species from 12 genera were found, including a number of Prorocentrum spp. not identified to the species level (Fig. 6, Table 3). The genus Prorocentrum Ehrenb. was represented by the largest number of species compared to the other genera. Two taxa were preliminarily identified as Pileidinium sp. and Togula sp.
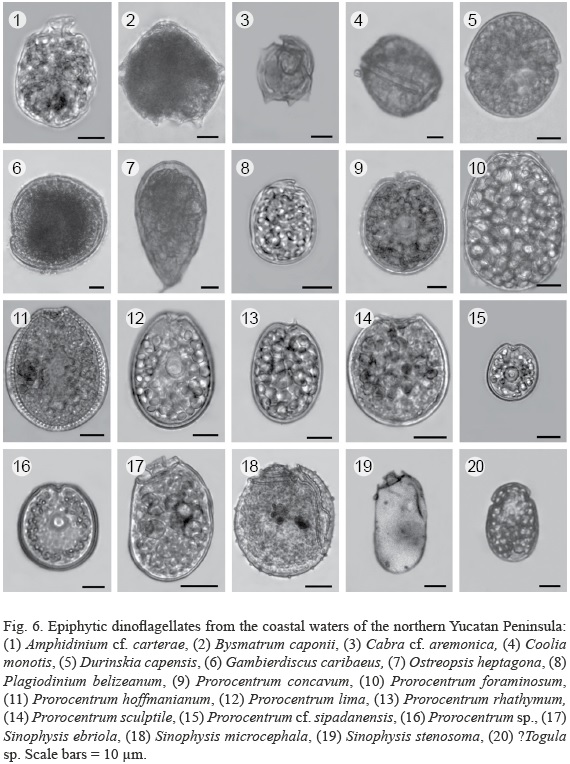
Dinoflagellate abundances
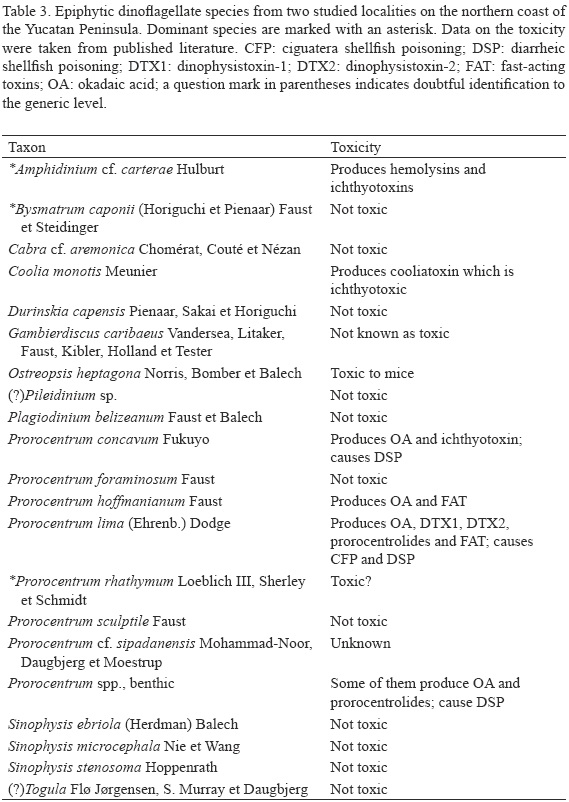
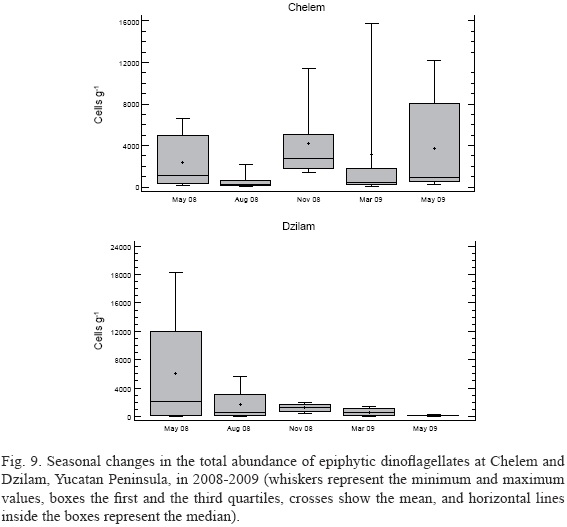
The minimum and the maximum values of dinoflagellate abundance in cells per gram of substrate wet weight (WW) at the same station varied markedly with substrate, the factor between these values ranging from 1.2 to 144.4 (32.1±41.2) in Chelem and from 2.2 to 131.8 (29.8±39.9) in Dzilam. At most stations (St. 1, 2, 5 and 6 in Chelem and St. 1, 2 and 3 in Dzilam), the epiphytic dinoflagellate assemblage did not show any noticeable seasonal variation in total abundance, with fewer than 7.00x103 cells g-1 WW (Figs. 7 and 8).Prorocentrum rhathymum was responsible for the peak value at St. 4 in Chelem in November (1.07x104 cells g-1 WW; it was also the dominant species at St. 1 and 6) and at St. 4 in Dzilam in May 2008 (2.41x104 cells g-1 WW; Fig. 8). Bysmatrum caponii accounted for the peak values at St. 3 in March (1.19 x104 cells g-1 WW) and at St. 4 in May 2009 (7.73 x104 cells g-1 WW), both in Chelem (Fig. 7). Amphidinium cf. carterae reached the highest population density in August at St. 4 in Dzilam (3.69x103 cells g-1 WW) and in May 2008 at St. 4 in Chelem (3.67x103 cells g-1 WW). At St. 2 and 4 in Chelem, Prorocentrum spp. (but not P. rhathymum)reached the highest abundance in November (up to 1.77 x103 cells g-1 WW; Fig. 7). A great similarity in the annual dynamics of the species composition was observed between stations only twice: between St. 1 and 6 in Chelem and between St. 2 and 3 in Dzilam, although the abundances differed by factor of 3 to 4 (Fig. 8). The epiphytic dinoflagellates usually showed one peak of total abundance although sometimes two peaks occurred (St. 4 in Chelem and St. 3 in Dzilam). Dinoflagellate abundance dynamics averaged from all the samples of a given survey differed noticeably between Chelem and Dzilam (Fig. 9). Among all the dinoflagellate species, only two, P. rhathymumand B. caponii, may be considered abundant in general.

