Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Acta botánica mexicana
versión On-line ISSN 2448-7589versión impresa ISSN 0187-7151
Act. Bot. Mex no.100 Pátzcuaro jul. 2012
Effect of the spider Peucetia viridans (Oxyopidae) on floral visitors and seed set of Cnidoscolus multilobus (Euphorbiaceae)
Efecto de la presencia de la araña Peucetia viridans (Oxyopidae) en los visitantes florales y la producción de semillas de Cnidoscolus multilobus (Euphorbiaceae)
Angélica M. Arango1,3, Jorge López-Portillo1, Victor Parra-Tabla2, Laura T. Hernández-Salazar4, Jorge E. Morales-Mávil4 & Victor Rico-Gray1,5,6
1 Instituto de Ecología, A.C., 91070 Xalapa, Veracruz, México.
2 Universidad Autónoma de Yucatán, 97000 Mérida, Yucatán, México.
3 Present address: Framboyanes 38, Fracc. Jacarandas, 91637 Las Trancas, Veracruz, México.
4 Universidad Veracruzana, Instituto de Neuroetología, 91190 Xalapa, Veracruz, México.
5 Present address: Universidad Veracruzana, Instituto de Neuroetología, 91190 Xalapa, Veracruz, México.
6 Author for correspondence: vrico@uv.mx, vricogray@yahoo.com
Recibido en enero de 2012.
Aceptado en marzo de 2012.
ABSTRACT
We studied the interaction between the plant Cnidoscolus multilobus, its floral visitors and the predator spider Peucetia viridans. The diet of P. viridans was composed exclusively of arthropods (spiders 32%, insects 68%). Body length of prey was 5.9 ± 1.0 mm, and prey size range was 11.0 ± 0.4 mm (i.e. 0.14-1.3 times larger than the spider). Based on feeding frequency and time available for prey capture and feeding, one spider may capture up to 3.9 prey items per day, depending on the time of year. From June to October 1998 we tested the number of floral visits affected by the presence or absence of spiders (visual effect). Four treatments were tested on inflorescences: (1) no spiders, (2) with spider, (3) with modified spider (carapace painted red), and (4) with decoy spider. We found two patterns depending on the response of floral visitors to "invisible" spider treatments (with and no spiders) and "visible" spider treatments (painted and decoy). These patterns were closely associated with the abundance of visitors. Using panicle enclosures, we estimated the effect of spider presence on seed set. In months with lower abundance of floral visitors (June, July and October), panicles without spiders had significantly more seeds than those with spiders. Whereas in August and September, the months with the highest number of floral visitors, there were no significant differences between treatments. Our results suggest that floral visitors were able to recognize visible spiders and avoid the inflorescences that have them, but were unable to recognize the presence of unpainted P. viridans. Since many of those visitors are potential pollinators, spider presence may indirectly decrease seed set by C. multilobus on months when floral visitors are less abundant.
Key words: extrafloral nectaries, Mexico, tritrophic systems, Veracruz.
RESUMEN
Estudiamos la interacción entre la planta Cnidoscolus multilobus, sus visitantes florales y la araña depredadora Peucetia viridans. La dieta de P. viridans estuvo compuesta exclusivamente por artrópodos (arañas 32%, insectos 68%). El tamaño del cuerpo de las presas fue de 5.9 ± 1.0 mm, y el ámbito de las presas fue de 11.0 ± 0.4 mm (i.e. 0.14-1.3 más grande que la araña). Basándonos en la frecuencia de alimentación y el tiempo disponible para capturar y alimentarse de las presas, una araña puede capturar hasta 3.9 presas por día, esto dependiendo de la época del año. Entre junio y octubre de 1998 probamos si el número de visitas a las flores era afectado por la presencia/ausencia de la araña (efecto visual). Probamos cuatro tratamientos: (1) sin araña, (2) con araña, (3) con araña modificada (carapacho pintado con rojo), y (4) araña falsa. Encontramos dos patrones dependiendo de la respuesta de los visitantes florales a la araña "invisible" (pintada y falsa). Estos patrones estaban cercanamente asociados con la abundancia de visitantes. Utilizando panículas cubiertas, estimamos el efecto de la presencia de las arañas sobre la producción de semillas. Durante los meses con menos abundancia de visitantes florales (junio, julio y octubre), las panículas sin arañas produjeron significativamente más semillas. Mientras que en agosto y septiembre, los meses con el mayor número de visitantes florales, no se encontraron diferencias significativas entre tratamientos. Los resultados sugieren que los visitantes florales pudieron evitar aquellas inflorescencias con arañas vivas visibles, pero no les fue posible reconocer a las arañas sin pintura. Ya que muchos visitantes florales son potenciales polinizadores, las arañas podrían indirectamente reducir el número de semillas en C. multilobus durante los meses cuando los visitantes florales eran menos abundantes y las arañas no estaban saciadas.
