Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Acta botánica mexicana
On-line version ISSN 2448-7589Print version ISSN 0187-7151
Act. Bot. Mex n.100 Pátzcuaro Jul. 2012
Direct seeding of Brosimum alicastrum Sw. (Moraceae) and Enterolobium cyclocarpum (Jacq.) Griseb. (Mimosaceae) in different habitats in the dry tropics of central Veracruz
Siembra directa de Brosimum alicastrum Sw. (Moraceae) y Enterolobium cyclocarpum (Jacq.) Griseb. (Mimosaceae) en diferentes habitats en el trópico seco del centro de Veracruz
Javier Laborde1, 2 & Isabel Corrales-Ferrayola1
1 Instituto de Ecología, A.C.carretera antigua a Coatepec 351, El Haya, 91070 Xalapa, Veracruz, Mexico.
2 Autor para la correspondencia:javier.laborde@inecol.edu.mx
Recibido en marzo de 2012.
Aceptado en mayo de 2012.
ABSTRACT
Secondary forest in the seasonal tropics is usually dominated by a few pioneer tree species (usually wind-dispersed), while animal-dispersed species with large seeds may be absent. Several studies have shown that directly seeding these tree species in abandoned pastures can be successful; however, information is lacking about the optimal habitat conditions for sowing. We selected two large-seeded zoochorous canopy tree species that are common in the semi-deciduous tropical forest of central Veracruz, Mexico: Brosimumalicastrum and Enterolobiumcyclocarpum. Their seeds were sown in seven habitats: six forming a gradient of increasing vegetation structure, from active pasture to 10-year-old secondary forest, and an old-growth forest. We assessed seed predation by granivores, protecting half of the seeds in wire cages. For a year we monitored seedling emergence, survival and growth, re-visiting the sites four-and-a-half years later. Seedling emergence was relatively high (75% in Brosimum, 60% in Enterolobium) and fairly even among habitats. Surprisingly, no seeds were removed by granivores. Enterolobium seedling survival and growth was higher in open habitats (around 60% survival up to a year) than in habitats shaded by woody plants (<10%). For Brosimum, the reverse was true; its seedlings survived and grew better under a dense woody canopy (>80% survival) than in open sites (0%). Our results show that abandoned pastures and secondary forests can be successfully enriched by directly seeding poorly-dispersed forest canopy tree species, if the right habitat for sowing is chosen with care and based on the ecology of seedling establishment of the desired species.
Key words: forest recovery, large-seeded trees, secondary forest, seed predation, seedling establishment, semi-deciduous tropical forest.
RESUMEN
Los acahuales (i.e. selvas secundarias) de zonas tropicales secas o estacionales, suelen ser pobres en especies arbóreas y dominados por unas cuantas especies de arbustos y árboles pioneros, usualmente dispersados por viento, siendo notable la ausencia de plantas arbóreas del dosel cuyas semillas relativamente grandes dependen de vectores animales para su dispersión. Varios estudios han encontrado que la siembra directa de especies arbóreas zoócoras con semillas grandes, en pastizales abandonados puede ser una práctica exitosa para enriquecer y acelerar la sucesión secundaria. Sin embargo, todavía no se conocen cabalmente las condiciones de hábitat o etapa sucesional óptima para realizar la siembra directa de semillas de árboles de fases sucesionales tardías. En este estudio seleccionamos dos especies arbóreas con semillas zoócoras relativamente grandes, que suelen formar parte del dosel de las selvas sub-caducifolias del centro de Veracruz: Brosimumalicastrum y Enterolobiumcyclocarpum. Las sembramos en siete hábitats; seis de ellos representando un gradiente de menor a mayor complejidad estructural o desarrollo sucesional, desde pastizal activo hasta acahual de 10 años y el hábitat restante fue selva mediana sub-caducifolia bien conservada. Evaluamos experimentalmente la importancia de la depredación de semillas, protegiendo la mitad de ellas sembradas dentro de jaulas diseñadas para excluir a vertebrados granívoros. Durante un año monitoreamos la emergencia, supervivencia y crecimiento de plántulas, marcando a las que sobrevivieron su primer año, para ser registradas cuatro años y medio después. Un porcentaje relativamente alto de plántulas emergió de las semillas sembradas (75% en Brosimum, 60% Enterolobium). No detectamos diferencias significativas en la emergencia de plántulas entre los siete hábitats, ni entre los dos tratamientos de exposición a granívoros (dentro vs. fuera de exclusorios). Ninguna de las semillas sembradas fue removida por granívoros. La supervivencia y crecimiento de Enterolobium durante el primer año fue mayor en hábitats abiertos sin cobertura de plantas leñosas (ca. 60%) que en los sombreados por arbustos y árboles (<10%). En contraste, las plántulas de Brosimum sobrevivieron y crecieron mucho mejor bajo la sombra de plantas leñosas (>80%) que en hábitats abiertos (0%). Nuestros resultados muestran que los pastizales abandonados y los acahuales pobres en especies arbóreas, pueden ser enriquecidos mediante la siembra directa de árboles de fases sucesionales tardías con baja capacidad de dispersión, siempre y cuando se elija cuidadosamente el hábitat (o etapa sucesional) óptimo para la siembra de semillas, con base en la ecología del establecimiento de plántulas de las especies involucradas. En el centro de Veracruz se puede acelerar la recuperación de la selva, sembrando semillas de Enterolobium desde el momento del abandono del pastizal, siempre y cuando se controle el crecimiento de los pastos durante los primeros dos a tres años de crecimiento de las plántulas. A su vez las semillas de Brosimum deberán sembrarse hasta que los arbustos o árboles pioneros hayan colonizado el sitio y sombreado a los pastos.
Palabras clave: árboles de semillas grandes, depredación de semillas, establecimiento de plántulas, regeneración forestal, selva mediana sub-caducifolia, selva secundaria.
INTRODUCTION
Over the last six decades an unprecedented area of old-growth and secondary forest has been cleared in the tropical Americas, most of which has been converted into pastures (Toledo, 1989; Chazdon, 2003; Griscom & Ashton, 2011). Concomitant with this massive deforestation is the severe fragmentation of the remaining tropical forest, which has left forest fragments of different sizes scattered in a landscape matrix of pastures and other deforested areas. The situation is so extreme that the very existence of tropical forests is in jeopardy in several regions (Challenger, 1998; Terborgh, 1999; Laurance et al., 2006). Clearly, deforestation has to be stopped and the reserves set up to protect tropical forest need to work properly. However, both of these strategies need to be complemented by the restoration of tropical forest in degraded areas if we want to achieve the long term persistence of tropical forest ecosystems and preserve their impressive biodiversity (Terborgh, 1999; Chazdon, 2003; Laurance et al., 2006; Griscom & Ashton, 2011).
Regrettably, the restoration of tropical forest in sites previously used as pastures is not an easy task. Pasture management practices, including grazing by cattle, quickly exhaust the re-sprouting potential of the roots and stumps of woody plants, also depleting the soil seed bank of woody plants (Kellman, 1974; Holl, 1999; Janzen, 2002). Therefore, the main and often the only route by which a tree or any other forest plant might establish in a pasture is the immigration (dispersal) of a seed into the site from a nearby seed source. However, due to the large size of pastures, suitable seed sources (i.e. forested sites) are usually too far away from the pasture to be effective. Furthermore, a large proportion of the tropical woody flora strictly depend on frugivorous animals for seed dispersal and most of these animals are reluctant to leave a forest patch and move into pastures (Holl, 1999; Janzen, 2002; Guevara et al., 2005). Consequently, secondary forests that grow on abandoned pastures are usually poor in tree species, and dominated by a handful of pioneer trees which produce an abundance of small seeds dispersed by the frugivorous birds and bats that are habitat generalists (Janzen, 2002; Guevara et al., 2005; Muscarella & Fleming, 2007). In tropical dry forest an important proportion of the tree flora is anemochorous (wind-dispersed), and their seeds can reach pastures much more easily than those of zoochorous (animal dispersed) tree species. A first wave of anemochorous trees that colonizes a pasture may dominate the site and delay the recruitment of zoochorous tree species for several decades (Janzen, 2002; Griscom & Ashton, 2011).