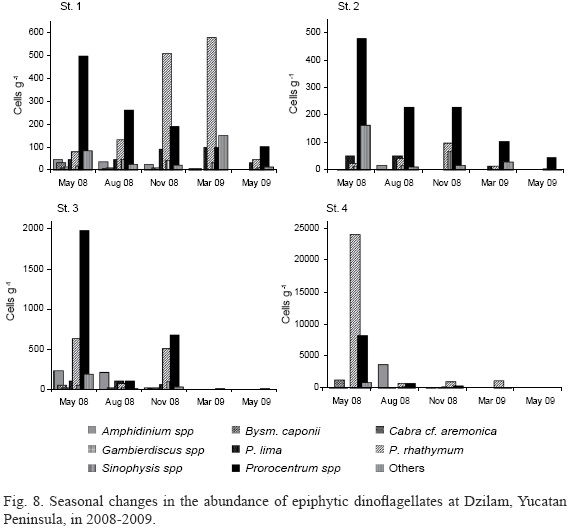
In Dzilam, Gambierdiscus species were sporadically found only in May and November 2008 and always in low numbers at two stations (St. 1 and 4) situated closer to the coast (Fig. 1). In Chelem, they were present all year round although appearing in only one sample in August 2008. The highest abundances of Gambierdiscus cellswere encountered in May and November (Table 4). Higher concentrations were found in Yucatan waters at temperatures of 24.5-30.2 oC. In the study region, G. caribaeus was the most common species, which was confirmed by numerous electron micrographs.
Species of the genera Ostreopsis Schmidt, (?)Pileidinium Tamura et Horiguchi, Plagiodinium Faust et Balech, S. Murray et Daugbjerg, CabraS. Murray et Patterson, Durinskia Carty et Cox, Coolia Meunier, (?)Togula Flø Jørgensen, S. Murray et Daugbjerg andSinophysis Nie et Wang were never abundant, although Coolia monotis and Sinophysis microcephala were found in the majority of samples, the former being more common and the latter always being rare. Among the genera mentioned above, Ostreopsis species were the scarcest, only being observed twice. Some species were identified tentatively, especially some small-sized thecate species and some Prorocentrum spp. which can only be distinguished by a thorough examination of the periflagellar area using SEM.
Statistical correlations
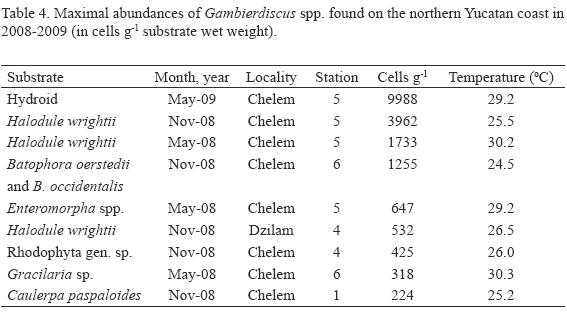
A principal component analysis based on physical-chemical characteristics, Chl-a and epiphytic dinoflagellate population densities indicated that Chelem lagoon is more homogenous than Dzilam (Fig. 10). The same analysis showed that hydrometeorological seasonal changes (referred to as northerly winds, dry season and rain season) largely determine species composition of epiphytic dinoflagellates in these systems (Fig. 11). Cluster analysis further confirmed that Chelem lagoon experiences smaller spatial and temporal variability in physical-chemical characteristics than Dzilam (Fig. 12).