Palabras clave: nectarios extraflorales, México, sistemas tritróficos, Veracruz.
INTRODUCTION
Species-level cascades occur within a subset of a community, such that changes in predator numbers affect the success of a subset of the plant species (Polis, 1999; Polis et al., 2000; Abdala-Roberts et al., 2010). Spiders are a major component of the predatory fauna, capturing a substantial fraction of insects in lower trophic levels (Wise, 1993). The predatory activity of spiders can potentially reduce the number of herbivorous insects on plants, either by feeding on them or by scaring them off. Some spiders (e.g., jumping spiders) also forage for the floral and extrafloral nectar offered by plants, which in turn may benefit from the presence of spiders (Pollard et al., 1995; Ruhren & Handel, 1999; see also Romero & Vasconcelos-Neto, 2004). Most spiders sit and wait for their prey, but jumping spiders move around and are aggressive (Ruhren & Handel, 1999). Thus spiders may also play a vital role in plant protection by decreasing the number of herbivorous insects, which in turn allows the plant to allocate more resources to reproduction resulting in a higher seed set (Ruhren & Handel, 1999). However, by feeding on flower visitors and decreasing the number of potential pollinators, spiders can simultaneously have an opposite effect on plant fitness and decrease seed set. For example, the activities of the green lynx spider (Peucetiaviridans, Oxyopidae) benefit the small shrub Haploppapus venetus (Asteraceae), since branches with spiders exhibited lower flower head damage (Louda, 1982). The number of pollinated flowers was also less, but the overall net effect of the spider on the plant was positive (Louda, 1982).
The spider Peucetia viridans exhibits a close relationship with nectaries (both floral and EFN) from shrubs of the genus Cnidoscolus (Euphorbiaceae) (e.g., C. aconitifolius and C. multilobus) in a large portion of its distribution in Mexico (Arango, 2001; Arango et al., 2000; Parra-Tabla et al., 2003). In the P. viridans-C. aconitifolius association, spiders choose plants potentially more attractive to floral visitors, and actively avoid intraspecific competition for territory by selecting isolated plants (Arango et al., 2000). Some populations of C. aconitifolius with P. viridans and ants exhibit low rates of herbivory, whereas other populations without the spider and ants exhibit high rates of leaf damage by geometrid caterpillars (Lepidoptera: Geometridae) (Arango et al., 2000; Carbajal-Rodríguez, 1998, Parra-Tabla et al., 2003). Different rates of herbivory exert compensatory effects in C. aconitifolius, although high rates of herbivory can have a detrimental effect on its leaf growth rate, fruit production, and sexual expression (Parra-Tabla et al., 2003; Parra-Tabla & Herrera, 2010). Female butterflies are able to visually recognize potential egg predators (e.g., ants, Freitas & Oliveira, 1996) and actively choose those sites that were safer for egg-laying, thus decreasing the risk of death for their offspring. In the case of herbivores like geometrid caterpillars, it is "quite possible that adult moths while laying eggs are able to recognize the presence of P. viridans, thus caterpillars are absent from these systems". We hypothesize the plant benefits from the presence of the spider because it prevents egg-laying by potential herbivores, indirectly decreasing herbivory rates. This benefit counteracts the detrimental effect of spiders on seed set due to predation of potential pollinators. Finally, a recent study has established that the effect of predatory spiders can be affected by the activity of other predators, such as ants (Nahas et al., 2012).
Here we describe the tri-trophic level interaction between the plant Cnidoscolusmultilobus, its floral visitors and the predatory spider Peucetia viridans. In particular, we emphasize which organisms comprise the diet of the spider, its daily rate of intake, and if floral visitors perceive the presence of the spiders on the inflorescences. In addition, determine if the presence of spiders affects floral visiting rates of potential pollinators, and how predation of the spider on pollinators affects seed set.