Once a tree seed arrives at a pasture it has to overcome new and different obstacles. The seeds could be heavily preyed upon by the granivorous animals that are abundant in abandoned pastures (Doust et al., 2006; García-Orth & Martínez-Ramos, 2008) or the emerged seedlings might be eaten by a variety of herbivores (Holl & Quiros-Nietzen, 1999; Griscom et al., 2005). Those seedlings that escape predators will have to compete with fast-growing grasses and other herbaceous plants, which might easily out-compete them (Hooper et al., 2002; Griscom et al., 2005; Ortega-Pieck et al., 2011). In addition to these two biotic barriers, tree seeds and their seedlings also have to cope with the harsh micro-environmental conditions prevalent in pastures that might hinder recruitment (Aide & Cavelier, 1994; Holl, 1999; Hooper et al., 2002; Janzen, 2002; Chazdon, 2003).
Recent restoration efforts as well as studies on tropical secondary succession indicate that, without assistance, in many cases late successional species might not be able to establish; in particular, large-seeded zoochorous tree species may fail to reach the site or take a very long time to colonize it (Holl, 1999; Janzen, 2002; Florentine & Westbrooke, 2004; Doust et al., 2006; Bonilla-Moheno & Holl, 2010; Cole et al., 2011). The enrichment of abandoned pastures and secondary forest with preferred tree species can be done in two ways: by transplanting seedlings produced in nurseries and by directly sowing their seeds (direct seeding) into the plot. The first has been shown to be successful in several studies (see reviews by Florentine & Westbrooke, 2004; Griscom & Ashton, 2011), however it is very expensive and usually limited to the few tree species that, owing to their high commercial or agricultural value, are available in nurseries (Cole et al., 2011). Direct seeding, on the other hand, is much cheaper and has been used more frequently in recent years (Campana-Camargo et al., 2002; Hooper et al., 2002; Doust et al., 2006; García-Orth & Martínez-Ramos, 2008; Bonilla-Moheno & Holl, 2010; Cole et al., 2011). Although several studies have shown that direct seeding is a promising practice for accelerating forest succession and enriching secondary forests, there have been some contradictory results. In most of the studies in which the sown seeds failed to become seedlings there was no explicit control of seed predation and so it is not possible to know whether establishment failure was due to seed predators or to unsuitable micro-environmental conditions. Additionally, most of the direct seeding studies have been done in recently abandoned pastures, with only a few in secondary forests that vary in age, vegetation structure and microclimate (Campana-Camargo et al., 2002; Bonilla-Moheno & Holl, 2010; Cole et al., 2011). Also most of these studies have been done in the humid tropics. Thus, more information is needed on whether direct seeding can be equally successful when carried out in different types of habitat, particularly in more seasonal or drier tropical regions, and whether the tree species of old growth forest have different optimal habitats where their seedling survival and growth are maximized. In addition, we need to discern to the best of our ability, the reasons that sown seeds fail to produce successful seedlings in different habitats and circumstances.
For this study, we selected Brosimumalicastrum and Enterolobiumcyclocarpum, two large-seeded zoochorous tree species that are common in the canopy of the original semi-deciduous tropical forest of central Veracruz, Mexico. Seeds of both species were sown in seven habitats that have contrasting vegetation cover and composition; six of them ranging from active pasture to a 10-year-old secondary forest. As a reference forest we included a remnant of old-growth forest that has been protected since 1977. We explicitly assessed the importance of seed predation by granivores, protecting half of the sown seeds inside wire cages. Over the course of a year we monitored seedling emergence, survival and growth, carefully recording the causes of seedling mortality in each of the seven habitats. We re-visited the sites to make final measurements of growth and survival four-and-a-half years later. Our aim was to compare seedling performance among the seven habitats and determine if there is an optimal habitat for direct seeding of large-seeded canopy tree species, and to assess whether the two tree species have different optimal habitats for seedling recruitment.
If seed removal by granivores is a major factor preventing the establishment of sown seeds, then we expect seedling emergence to be higher inside the wire cages than outside them. The intensity of seed removal may vary widely among the habitats studied, in accordance with the habitat preferences and densities of seed predators: in grass dominated habitats we expect a high rate of seed removal by rodents, however in the forest of the study site it has been reported that the red land crab (Gecarcinuslateralis) removes tree seeds and recently emerged seedlings from the forest floor (including Brosimum). Our results will reveal in which habitats seed predation is a strong impediment to direct seeding. Since Brosimum seeds are highly nutritious and unprotected, while those of Enterolobium are protected by a woody testa and not as palatable to granivores, we also expect a higher rate of seed removal for Brosimum. Differences in seedling survival and growth among habitats will reveal if there is an optimal habitat for sowing. Since differences in the floristic composition and vegetation structure of the seven studied habitats roughly resemble differences in forest recovery (i.e. secondary successional stage), the results of this study will show if there is an optimal moment in succession after pasture abandonment, when the selected species should be sown. Additionally, we did a field manipulation experiment during the first year of the study, removing the grass foliage from a grassland site without cattle, in order to minimize light competition between the grasses and emerged woody seedlings. This will tell us if this time consuming and demanding activity positively affects seedling growth or survival in abandoned pastures.
METHODS
Study site
This research was carried out in the "Centro de Investigaciones Costeras La Mancha" (CICOLMA) biological station located on the coast of the Gulf of Mexico in central Veracruz, Mexico (19°35'50" N; 96°22'45" W) and managed by the Instituto de Ecología, A.C. (INECOL). Climate is Aw2 tropical wet and dry or sub-humid with summer rains (Köppen modified by García, 1981; cited in Moreno-Casasola, 2006). Mean annual precipitation is 1286 mm/year (range: 899 to 1829 mm/year), the driest months are November to May (≤60 mm/month) and the wettest are June to September (>150 mm/month). Mean annual temperature is 25.6 ºC; mean values of the coldest and hottest month are 21.1 ºC (January) and 27.3 ºC (June), respectively (Moreno-Casasola, 2006; unpublished data from 1981 to 2006 collected at the CICOLMA meteorological station). There is an extensive system of Pleistocene-age sand dunes in the region and at the CICOLMA station a fossil dune dating from the Late-Glacial age has been overrun by a large N-S arm of a parabolic dune that is no more than a few hundred years old. In the fossil dune, sand-sized particles comprise 70 to 80% of the soil with noticeable clay formation, while in the recent dune soils are mostly pure sand (>95%). Both dune soils have very high water infiltration rates in comparison with most other soil types and both are also comparatively poor in nutrients (Kellman & Roulet, 1990; Moreno-Casasola, 2006).
The biological station and its protected area were established in 1977 when its 71.4 ha were mainly undisturbed native vegetation. In 1995 an adjacent, irregular 11.9 ha polygon (covered mainly by pastures) was added to the reserve. Currently, CICOLMA covers 83.3 ha, and the main vegetation types are semi-deciduous tropical forest (43.7 ha) and coastal dune scrub (24.5 ha), with the rest comprised of several small patches of different vegetation types, including wetlands and low stature tropical dry forest or scrub (Moreno-Casasola, 2006). In the area added to the reserve in 1995, there is a plot of approximately 6 ha (±200 × 300 m) that, prior its annexation, was used for over 20 years as a pasture for cattle and was planted with the African grass Panicummaximum. In 1995, the cattle were removed from this plot in order to start a long term study of vegetation change. Ten years later, in 2005 a secondary forest, 8 to 10 m tall, had covered most of the plot, except for a 90 × 60 m patch in one of the corners that still was covered by grasses. Five of the seven habitats in which seeds were sown in this study, were located inside this experimental plot (details below). The grassland patch within the 6 ha abandoned plot, allowed us to trace transects that represented a vegetation gradient of increasing structural complexity, from tree-less habitat covered by grasses, to habitats with incipient and recent tree colonization at the edge of the grassland-secondary forest, up to densely shaded habitat in the interior of the 10 year old secondary forest; which roughly resembles a successional gradient (Fig. 1).