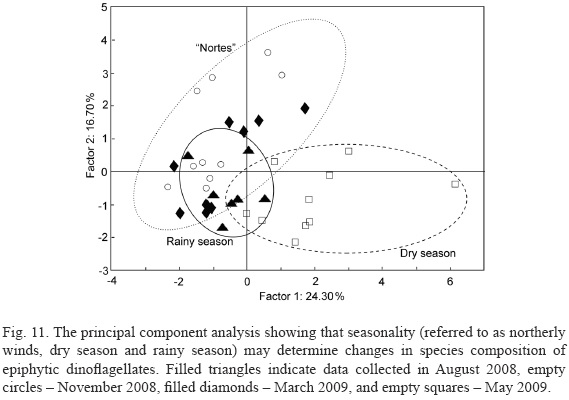

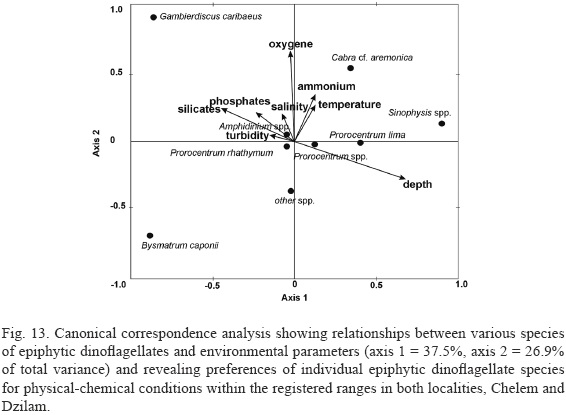
Canonical correspondence analysis showed relationships between various species of epiphytic dinoflagellates and environmental parameters (Montecarlo tests: first canonical axis F-ratio = 4.98, P = 0.027; all canonical axes F- ratio = 2.26, P = 0.001) (Fig. 13).Gambierdiscus spp. and Bysmatrum caponii are the species with the strongest response to environmental conditions. Gambierdiscus spp.are associated with high nutrient concentrations, high turbidity and salinity, and shallow water. Prorocentrum rhathymum prefers low nutrient concentrations, low turbidity and temperature, and shallow water. Prorocentrum lima abundance is correlated with high ammonium concentrations and medium temperature but low turbidity and medium depth. Amphidinium cf. carterae is associated with low nutrient concentrations, turbidity, temperature and depth. Bysmatrum caponii is associated with high nutrient concentrations except for ammonium, low turbidity, salinity, DO and temperature, and shallow water. Sinophysis microcephala is associated with high ammonium concentrations and high temperature, but low turbidity and salinity, and deeper sites. Cabra spp.are associated with high nitrogen-nutrient concentrations, temperature and salinity, and medium phosphate concentrations, and low turbidity and shallow sites. Abundances ofProrocentrum rhathymum and Amphidinium cf. carterae are not correlated with the gradients of environmental parameters studied.
DISCUSSION
Physical-chemical conditions
In general, the ranges of physical-chemical characteristics were similar in both studied localities. Only a few parameters such as DIN varied more at Dzilam than at Chelem, due to the higher nitrate and urea concentrations. Salinity was higher in Chelem than in Dzilam, probably due to higher evaporation in the lagoon (Fig. 2). Monthly precipitation had a seasonal pattern, with higher values from June through October, except for July in Dzilam. However, the salinity did not reflect this; moreover, May 2008 and May 2009 differed markedly. In contrast, the surface water temperature changed in a regular pattern, with the highest values in May-August and the lowest in early March. Changes in turbidity were similar to those in temperature, although within a narrower range. They do not seem to be related to the Chl-a contents in the water column, so the suspended detritus characteristic of both localities is likely responsible for the changes in turbidity.
DO concentrations were lower from May to August 2008 and higher from November through May 2009, with a more rapid increase between August and November that coincided with the increase in wind speed from 2.4-7.3 m s-1 in September to 8.2-18.2 m s-1 in October, the decrease in water temperature from 29-31 to 25 oC from August to November, and with monthly precipitation from September to November (a very steep drop in Dzilam and a rather slow one in Chelem). Differences in monthly precipitation permit us to distinguish a rainy season (May-June to October) and a dry season (November to April-May). The differences in DO content, which did not show a seasonal pattern, can be explained by changes in wind speed. Enhanced mixing of the water column resulted in higher DO concentrations during times of stronger winds: 3.5-9.4 m s-1 in May 2008, 2.4-5.9 m s-1 in August, and a range of 8.2 to 30.6 m s-1 from October through May 2009. These changes appear to be responsible for the significant difference in DO content between May 2008 and May 2009, as well as for the much lower epiphytic dinoflagellate abundances in May 2009 in the open shore in Dzilam. The latter is probably due to physical displacement of the cells from their host macrophytes.
In the semi-enclosed Chelem lagoon, temporal variation in DO content followed that of Chl-a concentration (an indicator of the phytoplankton biomass; Fig. 2, left), suggesting that the former is a good indicator of primary production in this locality. In contrast, in Dzilam this did not occur (Fig. 3, left), providing evidence for more complex relationships between the DO content and physical oceanographic phenomena such as coastal currents, tides and more intense vertical mixing due to the exposed character of the study zone.