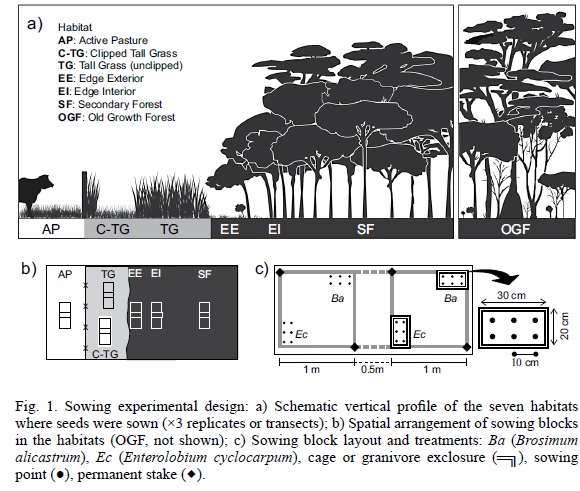
Species selected and experimental design
The two species selected -Brosimum alicastrum and Enterolobiumcyclocarpum- are among the most common and largest trees of the semi-deciduous tropical forest of the region. Brosimum (hereafter only the genus will be used) is a common canopy tree in both the humid and dry tropical forests of Mexico and Central America. Its seeds (13 to 20 mm in diameter) are dispersed by vertebrate frugivores, mainly bats and other mammals (Peters, 1991; Pennington & Sarukhán, 1998). Enterolobium is a deciduous tree common in lowland tropical areas from Mexico to the northern part of South America, and is also relatively common in moist and dry tropical forest. Even though it can form part of the canopy of old growth forest it is also common in open conditions, including savannas and pastures. Its presence in forested patches is usually taken as a sign of past disturbance and it is commonly regarded as a mid to late secondary species (Blain & Kellman, 1991; Pennington & Sarukhán, 1998; Williams-Linera et al., 2011). Enterolobium seeds (15 to 20 mm in length; 10 to 12 mm wide) are mainly dispersed by ruminants, including domestic livestock and occasionally by hoarder rodents (Janzen et al., 1985); hydrochory may also be an important dispersal mechanism where flooding occurs (Enterolobium pods float; Hunter, 1989).
Seeds were collected between mid-July and early-August in 2005. Most Brosimum seeds were collected from beneath the canopy of five large trees, and ca. 20% of them were collected under a compact group of mango (Mangifera indica) trees which were being used as feeding roosts by bats. Seeds were carefully picked, choosing only those from which the fruit pulp had been completely removed by frugivores and had recently fallen to the ground. Enterolobium pods were collected below the canopy of three large trees. At the time the seeds were collected, 25 to 30% of the ripe pods of the 2005 crop were still on the tree. Only large, recently fallen pods were collected. Seeds were extracted from the pods and cleaned under water to remove the thin pulp and only large, well formed seeds were selected.
Seeds were sown in seven distinct habitats which represent a gradient of vegetation cover and successional development from active pasture to 10-year-old secondary forest, and a protected old growth forest (Fig. 1). The 6 ha experimental plot described above was central to the layout of the experimental sowing blocks of this study. The first habitat was an active pasture (i.e. a pasture with grazing cattle; hereafter, AP) adjacent to the experimental plot and outside the limits of CICOLMA. The cattle were being raised using the management practices common in the region, with a stocking density of one adult cow per hectare (for more details see Moreno-Casasola, 2006). Pastures in the area have low densities of isolated shade trees and are dominated by an African grass Panicummaximum, which when grazed properly grows dense foliage 5 cm in height. When overgrazed, the cover of P. maximum is reduced while that of ruderal herbs and native grasses (Paspalum and Axonopus) increase. The other five habitats were located inside the 6 ha experimental plot: two within the patch still covered by grasses, another two at the border between the secondary forest and this grassland patch, and another inside the secondary forest (Figs. 1a, 1b). The grassland patch was dominated almost exclusively by P.maximum, which in the absence of cattle grows densely and surpasses 1 m in height, reaching up to 2 m during the rainy season. We called this the tall grass habitat (TG), and sowed seeds directly beneath the grass without disturbing it. Two meters away from TG, we established a paired sowing site where we regularly clipped the grassland turf to keep it at a height less than 5 cm during the first year of the study (i.e. until September 2006) and called this the clipped tall grass (C-TG) habitat, where competition with the grass foliage was minimized.
The next habitat was at the outer edge of the secondary forest, beneath the canopy of the outer line of colonizing trees, less than 5 m away from the open grassland, and we called this the edge exterior (EE) habitat. Seeds were sown in EE beneath the single, sparse layer of woody cover (1-3 m tall), under which sparse and relatively short (<20 cm tall) tufts of grass and heliophytic herbs (50 to 60% of ground cover) were growing, the rest was bare soil. The next habitat was the edge interior (EI), situated 10 to 15 m away from the grassland, on the inside of the outer line of trees that formed the forest edge. The sowing sites of this habitat were shaded by several trees up to 5 m tall, and on the ground heliophytic herbs and grasses were rare (<10% ground cover). The next habitat was the interior of the 10-year-old secondary forest (SF) for which we placed the sowing sites more than 50 m away from the forest edge. The tree canopy at SF sites was 10 m high, with a dense layer of tree foliage 5 to 10 m above the ground. The ground in SF sites was completely covered with leaf litter and devoid of heliophytic herbs and grasses. Vegetation sampling in this secondary forest in 2006 (eleven years after abandonment) recorded 55 species of woody plants with a DBH ≥2.5 cm and a stem density of 12.6 to 18.8 stems per 100 m² (unpublished data). Three anemochorous woody species -Diphysarobinioides, Mimosatricephala and Cedrelaodorata- were dominant, accounting for 36.6% of the abundance. A mere 10% of woody stems with DBH ≥2.5 cm belonged to zoochorous tree species, all of which have relatively small seeds (<5 mm in diameter), hence the need to enrich this stand with large-seeded zoochorous tree species. The seventh habitat was the interior of an und isturbed old growth forest (OGF) that has been protected since 1977 in CICOLMA (≈550 m away from the other six habitats). Sowing blocks in OGF were placed more than 70 m away from any canopy light gap or forest edge, and had a 20 m tall primary forest canopy over them that reached maximum foliage density between 12 and 18 m above the ground.
Each of our experimental sowing blocks measured 2.5 × 1 m and consisted of two 1 m² square quadrats separated by 0.5 m (Fig. 1c). Within each 1 m² quadrat, Brosimum and Enterolobium were sown in opposite corners placing six seeds of each in two rows of three sowing points, 10 cm apart from each other. At each sowing point one seed was carefully buried 1 cm below the ground. To protect seeds from vertebrate seed eaters, in one quadrat the seeds were protected by a rectangular wire cage measuring 30 × 20 cm and 20 cm in height with a 0.5 cm pore size (granivore exclosure), and in the accompanying quadrat seeds were also buried but left unprotected. Four permanent stakes (Fig. 1c) marked the sowing block's position and allowed for the exact re-location of each sowing point (i.e. all seeds sown). In the different habitats, the sowing blocks were loosely arranged along three transects, each extending from the grassland patch into the secondary forest interior (Fig. 1b), i.e. there were three replicates (sowing blocks) for each habitat. In total, 252 seeds were sown per species. The distance between replicates in the same habitat varied: 40 to 45 m for the tall grass habitats (TG and C-TG); 50 to 55 m for the forest edge habitats (EE and EI) and 90 to 100 m for the OGF, SF and AP habitats.
All seeds were sown from 19 to 22 of August 2005. After sowing, each block was visited weekly during the 1st month, then every 15 days from the 2nd to 4th month and then once per month until May 2006, the last survey of the first year was done in mid-September 2006. In May 2010, almost five years after sowing, the experimental blocks were re-visited for final inspection. It is important to mention that the active pasture (AP) was abandoned in 2008 and its owner stopped raising cattle in there, so in 2010 when we re-visited this site, it had been abandoned for 2 years.
On every field visit, for each sowing point we recorded whether a seedling had emerged or not. Measurements for each emerged seedling were stem diameter (mm) at 3 cm above ground; height (cm) to the final meristem; and seedling leaf area (L.A. in cm²), estimated by counting the number of leaves in each of three size categories (see below). The date of the field visit when a seedling was found dead was assumed to be the date of death for the survival analysis. When possible, the most likely cause of death was recorded; i.e. herbivory by vertebrate or invertebrate; dehydration; etc.; but if the cause of death was not clear, it was recorded as unknown. To estimate seedling leaf area, we counted the number of leaves in three size categories: small, medium and large; which, for each species, equally divided the range of leaf sizes up to the mean size of the adult leaf into thirds. Only leaves larger than 4 cm long and 1.5 cm wide were taken into account. The outline of several leaves of each size category (20 to 30 per category) was drawn on a piece of paper, directly from the seedling without damaging the leaf. The silhouette of each leaf was placed on a sheet of millimeter graph paper to measure its area to the nearest mm2 and estimate the mean area of each size category for each species: 15.96, 27.96 and 47.13 cm², respectively for the small, medium and large leaves of Brosimum, and 27.66, 76.30 and 183.68 cm² for Enterolobium. The number of leaves in each size category was multiplied by the respective mean area value to obtain the L.A. of each seedling.
Data analysis
Seedling emergence was analyzed separately for each species using a two-way ANOVA, with habitat (7 sites) as one factor and the exclosure treatment (inside vs. outside the cage) as the other. Average values of % emergence per habitat-treatment were arc-sin transformed to meet the assumptions of the ANOVA. When statistical differences were detected, a post-hoc multiple contrast Tukey test was used to compare all habitat-treatment combinations. Regardless of emergence date, all emerged seedlings were taken into account for the analyses. Since the wire cages had been placed in the field for the purpose of excluding seed eaters, they were removed when at least one seedling was close to reaching the cage roof. At the end of November 2005 (3 months after sowing) all of the cages had been removed and all seedlings (i.e. from both inside and outside the cages) were used in the seedling survival analysis.
Seedling survival during the first year (September 2005-2006) was analyzed with Kaplan-Meier non-parametric test, comparing survival curves among the 7 habitats for each species separately. The analysis was done in MINITAB© (Release 14.2) using the right-censored non-parametric survival analysis module. Since we do not know what happened between September 2006 and May 2010 (sowing blocks were not visited), the survival data for that period were excluded from the statistical comparison of survival. However, we describe and illustrate the survival results up to May 2010 for each species.
For the comparison of seedling size among habitats, only those habitats with live seedlings in 5 or 6 of their sub-blocks (i.e. groups of 6 sowing points) were included in the statistical analysis. In order to ensure the total independence of measured seedlings and reliable representativeness per habitat, only one seedling from each of the six sub-blocks was used to calculate the average seedling size per habitat, selecting the seedling with the least apparent damage and that was least overshadowed by neighboring seedlings. In spite of the small sample size per habitat (n = 5 or 6), we expected to detect statistical differences in seedling size if the conditions for growth were different among habitats. Since the seeds of both Brosimum and Enterolobium are relatively large, and their seedlings are nurtured by their cotyledons for several months, we only compared seedling sizes after one year of growth (September 2006), and after 4.7 years (May 2010). Comparisons among habitats for each species and size variable were done separately using an ANOVA. In habitats with live seedlings in only four independent sub-blocks, average size values are given in Results for illustrative purposes, but were excluded from statistical comparisons. In habitats where there were fewer than four independent seedlings alive, the actual values of the two largest seedlings are given.
RESULTS
Seedling emergence
A total of 189 (75%) seedlings of Brosimum emerged from the 252 seeds sown. For Enterolobium, 151 (60%) seedlings emerged. Average percent emergence of Brosimum seedlings was significantly different among habitats (2-way ANOVA; F(6,28) = 6.46; P< 0.001), but did not differ between exclosure treatments (outside vs. inside cages; F(1,28) = 2.59; P = 0.119). The interaction between these factors (habitat and exclosure) was not significant (F(6,28) = 0.55; P = 0.763). Brosimum's emergence was significantly lower in the C-TG habitat (avg. emergence <40%) than in all other habitats, which had no significant differences among them. Average emergence for Brosimum surpassed 90% in two habitats: EI and EE (Fig. 2a). Seedling emergence of Enterolobium was not significantly different among habitats (F(6,28) = 1.57; P = 0.194) or between exclosure treatments (F(1,28) = 0.93; P = 0.344). Emergence tended to be high in AP (>80%), followed by SF (>70%), and in the remaining habitats average emergence was 40 to 70%, however within each of these habitats emergence success varied widely (as shown by the length of S.E. bars in Fig. 2b), explaining why there were no statistical differences among habitats for Enterolobium emergence.
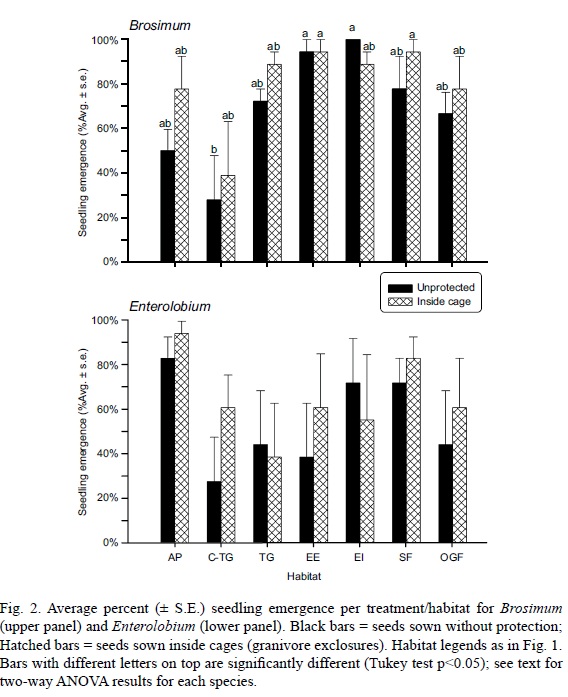
To learn the fate of the seeds which failed to emerge, we did the following in September 2006 (one year after sowing): in the sub-blocks where three or more of the six seeds failed to emerge (10 of 42 sub-blocks in Brosimum; 16 of 42 in Enterolobium) two of the failed sowing points were randomly chosen to look for the seed and inspect it. In all cases the seed was recovered and had no apparent damage (no rotting, no herbivore attack). It was not possible to determine why these seeds failed to germinate, while other conspecific seeds, sometimes in neighboring sowing points, germinated successfully.
Seedling survival
Overall survival after one year (in September 2006) was 58.5% and 21.9% of emerged seedlings for Brosimum and Enterolobium, respectively. In Brosimum, seedling emergence started during the fourth week of September 2005 (5 weeks after sowing), by the first week of November 94% of total emergence had occurred and one week later emergence was almost complete. To facilitate the survival analysis, October 15, 2005 was designated as the start of emergence of Brosimum seedlings, with their first survival survey taken one month later. For Enterolobium, emergence started in the first week of September, 2005 (2 weeks after sowing), by the end of September 81% of total emergence had occurred and one week later was almost complete. September 12, 2005 was designated as the start of emergence for Enterolobium, with their first survival survey taken one month later. Seedlings that emerged after November 2005 (two Brosimum, five Enterolobium) were regarded as late emergences and excluded from survival analysis.