In the Chelem lagoon, DIN was relatively high most of the time at almost all the stations (Fig. 4). At Dzilam, the offshore sites (St. 2 and 3) showed lower but still substantial amounts of DIN. The inshore sites (St. 1 and 4), however, received high nutrient inputs from land during the rainy season. Elevated concentrations of nitrates (up to 18.18 µM and 26.56 µM at St. 1 and 4, respectively) were found at the stations closest to the coast. Phosphates were moderately low throughout the year except for May 2009 at both localities and May 2008 at St. 1 at Dzilam. The higher N:P ratio found in Dzilam in November 2008 and March 2009 at St. 1 and 4 was likely due to the proximity of the coast, where a thicker layer of detritus was observed on the seafloor, and to the northerlies that easily resuspend the accumulated detritus in shallow water during this time. At Chelem, the N:P ratios, in general, are comparable with those at Dzilam, and microalgae are N-limited at nearly all the stations except St. 5 in the channel of the lagoon. At Dzilam, the system seems to be P-limited inshore (St. 1 and 4). The conclusions given above are based on a traditional Redfield ratio (N:P = 16:1) interpretation. However, recently published literature (Martiny et al., 2013) provides evidence for the existence of a clear latitudinal gradient in the geographical distribution of the ratio values throughout the oceans and that the ratio is usually 28:1 in the warm nutrient-depleted low-latitude gyres, and 18:1 in warm nutrient-rich upwelling zones. From this point of view, microalgae from the study zone are N-limited with only one exception (the inshore St. 4 at Dzilam in November).
Macrophyte substrates
The northern Yucatan region is characterized by the marked predominance of seagrasses and green algae, especially those belonging to the genus Caulerpa J. V. Lamour.and to a lesser extent to the genera Halimeda J. V. Lamour., Penicillus Lam. and Udotea J. V. Lamour. Larger red and brown algae were scarce (except for large masses of Heterosiphonia gibbesii). In contrast, the Veracruz reef zone, the only area in the southern Gulf of Mexico where the epiphytic dinoflagellate assemblage has been studied, is distinguished by the prevalence of the green alga Halimeda opuntia (L.) J. V. Lamour. and a greater occurrence of larger red and brown algae (especially of the genera Galaxaura J. V. Lamour., Tricleocarpa Huisman et Borowitzka, Acanthophora J. V. Lamour., Dictyota J. V. Lamour., and Padina Adanson, as well as turf Hypnea spp. covering hermatypic corals). Evidently, big coral heads and coral rubble in Veracruz are responsible for a very peculiar marinescape, also offering a solid surface so that macroalgae, especially the red and brown algae, can attach to it and grow. According to our observations in Yucatan waters, green algae from the genera Caulerpa, Udotea, Penicillus and Avrainvillea Decaisne and the common species Halimeda incrassata do not need a solid surface and grow in sand. Many of them have a large submerged bulbous siphonous holdfast with adhering sand particles, or even a well-developed rizomatous portion of a thallus as in Avrainvillea longicaulis f. laxa. In contrast, Acetabularia spp. and Batophora spp. in the study area obligatorily grow attached to mangrove roots and fallen tree branches, wooden piers, submerged dead gorgonian corals and to some typical elements of the anthropogenic marinescape such as plastic bags and buckets, glass bottles and automobile tires.
Species composition and abundances of dinoflagellates
Species composition of epiphytic dinoflagellates in the study area was typical in terms of the prevalence of Prorocentrum spp. Several species occur in shallow subtropical waters and mangrove lagoons (Carlson & Tindall, 1985; Mitchell, 1985; Faust, 1996, 2004; Tindall & Morton, 1998; Turquet et al., 2001; Faust et al., 2005). The contribution of Prorocentrum to the epiphytic dinoflagellate assemblage was important at nearly all the sampled stations. The high abundance of Prorocentrum rhathymum observed at five stations (St. 1, 3 and 6 in Chelem and St. 1 and 4 in Dzilam), which to a greater extent determined the annual dynamics of the whole assemblage, has not been previously reported in the literature. However, there is a report of a maximum population density of 1.52×106 P. mexicana cells g-1macroalgae fresh weight (Carlson & Tindall, 1985); this could have been a mistakenly identified P. rhathymum. Based on the Dzilam samples, this species clearly showed a preference for a proximity to the coast (Fig. 8). Another species, Bysmatrum caponii, also contributed significantly to the epiphytic assemblage but locally, only at St. 3 and 4 in Chelem (Fig. 7), where it was sometimes dominant (with a maximum abundance of 1.20x104 cells g-1 WW at St. 3 in Chelem). This is the first record of such a high abundance of B. caponii. This dinoflagellate species is known to form blooms in the water column in coral-reef mangrove lagoons in Belize, reaching 1.85x102 cells l-1 (Faust et al., 2005).
The maximum total abundance of epiphytic dinoflagellates was almost the same in Yucatan and Veracruz, reaching 2.6×104 and 3.2×104 cells g-1 WW, respectively, in both locations in May, at the station nearest to the coast (Fig. 8; Okolodkov et al., 2007: Fig. 7B). In comparing these two regions in the southern Gulf of Mexico, it is interesting to note that Prorocentrum lima dominates the epiphytic dinoflagellate assemblage in Veracruz and P. rhathymum in Yucatan. Unlike in Veracruz, in Yucatan Coolia monotis is not as numerically important, although it also occurs frequently. Similarly, Ostreopsis spp. are much more abundant in Veracruz.