Comparison of survival among habitats
The survival curves for the two species were significantly different among habitats (Fig. 3). For Brosimum, the Kaplan-Meier survival analysis shows that up to September 2006, survival curves were significantly different among habitats (log rank test; Xi2 = 95.98; d.f. = 6; P<<0.001). The probability of survival to September 2006 (341 days after the start of emergence) was higher than 80% in SF, OGF and EI (93.6%; 92.3%; 82.3%, respectively), and significantly higher than in the other four habitats (non-overlapping 95% Confidence Intervals). The probability of survival to 341 days was moderate in the EE and TG habitats (48.5% and 44.8%, respectively), while in AP and C-TG none of the Brosimum seedlings survived to this date (Fig. 3a). It is noteworthy that up to May 2006 (222 days after emergence), the probability of survival was very high in TG (82.8%), but dropped dramatically (by 50%) four months later. Brosimum survival to May 2010 (4.7 years after emergence) was highest in EI and SF (73.5% and 71.0%, respectively), intermediate in OGF (53.8%) and lowest in EE (36.4%). None of the Brosimum seedlings survived to May 2010 in TG.
Enterolobium survival curves to September 2006 were also significantly different among habitats (log rank test; Xi2 = 99.65; d.f. = 6; P<0.001). The probability of survival to September 2006 (371 days after the start of emergence) was highest in C-TG and AP (62.5% and 59.4%, respectively), and significantly higher than in the other five habitats (non-overlapping 95% C.I.). Survival probability to 371 days was 7.4% in SF and 5.3% in EI, none of the seedlings survived to this date in TG, EE or OGF (Fig. 3b). Up to May 2006 Enterolobium survival was much higher in the non-woody habitats (AP, C-TG and TG). In the shaded woody habitats (see Fig. 1), seedlings of Enterolobium that had emerged, died relatively quickly, usually surviving less than four months. The latter was particularly apparent in OGF (Fig. 3b). Enterolobium seedlings only survived to May 2010 (4.7 years after emergence) in AP and in C-TG, with just a few live seedlings in each habitat (15.6% and 12.5%, respectively).
Causes of seedling mortality during the first year
By September 2006, 77 Brosimum seedlings had died. The most common cause of death in this species was attack by herbivores (51% of deaths), which was particularly high in AP and EE (Fig. 4a). Cattle was responsible for all of the herbivory deaths in AP, while in EE the main herbivore was an undetermined vertebrate (possibly rabbits or iguanas, though we were unable to identify the animal that had bitten and killed the seedlings). Caterpillars and snails also killed a few Brosimum seedlings in EE, and in EI, SF and OGF. The second most important cause of death in this species was wilting during the dry season (35% died of dehydration), which was particularly common in non-wooded habitats (TG, C-TG and AP).
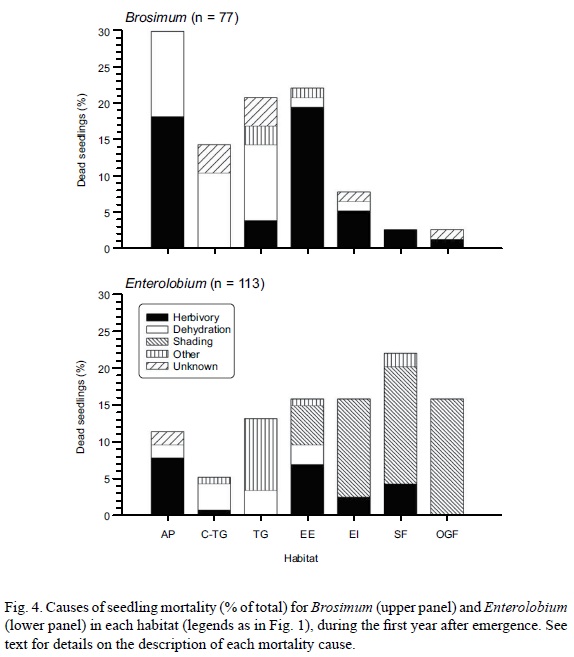
One hundred and thirteen Enterolobium seedlings had died by September 2006. Insufficient light (shading) was the main cause of death (50%), and was particularly high in habitats with dense woody cover (OGF, SF and EI) when tree foliage was densest. Herbivory was second in importance (23%), again notably higher in AP (repeated grazing by cattle) and EE (unknown vertebrate). In TG several Enterolobium seedlings died during the 2006 rainy season (between June and September), outcompeted by the dense growth of the P. maximum foliage during those months (classified as 'other' causes of death in Fig. 4b). Dehydration during the dry season of 2006 was a less important cause of death (11.5%) for Enterolobium seedlings (Fig. 4b) than for Brosimum (Fig. 4a).
Seedling growth
In September 2006 there were no Brosimum seedlings alive in either AP or C-TG. In the remaining five habitats the average size of 1-year-old Brosimum seedlings was significantly different in stem diameter (F(4,23) = 3.41; P = 0.025) and in L.A. (F(4,23) = 3.91; P = 0.015), but not in average height (F(4,23) = 1.08; P = 0.388). Taking all three size measurements together, Brosimum seedlings performed better in the EI habitat (Table 1), while the least robust 1-year-old seedlings were found in TG and in OGF. After four years, in May 2010 there were no live seedlings in TG, and the largest and most robust 4-year-old seedlings now were those of EE and SF, while those growing in OGF were the smallest (Table 1).
Only two habitats (AP and C-TG) had enough live Enterolobium seedlings in September 2006 to compare seedling size (see Methods). After one year of growth, Enterolobium seedling size was not significantly different between AP and C-TG for any of the measured variables: stem diameter (t = 1.43; d.f. = 8; P = 0.190), height (t = 0.54; d.f. = 9; P = 0.601) or L.A. (t = 0.30; d.f. = 6; P = 0.773). In September 2006, there were no live Enterolobium seedlings in TG, EE or OGF, and only a few had survived in EI and SF so these habitats could not be included in the statistical comparisons; however the two largest seedlings in each EI and SF were clearly smaller than those growing in AP and C-TG (Table 1). Four years later, in May 2010 there were too few seedlings still alive to make valid comparisons of seedling size between habitats. However, the four independent sub-blocks of AP with live 4-year-old Enterolobium seedlings had the highest average values of seedling size for the whole experiment. In C-TG the two largest seedlings of Enterolobium that survived to May 2010 had the widest stem diameter (>40 mm at 3 cm from the ground), were the tallest (>150 cm) and had the largest leaf area (>2 m2 of canopy cover) recorded in this study.
DISCUSSION
Seedling emergence
In comparison to similar studies, we detected a relatively high success in seedling emergence for both Enterolobium and Brosimum. This is explained in part by our careful selection of viable seeds, which involved collecting only fruit or seeds recently fallen or dispersed from the mother plant, sowing only those with good appearance and size. Another contributing factor was that 2005, the year we sowed the seeds, was quite favorable for seed germination. That year precipitation (1623 mm) was 27% higher than the annual mean (1286 mm/yr), and the rain that fell from August to October (1034 mm in three months) was double the norm for that period (515.3 mm for Aug-Oct; based on the monthly means from 23 years of data). At the time we sowed the seeds (August 2005) the soil was saturated and two months later most of the seedlings (97%) had emerged.
Several studies report that the hard, woody Enterolobium seeds must be scarified in order to germinate (Janzen et al., 1985; Blain & Kellman, 1991; Salinas, 1992). However, in this study, we sowed Enterolobium seeds without scarifying them and emergence was high. This could also be related to the abundant precipitation of 2005 since it has been reported that the pod of this species can absorb a lot of water and transfer it to the seeds which become imbibed before the seed coat hardens (Hunter, 1989). On collecting the pods from the ground, we noted they were swollen with water as were the seeds within them and this may have made scarification unnecessary given that the seed coat was not hard enough to be impermeable.
The lack of seed removal or predation by granivores in our study is an unexpected result. Predation on tree seeds by granivores has been repeatedly mentioned as one of the main barriers to forest regeneration in abandoned cropfields and pastures (Holl, 1999; Janzen, 2002; Peña-Claros & de Boo, 2002) and one of the main impediments to directly sowing large seeds during the early phases of secondary succession (Campana-Camargo et al., 2002; Doust et al., 2006; García-Orth & Martínez-Ramos, 2008). Previous studies within the semi-deciduous tropical forest of our study area have reported high rates of seed predation for different tree species, including Brosimum and Enterolobium by the red land crab Gecarcinuslateralis (Delfosse, 1990), which also preys on the recently emerged seedlings of both tree species (Salinas, 1992; Capistrán-Barradas et al., 2006; see also Lindquist & Carrol 2004 for an example in coastal tropical forest of Costa Rica). Our sowing blocks in the OGF habitat were situated in the same forest patch studied by Delfosse (1990) and later by Capistrán-Barradas and colaborators (2006), so we were expecting a high rate of seed predation by this land crab, at least in the OGF habitat. The granivorous rodents Peromyscusmexicanus and Lyomispictuspictus are abundant at our study site, and the latter is particularly abundant in agricultural habitats dominated by grasses (Cervantes & Hortelano, 1991), however during our study none of the sowed seeds were removed by these rodents or any other animal. Our results indicate that seed predation is not always an impediment to direct seeding or forest recovery. Some authors have found that seed burial substantially reduces the rate of seed removal and predation by granivores (Doust et al., 2006; Cole et al., 2011), however, the reduction they report is not absolute, much less in the case of highly nutritional seeds such as those of Brosimum which are actively sought by rodents (Sánchez-Cordero & Martínez-Gallardo, 1998; García-Orth & Martínez-Ramos, 2008). Therefore, the lack of seed removal in our study, might be explained by a very low density of granivores during 2005, however we do not have field data on rodent or land crab abundance for our study period to test this possibility. Even if the lack of predation was particular to the year we did our study, it represents an important window of opportunity for the seedlings that have managed to overcome other barriers to establishment. However, seed predation is notoriously site specific and variable over time (Hulme & Benkman, 2002) and therefore this aspect of our results should be interpreted with caution.
Of the seven habitats, only in C-TG was Brosimum seedling emergence significantly lower than in the other habitats. In C-TG, it appears that frequently cutting the grass had a negative effect on Brosimum's germination, however we cannot offer a conclusive explanation for this. One possibility is that cutting the grass down to 5 cm exposed the soil of these blocks to extreme desiccation at midday, even during the rainy season, and that this affected the Brosimum seeds, but not those of Enterolobium. In agreement with Cole et al. (2011), we found that germination success was relatively uniform among different types of habitat. Other studies in degraded tropical sites have found differential success in seedling emergence among different habitats (Campana-Camargo et al., 2002; Bonilla-Moheno & Holl, 2010), however these studies did not control for granivory so it is not possible to know whether the differences resulted from variation in seed predation intensity or differing germination conditions.
Our results suggest that directly seeding large-seeded forest canopy trees could be highly successful during wet years and that burying the seeds, in the way we did (1 cm below ground), is a good strategy. Seed burial also enhances germination by promoting better contact between the seed and the humid soil (Doust et al., 2006; García-Orth & Martínez-Ramos, 2008). In areas with high rates of seed removal or intense predation by granivores, we strongly recommend protecting the seeds with exclosures similar to ours (see Methods), as they are relatively cheap to make and easy to install, and can be easily removed after seedling emergence and reused. In spite of the relatively high germination rates of both tree species in practically all of the habitats studied, there were differences in seedling survival and growth.
Seedling survival and growth
The habitats included in our study represent both a spatial and a temporal gradient with clear contrasts in vegetation structure and microclimatic conditions (Fig. 1), and the two tree species studied showed divergent trends in survival and growth across these habitats. Survival and growth for Enterolobium seedlings was much better in open habitats than under woody cover. Even the light shade of the EE sites notably reduced Enterolobium survival. For Brosimum, the reverse trend was found; its seedlings survived and grew better under a woody canopy than in the open. Several researchers have pointed out that the dichotomy of shade-tolerant vs. intolerant tree species, so important in gap-dynamics and the regeneration of humid tropical rainforest, is not as important in tropical dry forest which sheds most of its leaves during the dry season, thus allowing direct sunlight to reach the forest floor where tree seedlings are growing (see reviews by Quesada et al., 2009 and by Griscom & Ashton, 2011). Thus, our divergent results on the shade tolerance of Enterolobium and Brosimum seedlings may be specific to the selected species and the semi-deciduous forest of our study site. Some non-deciduous tree species attain relatively high densities in the forest canopy and sub-canopy of our study site (Moreno-Casasola, 2006), and keep understory light levels low throughout the year. This may limit the extrapolation of our results to more strongly seasonal forests at drier sites. However, we agree with Griscom and Ashton (2011) and would like to point out that more site specific experimental studies like ours are needed in different regions of the dry tropics to fully document and understand the seedling ecology and regeneration requirements of native tree species in these regions. We would also like to emphasize that tree seedlings in seasonal tropical forest emerge at the start of the rainy season (Quesada et al., 2009), and soon afterwards the forest canopy reaches maximum foliage density. Therefore during the first months of their lives tree seedlings have to cope with low levels of solar radiation even in totally deciduous tropical dry forest.
Our results for Enterolobium's survival coincide with those of Blain and Kellman (1991) in the same study area, and show that the seedlings of this tree do not tolerate the shade of other woody plants and need direct sunlight to survive. In contrast, on the Yucatan peninsula -where tropical dry forests are more seasonal and deciduous- Bonilla-Moheno and Holl (2010) found that Enterolobium survival was higher in young secondary forest (8-15 years old) and older forest (>50 years) than in more open recently abandoned (<5 years) milpa fields. In our study the optimal habitats for Enterolobium seedlings were AP and C-TG where, in addition to full light conditions there was relatively little interference by the grass foliage (the growth of which was repeatedly interrupted by grazing cattle in AP and experimentally by us in C-TG). This was true during the first year of growth, however, four years later Enterolobium survival decreased considerably in both habitats. The turf in C-TG was clipped until September 2006, after which the grass quickly recovered, surpassing 1 m in height during the rainy season of 2007, and outcompeting most of our seedlings by 2010. The benefits of clipping the turf during the first year did not last long. Our results suggest that at least another year of clipping would be necessary to enable the Enterolobium seedlings to grow above the tall grass foliage and sustain a high survival rate. Nevertheless in the grassy habitat of C-TG (with clipped turf during the first year of growth and no livestock), the few Enterolobium seedlings that reached four years of age were the largest and most robust of the whole experiment, and at four years their crowns had already completely shaded the surrounding grasses. Of all the seeds of both species that we sowed, these seedlings had the highest probability of being recruited as adults. In AP mortality increased after September 2006, in part because the cattle kept browsing on the live seedlings during 2007, but also because the owner of the pasture cut our seedlings with a machete that same year, reducing them to naked stumps 15 to 20 cm in height, before this pasture was abandoned in 2008.
Brosimum survival was higher in habitats with the densest woody shade (EI, SF and OGF), while the largest seedlings after four years of growth were found in EE and SF. The best habitat for Brosimum was the interior of the secondary forest (SF) where its survival and growth were highest, coinciding with results of Bonilla-Moheno and Holl (2011) for Brosimum seedlings in secondary forests of the Yucatan peninsula. In spite of the relatively high survival in the undisturbed forest (OGF) four-year-old seedlings in this habitat were notoriously smaller than those growing in secondary forest habitats (EE and SF). The foliage of the EI canopy was very dense, particularly between 3 and 5 m above ground, and produced dense shade. In SF, the foliage was also very dense but between 5 and 10 m above ground. This canopy allowed oblique rays of light to reach the ground and these favored the growth of Brosimum. The plants that survived four years in EE where arboreal shade was very light, were as large as the seedlings in SF, and in these two habitats Brosimum grew the most.