Toxic dinoflagellate risk assessment
Gambierdiscus spp. clearly showed a preference for the lagoon in Chelem (Table 4), although the shallow-water coastal environment near mangroves in Dzilam characterized by stronger wave action also seemed to favour their appearance. At Johnston Atoll in the Pacific Ocean, the abundance of Gambierdiscus spp. was negatively correlated with water motion (Richlen & Lobel, 2011). The absence of Gambierdiscus cells in early March 2009 at Dzilam and very low concentrations (only up to 17 cells g-1 WW) at Chelem can be explained by comparatively low water temperatures (20.5-22.7 oC). Curiously, the absence of Gambierdiscus spp. in early March samples at Dzilam coincides with the minimal recorded residual nitrate, nitrite and ammonium concentrations. In general, the distribution of abundance ofGambierdiscus spp. in the two study areas was very heterogeneous; the same was observed at Johnston Atoll, where Gambierdiscus spp. reached 4.00x102 cells g-1 WW (Richlen & Lobel, 2011). The maximum population densities observed in Yucatan waters were comparable to maxima reported for Gambierdiscus spp. from the western Caribbean (Morton & Faust, 1997; up to 4.00x103 cells g-1 dry weight macroalgae), Tahiti, French Polynesia (Chinain et al., 1999; up to 5.00x103 cells g-1 WW), and Hawaii (Parsons & Preskitt, 2007; up to 1.15x103 cells g-1 WW), and were much lower compared to the highest concentrations observed in other tropical sites. Yasumoto et al. (1980) found 3.18x105 cells g-1 WW in Gambier Island, French Polynesia, Carlson and Tindall (1985) reported 7.58x104 cells g-1 WW from the Virgin Islands, East Caribbean, and Turquet et al. (2001) found 6.05x104 cells g-1 WW in the Comoros archipelago, SW Indian Ocean. Elevated concentrations of Gambierdiscus spp. were encountered in Yucatan at the beginning of the hot and rainy season, and later, at temperatures of 24.5-32 oC, which are higher than those reported by other authors for G. "toxicus". Litaker et al. (2010), who reviewed the ecophysiological preferences of Gambierdiscus spp., concluded that they prefer the habitats where annual water temperatures range from 21 to 31 oC, with an optimum between 25 and 29 oC. According to Kibler et al. (2012), who examined cultures of eight Gambierdiscus spp. from North Carolina and Hawaii, USA, and Belize, depending on the species, the range of the maximal growth varied between 26.5 oC and 31.1 oC. Because of various temperature tolerances exhibited by different Gambierdiscus spp., seasonal patterns of the abundance dynamics may be complicated (Parsons et al., 2012). Gambierdiscus caribaeus (the most abundant species of this genus that we found in Yucatan samples) growth was limited at temperatures below 26 oC and above 29-31 oC (Parsons et al., 2010; Kibler et al., 2012). This species is prevalent in the summer months in Florida waters (Parsons et al., 2012). Gillespie et al. (1985) found a peak in population density that corresponded to water temperatures lower than 22 oC in Queensland, NE Australia. While Carlson and Tindall (1985) found that precipitation corresponded to an increase in abundance of G. "toxicus", in our study the high population densities are definitely not characteristic of the rainy season; the highest concentrations were in May and November (Table 4). These results match well with those presented by Kibler et al. (2012), who found that the salinity range of the maximal growth of eight Gambierdiscus spp. occurred between 24.7 and 35, although they showed tolerance both to lower (<14 to 20.9) and higher (41) salinities.
The most striking difference between the epiphytic dinoflagellate assemblages of the northern Yucatan Peninsula and that in the coral-reef zone in Veracruz (Okolodkov et al., 2007) is the almost complete absence of the genus Ostreopsis J. Schmidt. Ostreopsis species are usually a common component of the epiphytic assemblage in tropical and subtropical waters (Bomber et al., 1985, 1988; Carlson & Tindall, 1985; Gillespie et al., 1985; Ballantine et al., 1988; Quod, 1994; Faust, 1996, 2004; Faust et al., 1996, 2005; Tindall & Morton, 1998; Rhodes et al., 2000; Turquet et al., 2001; Lenoir et al., 2004; Shears & Ross, 2009; Kim et al., 2011), including the Mediterranean Sea (Tognetto et al., 1995; Vila et al., 2001; Aligizaki & Nikolaidis, 2006; Totti et al., 2010). This fact may be explained by the preference of Ostreopsis species for a particular substrate. Furthermore, it was shown that Ostreopsis species in general are characteristic of high energy reef sites, being positively correlated with water motion (Richlen & Lobel, 2011).
The studied localities in the northern Yucatan waters can be considered a ciguateric region with a high risk of human poisoning by consumption of carnivorous fish or herbivorous gastropods, especially in Chelem area. In April 2010, a dozen people were reported to have been poisoned by eating the great barracuda, Sphyraena barracuda (Walbaum), supposedly caught in the Chuburna region (located 11.3 km from Chelem), according to information from the Secretary of Public Health in Yucatan (Pilar E. Granja-Pérez, Duly M. Marrufo-Estrada, pers. comm.). On 9 April 2010, in the "Lucas de Gálvez" market in Mérida, toxic fried barracuda was suspected. Raw and fried fish and human urea samples from 11 patients were tested the next day by the mouse bioassay technique; samples from eight patients were positive for ciguatera-related biotoxins, and the rest were negative. Whether the barracudas are a part of a local population or they came from the Caribbean where they are more abundant, it is obvious that the causative agent of ciguatera may reach high abundances in the northern waters of Yucatan. Consumption of the molluscs Melongena corona bispinosa (Philippi) and M. melongena (L.) (Gastropoda: Melongenidae) is probably the main threat at present both for local inhabitants and tourists. Although there is a permanent closed season for these gastropods, 256 tons are extracted annually from the lagoon (Patiño-Suárez et al., 2003). Additional monitoring of epiphytic assemblages along the coasts of the state of Quintana Roo (the Caribbean Sea) in June and the state of Yucatan (the Gulf of Mexico) in August, from El Cuyo to Celestun, demonstrated the presence of Gambierdiscus spp. in the Caribbean samples taken at Xcalac and Puerto Morelos and in the eastern sector of the northern Yucatan coast, between El Cuyo and Dzilam, but not westward on the seaside (Okolodkov, unpubl. data). Thus, at present, Chelem seems to be the westernmost locality where Gambierdiscus spp. were found, most likely due to specific hydrochemical and biological conditions of abundant submerged vegetation and high nutrients. However, the problem of a causative agent of ciguatera in Yucatan waters in 2010 still remains. A weekly monitoring is desirable, considering the maximum growth rate of 0.25 to > 0.5 division day-1 for the fastest growing clones of Gambierdiscus "toxicus" (Bomber et al., 1988), between ~0.2 and ~0.4 division day-1 for eight Gambierdiscus spp. (Kibler et al., 2012), and 0.5 division day-1 for Prorocentrum lima (Jackson et al., 1993). Despite some deficiencies, our pilot monitoring study has been useful and efficient enough to answer some basic questions, and it can serve as a guide for further studies of the epiphytic dinoflagellate assemblage and ciguatera in the coastal waters of the Yucatan Peninsula.
ACKNOWLEDGEMENTS
We thank Manuel A. González-Salas (Dzilam de Bravo) and Javier Ramírez-Ramírez (CINVESTAV) for logistic support, Iliana Osorio-Moreno (CINVESTAV) for chemical analyses, Isabel Sánchez-Molina (Universidad Autónoma de Yucatán) and Citlalli Galicia-García (ITBoca, Veracruz) for identification of macroalgae, R. Wayne Litaker (NOS/NOAA, Beaufort, North Carolina, USA) for thorough verification of all the photos with Gambierdiscus, Pilar E. Granja-Pérez and Duly M. Marrufo-Estrada (Laboratorio Estatal de Salud Pública de Yucatán, Servicios de Salud de Yucatán) for sharing the information about the ciguatera case, Mindy L. Richlen (Woods Hole Oceanographic Institution, Massachusetts, USA) for help with the literature, Marcia M. Gowing (University of California at Santa Cruz, California, USA) for improving the writing style, Natalia A. Okolodkova and Fernando Aguirre-Bahena (CICIMAR-IPN, La Paz) for technical assistance with illustrations, two anonymous reviewers for their valuable critical comments, Consejo Nacional de Ciencia y Tecnología (CONACYT), Mexico, and Gobierno del Estado de Yucatán for financial support to the FOMIX CONACYT-Yucatán project "Análisis de las causas, dispersión y consecuencias ambientales de la marea roja en Yucatán" (No. 108897; 2009-2011) given to JAHS.
LITERATURE CITED
Aligizaki, K. & G. Nikolaidis. 2006. The presence of the potentially toxic genera Ostreopsis and Coolia (Dinophyceae) in the North Aegean Sea, Greece. Harmful Algae5: 717-730. [ Links ]
Bagnis, B., J. Bennett, C. Prieur & A. M. Legrand. 1985. The dynamics of three toxic benthic dinoflagellates and the toxicity of ciguateric surgeonfish in French Polynesia. In: Anderson, D. M., A. W. White & D. G. Baden (eds.). Toxic dinoflagellates. Elsevier Science Publishing. New York, USA. pp. 177-182. [ Links ]