Herbivory considerably reduced seedling survival for both species in almost all of the habitats, but more notably so in AP and EE. Brosimum was more affected because its seedlings have a much lower resprouting capacity after being attacked by herbivores than do those of Enterolobium, all of which needed repeated attacks before they failed to resprout, and died. Some Enterolobium seedlings even re-sprouted after being cut by machete in 2007 in the AP habitat and grew to a height of over 1 m by 2010. Protection from herbivory during the first years of seedling growth would drastically increase survival, therefore the use of fences, exclosures or other devices that deter herbivores is highly recommended in forest restoration efforts (Holl & Quiros-Nietzen, 1999; Griscom et al., 2005; Ortega-Pieck et al., 2011). Our results also show that Enterolobium seedlings were more drought resistant than those of Brosimum. During the dry season of 2006 several Brosimum seedlings were not able to survive in the habitats with the shortest and thinnest vegetation cover (AP and C-TG), where the loss of soil humidity at midday was highest (pers. obs. JL).
The tall, dense foliage of the African grass Panicum maximum had a peculiar effect on seedling survival for both species in TG. During the dry season of 2006 (February to May) it had a positive effect on Brosimum but no effect on Enterolobium, as revealed by comparative data from the clipped treatment in C-TG (Fig. 3). The foliage of this grass reduces the solar radiation that arrives at the soil, and ameliorates the harsh and extreme conditions (wide fluctuations in temperature and humidity) that prevail at totally open sites (see also Ortega-Pieck et al., 2011). Some studies report that herbaceous plant cover in open sites can have a favorable effect on the survival of woody plants' seedlings by improving an adverse microclimate (Aide & Cavelier, 1994; Hooper et al., 2002). This seems to have happened at our study site in TG until May 2006, which is when the dry season ends. However, the positive effect of the exotic grass was only temporary given that once the rains and the growing season began, the survival of both Brosimum and Enterolobium seedlings decreased notably in TG, due to the quick and massive growth of the exotic grass that outcompeted most of our seedlings. Any potential benefit during the dry season would be reversed during the rainy season if the growth of the grasses is not checked, particularly if the woody seedlings have not yet grown taller than the grass foliage. The African grass species which are commonly cultivated in man-made pastures in the Neotropics have very fast growth rates and accumulate foliage biomass very quickly once domestic herbivores are removed, thus hampering the establishment of woody seedlings in abandoned pastures (Hooper et al., 2002; Janzen, 2002; Ortega-Pieck et al., 2011; see also review in Griscom & Ashton, 2011).
Enrichment of secondary forest by direct seeding: implications for forest recovery
In agreement with other studies done in different tropical regions our results show that direct seeding is a reliable mechanism for accelerating and directing secondary succession into desired outcomes, markedly increasing the potential of attaining floristic composition and relative abundances similar to those of old growth forest more quickly (García-Orth & Martínez-Ramos, 2008; Bonilla-Moheno & Holl, 2010; Cole et al., 2011). In comparison with transplanting seedlings produced in nurseries, direct seeding is a much cheaper practice for accelerating forest recovery (Cole et al., 2011) and can be repeated by the local inhabitants as often as neccesary until there is a good wet year. Our results also show that different habitat types influence the success of direct seeding depending on the ecology of seedling establishment of the tree species involved.
As mentioned earlier, the preferred tree species for the enrichment of secondary forest are usually large-seeded trees that reach the canopy of old growth forest. The two species chosen in our study have contrasting requirements for the successful establishment of their seedlings and could thus be regarded as models for other tree species. Enterolobium cyclocarpum represents mid to late secondary species which can gain a place in the secondary canopy and occupy it for several decades or even centuries. The seedlings of these tree species usually thrive in full light conditions and are absent from the shaded understory of undisturbed forest. Their seeds could be sown in the agricultural plots or degraded sites to be restored, during or soon after abandonment, when the plot is covered by herbaceous plants and woody cover is minimal or absent. In abandoned pastures, the biomass of fast growing grasses needs to be reduced around the seedlings until they can grow taller than the grasses. If a closed woody canopy has already formed, but enrichment with late secondary tree species similar to Enterolobium is the goal, then a canopy gap must be opened in the secondary forest when the seeds are sown. This gap should be large enough to allow direct light to reach the ground and it must be kept open for several years without affecting the established seedlings, by carefully removing or girdling the shading trees (see Ramos & del Amo, 1992).
Large canopy tree species that are ecologically similar to Brosimumalicastrum, have shade tolerant seedlings and saplings which usually form seedling banks in the forest understory. These could be seeded as soon as a woody cover has formed in the abandoned pasture or cropfield to be restored. If the secondary canopy becomes too dense, seedling survival may be high but growth could slow down or stop. In this case, selective thinning of the secondary canopy may be required to accelerate seedling and sapling growth, the idea being to eliminate abundant secondary species until a thin canopy shade is left over the desired tree seedlings.
Local farmers use and manage secondary forests in many ways, and obtain many plant and animal products along with other environmental services from them (Challenger, 1998; Chazdon, 2003). Firewood is extracted from secondary forest throughout the tropics, and this practice could be easily adjusted or re-directed toward the selective thinning of the secondary canopy mentioned earlier. Patches of secondary forest that are poor in tree species and dominated by a few fast growing species, could be targeted for firewood extraction performed in such a way as to leave a thin secondary canopy under which the seeds of tree species with shade tolerant seedlings (such as Brosimum) could be directly seeded, with recurrent and selective thinning done until the desired slow-growing tree species are well on their way to becoming adults. Firewood can also be extracted in ways that create and maintain relatively large canopy gaps in secondary forests in order to sow the seeds of tree species whose seedlings and saplings need direct sunlight, such as those of Enterolobium. Future manipulations of the secondary canopy at our study site will allow us to make more specific recommendations and fine tune the degree to which the secondary canopy can be thinned without compromising the survival and growth of the preferred tree species.
ACKNOWLEDGEMENTS
Many thanks to the La Mancha Ecoguides (David Díaz, Enrique Romero, Omar Romero, Alejandro Sandria, Agueda Díaz, Hector Barradas) and to Graciela Sánchez-Rios for help in the field. We are grateful to CICOLMA's dedicated personnel: Enrique López-Barradas, Anastacio García and Fernando Aguilar, for logistic support. We are grateful to Bianca Delfosse for translating parts of the manuscript into English, style revision, and for helpful comments. Thanks to Kerenha Hernández for drawing the vegetation profile of Figure 1. This study was generously supported by the Consejo Nacional de Ciencia y Tecnología (CONACyT project: DDAJI-100/029/06) and by the Instituto de Ecología A.C. (project: INECOL 902-11-281).