Ballantine, D. L., T. R. Tosteson & A. T. Bardales. 1988. Population dynamics and toxicity of natural populations of benthic dinoflagellates in southwestern Puerto Rico. J. Exp. Mar. Biol. Ecol. 119: 201-212. [ Links ]
Bomber, J. W., D. R. Norris & L. E. Mitchell. 1985. Benthic dinoflagellates associated with ciguatera from the Florida Keys. II. Temporal, spatial and substrate heterogeneity of Prorocentrum lima. In: Anderson, D. M., A. W. White & D. G. Baden (eds.). Toxic dinoflagellates. Elsevier Science Publishing. New York, USA. pp. 45-50. [ Links ]
Bomber, J. W., R. R. L. Guillard & W. G. Nelson. 1988. Roles of temperature, salinity, and light in seasonality, growth, and toxicity of ciguatera-causing Gambierdiscus toxicus Adachi et Fukuyo (Dinophyceae). J. Exp. Mar. Biol. Ecol. 115: 53-65. [ Links ]
Braak, C. J. F. ter & P. Šmilauer. 2002. CANOCO reference manual and CanoDraw for Windows user's guide: software for Canonical Community Ordination (version 4.5). Section on permutation methods. Microcomputer Power, Ithaca, USA. 500 pp. [ Links ]
Carlson, R. D. & D. R. Tindall. 1985. Distribution and periodicity of toxic dinoflagellates in the Virgin Islands. In: Anderson, D. M., A. W. White & D. G. Baden (eds.). Toxic dinoflagellates. Elsevier Science Publishing. New York, USA. pp. 171-176. [ Links ]
Chinain, M., M. Germain, X. Deparis, S. Pauillac & A.-M. Legrand. 1999. Seasonal abundance and toxicity of the dinoflagellate Gambierdiscus spp. (Dinophyceae), the causative agent of ciguatera in Tahiti (French Polynesia). Mar. Biol. 135: 259-267. [ Links ]
Faust, M. A. 1996. Dinoflagellates in a mangrove ecosystem, Twin Cays, Belize. Nova Hedw. Beih. 112: 447-460. [ Links ]
Faust, M. A. 2004. The dinoflagellates of Twin Cays, Belize: biodiversity, distribution, and vulnerability. Atoll Res. Bull.514: 1-20. [ Links ]
Faust, M. A., S. L. Morton & J. P. Quod. 1996. Further SEM study of marine dinoflagellates: the genus Ostreopsis (Dinophyceae). J. Phycol.32: 1053-1065. [ Links ]
Faust, M. A., W. Litaker, M. W. Vandersea, S. R. Kibler & P. A. Tester. 2005. Dinoflagellate diversity and abundance in two Belizean coral-reef mangrove lagoons: a test of Margalef's Mandala. Atoll Res. Bull.534: 105-131. [ Links ]
Gillespie, N. C., M. J. Holmes, J. B. Burke & J. Doley. 1985. Distribution and periodicity of Gambierdiscus toxicus in Queensland, Australia. In: Anderson, D. M., A. W. White & D. G. Baden (eds.). Toxic dinoflagellates. Elsevier Science Publishing. New York, USA. pp. 183-188. [ Links ]
Herrera-Silveira, J. A. 1993. Ecología de los productores primarios en la laguna de Celestún, México. Patrones de variación espacial y temporal. Ph. D. thesis. Universitat de Barcelona, Spain. 233 pp. [ Links ]
Jackson, A. E., J. C. Marr & J. L. McLachlan. 1993. The production of diarrhetic shellfish toxins by an isolate of Prorocentrum lima from Nova Scotia, Canada. In: Smayda, T. J. & Y. Shimizu (eds.). Toxic phytoplankton blooms in the sea. Elsevier Scientific Publishing. Amsterdam, The Netherlands. pp. 513-518. [ Links ]
Kibler, S. R., R. W. Litaker, W. C. Holland, M. W. Vandersea & P. A. Tester. 2012. Growth of eight Gambierdiscus (Dinophyceae) species: Effects of temperature, salinity and irradiance. Harmful Algae 19: 1-14. [ Links ]
Kim, H. S., W. Yih, J. H. Kim, Myung & G. H. J. Jeong. 2011. Abundance of epiphytic dinoflagellates from coastal waters off Jeju Island, Korea during autumn 2009. Ocean. Sci. J. 46(3): 205-209. [ Links ]
Lenoir, S., L. Ten-Hage, J. Turquet, J.-P. Quod, C. Bernand & M.-C. Hennion. 2004. First evidence of palytoxin analogues from an Ostreopsis mascarenensis (Dinophyceae) benthic bloom in the southwestern Indian Ocean. J. Phycol.40: 1042-1051. [ Links ]
Levasseur, M., J.-I. Couture, A. M. Weise, S. Michaud, M. Elbrächter, G. Sauvé & E. Bonneau. 2003. Pelagic and epiphytic summer distributions of Prorocentrum lima and P. mexicanum at two mussel farms in the Gulf of St. Lawrence, Canada. Aquat. Microb. Ecol.30: 283-293. [ Links ]
Litaker, R. W., M. W. Vandersea, M. A. Faust, S. R. Kibler, A. W. Nau, W. C. Holland, M. Chinain, M. J. Holmes & P. A. Tester. 2010. Global distribution of ciguatera causing dinoflagellates in the genus Gambierdiscus. Toxicon 56: 711-730. [ Links ]
Martiny, A. C., C. T. A. Pham, F. W. Primeau, J. A. Vrugt, J. K. Moore, S. A. Levin & M. W. Lomas. 2013. Strong latitudinal patterns in the elemental ratios of marine plankton and organic matter. Nature Geoscience 6: 279-283. [ Links ]