LITERATURE CITED
Aide, T. M. & J. Cavelier. 1994. Barriers to lowland tropical forest restoration in the Sierra Nevada de Santa Marta, Colombia. Restor. Ecol. 2: 219-229. [ Links ]
Blain, D. & M. Kellman. 1991. The effect of water supply on tree seed germination and seedling survival in a tropical seasonal forest in Veracruz, Mexico. J. Trop. Ecol. 7: 69-83. [ Links ]
Bonilla-Moheno, M. & K. D. Holl. 2010. Direct seeding to restore tropical mature-forest species in areas of slash-and-burn agriculture. Restor. Ecol. 18: 438-445. [ Links ]
Campana-Camargo, J. L., I. D. Kossman & A. M. Imakawa. 2002. Rehabilitation of degraded areas of central Amazonia using direct sowing of forest tree seeds. Restor. Ecol. 10: 636-644. [ Links ]
Capistrán-Barradas, A., P. Moreno-Casasola & O. Defeo. 2006. Post-dispersal fruit and seed removal by the crab Gecarcinus lateralis in a coastal forest in Veracruz, Mexico. Biotropica 38: 203-209. [ Links ]
Challenger, A. 1998. Utilización y conservación de los ecosistemas terrestres de México: pasado, presente y futuro. Comisión Nacional para el Conocimiento y Uso de la Biodiversidad, Universidad Nacional Autónoma de México, Agrupación Sierra Madre S.C. México, D.F., Mexico. 847 pp. [ Links ]
Chazdon, R. 2003. Tropical forest recovery: legacies of human impact and natural disturbances. Perspect. Plant Ecol. Evol. Syst. 6: 51-71. [ Links ]
Cervantes, F. A. & Y. Hortelano. 1991. Mamíferos pequeños de la estación biológica El Morro de La Mancha. Anales Inst. Biol. Univ. Nac. Autón. Mex., Ser. Zool. 62: 129-136. [ Links ]
Cole, R. J., K. D. Holl, C. L. Keene & R. A. Zahawi. 2011. Direct seeding of late-successional trees to restore tropical montane forest. For. Ecol. Manage. 261: 1590-1597. [ Links ]
Delfosse, B. 1990. The effect of the red land crab, Gecarcinuslateralis, on the litter layer, nutrient availability and seedling recruitment in a semi-deciduous seasonal tropical forest. MSc Dissertation. York University. Toronto, Canada. 176 pp. [ Links ]
Doust, S. J., P. D. Erskine & D. Lamb. 2006. Direct seeding to restore rainforest species: microsite effects on the early establishment and growth of rainforest tree seedlings on degraded land in the wet tropics of Australia. For. Ecol. Manage. 234: 333-343. [ Links ]
Florentine, S. K. & M. E. Westbrooke. 2004. Restoration on abandoned tropical pasturelands– do we know enough? J. Nat. Conserv. 12: 85-94. [ Links ]
García-Orth, X. & M. Martínez-Ramos. 2008. Seed dynamics of early and late successional tree species in tropical abandoned pastures: seed burial as a way of evading predation. Restor. Ecol. 16: 435-443. [ Links ]
Griscom, H. P. & P. M. S. Ashton. 2011. Restoration of dry tropical forest in Central America: A review of pattern and process. For. Ecol. Manage. 261: 1564-1579. [ Links ]
Griscom, H. P., P. M. S. Ashton & G. P. Berlyn. 2005. Seedling survival and growth of native tree species in pastures: Implications for dry tropical forest rehabilitation in Central Panama. For. Ecol. Manage. 218: 305-318. [ Links ]
Guevara, S., J. Laborde & G. Sánchez-Ríos. 2005. Los árboles que la selva dejó atrás. Interciencia 30: 595-601. [ Links ]
Holl, K. D. 1999. Factors limiting tropical rain forest regeneration in abandoned pastures: seed rain, seed germination, microclimate and soil. Biotropica 31: 229-249. [ Links ]
Holl, K. D. & E. Quiros-Nietzen. 1999. The effect of rabbit herbivory on reforestation of abandoned pasture in southern Costa Rica. Biol. Conserv. 87: 391-395. [ Links ]
Hooper, E., R. Condit & P. Legendre. 2002. Responses of 20 native tree species to reforestation strategies for abandoned farmland in Panama. Ecol. Appl. 12: 1626-1641. [ Links ]
Hulme, P. E. & C. W. Benkman. 2002. Granivory. In: Herrera, C. M. & O. Pellmyr (eds.). Plant-animal interactions: An evolutionary approach. Blackwell. Oxford, UK. pp. 132-154. [ Links ]
Hunter, J. R. 1989. Seed dispersal and germination of Enterolobiumcyclocarpum (Jacq.) Griseb. (Leguminosae: Mimosoideae): are megafauna necessary? J. Biogeogr. 16: 369-378. [ Links ]
Janzen, D. H. 2002. Tropical dry forest: Área de Conservación Guanacaste, northwestern Costa Rica. In: Perrow, M. R. & A. J. Davy (eds.). Handbook of ecological restoration, volume 2. Cambridge University Press. Cambridge, UK. pp. 559-583. [ Links ]
Janzen, D. H., M. W. Demment & J. B. Robertson. 1985. How fast and why do germinating Guanacaste seeds (Enterolobiumcyclocarpum) die inside cows and horses? Biotropica 17: 322-325. [ Links ]
Kellman, M. 1974. The viable weed seed content of some tropical agricultural soils. J. Appl. Ecol. 11: 669-677. [ Links ]
Kellman, M. & N. Roulet. 1990. Nutrient flux and retention in a tropical sand-dune succession. J. Ecol. 78: 664-676. [ Links ]
Laurance, W. F., H. E. M. Nascimento, S. G. Laurance, A. C. Andrade, P. M. Fearnside, J. E. L. Ribeiro & R. L. Capretz. 2006. Rain forest fragmentation and the proliferation of successional trees. Ecology 87: 469-482. [ Links ]
Lindquist, E. S. & C. R. Carrol. 2004. Differential seed and seedling predation by crabs: Impacts on tropical forest composition. Oecologia 141: 661-671. [ Links ]
Moreno-Casasola, P. (ed.). 2006. Entornos veracruzanos: la costa de La Mancha. Instituto de Ecología, A.C. Xalapa, Mexico. 574 pp. [ Links ]
Muscarella, R. & T. H. Fleming. 2007. The role of frugivorous bats in tropical forest succession. Biol. Rev. 82: 573-590. [ Links ]
Ortega-Pieck, A., F. López-Barrera, N. Ramírez-Marcial & J. G. García-Franco. 2011. Early seedling establishment of two tropical montane cloud forest tree species: The role of native and exotic grasses. For. Ecol. Manage. 261: 1336-1343. [ Links ]
Pennington, T. D. & J. Sarukhán. 1998. Árboles tropicales de México. Manual para la identificación de las principales especies. 2a ed. Universidad Nacional Autónoma de México-Fondo de Cultura Económica. México, D.F., Mexico. 521 pp. [ Links ]
Peña-Claros, M. & H. de Boo. 2002. The effect of forest successional stage on seed removal of tropical rain forest tree species. J. Trop. Ecol. 18: 261-274. [ Links ]
Peters, C. M. 1991. Plant demography and the management of tropical forest resources: a case study of Brosimumalicastrum in Mexico. In: Gómez-Pompa, A., T. C. Whitmore & M. Hadley (eds.). Rainforest regeneration and management. United Nations Educational, Scientific and Cultural Organization. Paris, France. pp. 265-272. [ Links ]
Quesada, M., G. A. Sanchez-Azofeifa, M. Alvarez-Añorve, K. E. Stoner, L. Ávila-Cabadilla, J. Calvo-Alvarado, A. Castillo, M. M. Espírito-Santo, M. Fagundes, G. W. Fernandes, J. Gamon, M. Lopezaraiza, D. Lawrence, L. P. Cerdeira, J. S. Powers, F. S. Neves, V. Rosas-Guerrero, R. Sayago & G. Sanchez-Montoya. 2009. Succession and management of tropical dry forest in the Americas: review and new perspectives. For. Ecol. Manage. 258: 1014-1024. [ Links ]
Ramos, J. M. & S. del Amo. 1992. Enrichment planting in a tropical secondary forest in Veracruz, Mexico. For. Ecol. Manage. 54: 289-304. [ Links ]
Salinas, P. M. G. 1992. Crecimiento de especies arbóreas de dunas costeras bajo diferentes condiciones de suelo y cobertura. BSc Dissertation. Facultad de Ciencias, Universidad Nacional Autónoma de México. México, D.F., Mexico. 137 pp. [ Links ]
Sánchez-Cordero, V. & R. Martínez-Gallardo. 1998. Postdispersal fruit and seed removal by forest-dwelling rodents in a lowland rainforest in Mexico. J. Trop. Ecol. 14: 139-151. [ Links ]
Terborgh, J. 1999. Requiem for Nature. Island Press. Washington, DC., USA. 256 pp. [ Links ]
Toledo, V. M. 1989. Bio-economic costs of transforming tropical forest to pastures in Latinoamérica. In: Hecht, S. (ed.). Cattle ranching and tropical deforestation in Latinoamerica. Westview Press. Boulder, USA. pp. 67-93. [ Links ]
Williams-Linera, G., C. Alvarez-Aquino, E. Hernández-Ascención & M. Toledo. 2011. Early successional sites and the recovery of vegetation structure and tree species of the tropical dry forest in Veracruz, Mexico. New Forests 42: 131-148. [ Links ]