Mitchell, L. E. 1985. Ecological studies of benthic dinoflagellates associated with ciguatera in the Florida Keys: the 0-38 micrometer size fraction. M. Sc. Thesis. Department of Oceanography and Ocean Engineering, Florida Institute of Technology. Melbourne, USA. 46 pp. [ Links ]
Morton, S. L. & M. A. Faust. 1997. Survey of toxic dinoflagellates from the Belizean barrier reef ecosystem. Bull. Mar. Sci.61(3): 899-906. [ Links ]
Mulvenna, P. F. & G. Savidge. 1992. A modified manual method for the determination of urea in seawater using diacetylmonoxime reagent. Estuar. Coast. Shelf Sci.34: 429-438. [ Links ]
Okolodkov, Y. B., G. Campos-Bautista, I. Gárate-Lizárraga, J. A. G. González-González, M. Hoppenrath & V. Arenas. 2007. Seasonal changes of benthic and epiphytic dinoflagellates in the Veracruz reef zone, Gulf of Mexico. Aquat. Microb. Ecol.47(3): 223-237. [ Links ]
Parsons, M. L. & L. B. Preskitt. 2007. A survey of epiphytic dinoflagellates from the coastal waters of the island of Hawai'i. Harmful Algae 6: 658-669. [ Links ]
Parsons, D. L., K. Aligizaki, M.-Y. Dechraoui Bottein, S. Fraga, S. L. Morton, A. Penna & L. Rhodes. 2012. Gambierdiscus and Ostreopsis: Reassessment of the state of knowledge of their taxonomy, geography, ecophysiology, and toxicology. Harmful Algae 14: 107-129. [ Links ]
Parsons, M. L., C. J. Settelmier & P. K. Bienfang. 2010. A simple model capable of stimulating the population dynamics of Gambierdiscus, the benthic dinoflagellate responsible for ciguatera fish poisoning. Harmful Algae 10: 71-80. [ Links ]
Patiño-Suárez, V., A. G. Zamora, M. Tapia-Arjona & D. Aldana. 2003. La nobleza, belleza y via crucis del recurso pesquero "chivita" en Yucatán. Ciencia 54(3): 66-72. [ Links ]
Quod, J.-P. 1994. Ostreopsis mascarenensis sp. nov. (Dinophyceae), dinoflagellé toxique associé à la ciguatera dans l'océan Indien. Cryptogamie Algol.15(4): 243-251. [ Links ]
Richards, F. A. & T. F. Thompson. 1952. The estimation and characterization of plankton populations by pigment analyses. II. A spectrophotometric method for the estimation of plankton pigments. J. Mar. Res.11: 156-172. [ Links ]
Rhodes, L., J. Adamson, T. Suzuki, L. Briggs & I. Garthwaite. 2000. Toxic marine epiphytic dinoflagellates, Ostreopsis siamensis and Coolia monotis (Dinophyceae), in New Zealand. N. Z. J. Mar. Fresh. Res. 34: 371-383. [ Links ]
Richlen, M. L. & P. S. Lobel. 2011. Effects of depth, habitat, and water motion on the abundance and distribution of ciguatera dinoflagellates at Johnston Atoll, Pacific Ocean. Mar. Ecol. Prog. Ser. 421: 51-66. [ Links ]
Shears, N. T. & P. M. Ross. 2009. Blooms of benthic dinoflagellates of the genus Ostreopsis; an increasing and ecologically important phenomenon on temperate reefs in New Zealand and worldwide. Harmful Algae 8: 916-925. [ Links ]
Strickland, J. D. H. & T. R. Parsons. 1972. A practical handbook of seawater analysis. Fisheries Research Board of Canada. Ottawa, Canada. 310 pp. [ Links ]
Tindall, D. R. & S. L. Morton. 1998. Community dynamics and physiology of epiphytic/benthic dinoflagellates associated with ciguatera. In: Anderson, D. M., A. D. Cembella & G. M. Hallegraeff (eds.). Physiological ecology of harmful algal blooms. NATO ASI Series, Series G: Ecological Sciences 41. Springer-Verlag. Berlin, Germany. pp. 293-313. [ Links ]
Tognetto, L., Bellato, S., Moro & I. C. Andreoli. 1995. Occurrence of Ostreopsis ovata (Dinophyceae) in the Tyrrhenian Sea during summer 1994. Bot. Mar.38: 291-295. [ Links ]
Totti, C., S. Accoroni, F. Cerino, E. Cucchiari & T. Romagnoli. 2010. Ostreopsis ovata bloom along the Conero Riviera (northern Adriatic Sea): Relationships with environmental conditions and substrata. Harmful Algae9: 233-239. [ Links ]
Turquet, J., J.-P. Quod, L. Ten-Hage, Y. Dahalani & R. Wendling. 2001. Example of a Gambierdiscus toxicus flare-up following the 1998 coral bleaching event in Mayotte Island (Comoros, south-west Indian Ocean). In: Hallegraeff, G. M., S. I. Blackburn, C. J. Bolch & R. J. Lewis (eds.). Harmful algal blooms 2000. Intergovernmental Oceanographic Commission of UNESCO. Paris, France. pp. 50-53. [ Links ]
Vila, M., E. Garcés & M. Masó. 2001. Potentially toxic epiphytic dinoflagellate assemblages on macroalgae in the NW Mediterranean. Aquat. Microb. Ecol.26: 51-60. [ Links ]
Yasumoto, T., A. Inoue, O. Tadashi, K. Fujimoto, Y. Oshima, Y. Fukuyo, R. Adachi & R. Bagnis. 1980. Environmental studies on a toxic dinoflagellate responsible for ciguatera. Bull. Jap. Soc. Sci. Fish.46: 1397-1404. [ Links ]