STUDY SITE AND METHODS
Field work was conducted in an area of secondary vegetation 1.5 km Southeast of Las Trancas, near Xalapa, Veracruz, Mexico (19º14'N, 96º19' W, altitude 1300 m). The vegetation is composed of a mixture of secondary species derived from elements of tropical deciduous forest and montane cloud forest, surrounded by sugarcane and coffee plantations (Rzedowski, 1978). The climate is warm sub-humid, annual precipitation is ca. 2000 mm, with the main rainy season occurring in summer, and winter precipitation 5-10% of the total rainfall, mean temperature of 20 ºC (maximum 34 ºC, minimum 6 ºC) (Soto & García, 1989).
The green lynx spider (Peucetiaviridans) is a cursorial hunting spider, foraging by day and night on a wide variety of prey commonly living on wild flowers, grasses, low shrubs or weeds (Brady, 1964; Nyffeler et al. 1987a; 1987b; 1992; Simon, 1980; van Niekerk & Dippenaar-Schoeman, 1994; Vasconcelos-Neto et al. 2006; Weems & Whitcomb, 1977; Whitcomb & Eason, 1967; Whitcomb et al., 1966). It is a dominant polyphagous predatory arthropod, its diet includes several insect orders and spiders (including its own species), and may prey on individuals up to 2.5 times larger than itself (Nyffeler et al., 1987a, 1992). Peucetiaviridans is considered an annual univoltine species, with a reproductive season between June and September. Oviposition (25-600 eggs) occurrs between September and December, and with hatching and dispersal of juveniles by ballooning in December to March, growth of juveniles takes place between March and June (Arango et al., 2000; Exline & Whitcomb, 1965; Whitcomb & Eason, 1965). In Texas and Florida, P. viridans is frequently associated with Crotoncapitatus (Euphorbiaceae), Gossypium spp. (Malvaceae) and Helianthus spp. (Asteraceae), where it plays an important role as predator of noxious fauna (Randall, 1982; Simpson, 1995). In Mexico, P. viridans is associated with C. aconitifolius and C. multilobus (Arango, 2001; Arango et al., 2000; Carbajal-Rodríguez, 1998; Parra-Tabla et al., 2003).
We used two sampling methods to estimate the diet of P. viridans: (1) using a 7 cm diameter plastic cup, we captured spiders with prey, and released the spiders but preserved the prey in 70% alcohol; and (2) we placed mesh traps close to the ground under spider nests located on C. multilobus plants (30 traps per month), and collected all fallen corpses discarded by the spider. In order to exclude ants from stealing the corpses, the bottom of the mesh traps were covered with tanglefoot (The Tanglefoot Co., Grand Rapids, MI, U.S.A.); which was replaced as needed (i.e. when dry or with excess of debris). Cups and traps were visited every 5 days from June to October 1998, and all prey corpses were identified to Order level.
To establish the potential of spiders as predators, we estimated their daily rate of intake as number of prey items per spider per day, which was suggested as the best way to characterize the impact of spiders on the population of prey (Nyffeler et al., 1987a; 1987b). The rate of prey capture of P. viridans (b) was computed using the method for wolf spiders developed by Edgar (1970) and modified by Nyffeler et al. (1987a; 1987b):
b = (Tf x ω) / (Th x 100)
where Tf is the proportion of time of day in minutes (min d-1) available for prey capture and feeding in the field, ω is the percentage of spiders with prey in a sample, and Th is the average handling time (min). Since spider size/age structure is seasonal, prey capture rate was only computed for August, when >90% of the spiders fitted the adult size range (10-24.7 mm). Feeding frequency (ω) and the time available for prey capture and feeding in the field (Tf) were assessed along a 30 m transect from June to October (a 1 h sampling effort per survey). We walked the transect at a slow pace and at different times of the day (0600, 1200, 1800, 2400) during three days, and recorded the number of spiders on C. multilobus with and without prey. Handling time (Th) was defined as the period between the start of an attack and cessation of feeding by a spider, and was estimated in the laboratory using a sample of 20 spiders randomly collected in the study site. We measured the handling time in sub-adult and adult P. viridans regardless of gender while feeding each spider with Apis mellifera (a common flower visitor and prey) on one day per week for three consecutive weeks.
To test if there was a visual effect of the spiders on the visiting rate of insects to flowers (i.e., if butterflies, bees, wasps or flies can distinguish and avoid spiders), we marked three groups of four plants and considered each group as an experimental block. Plants within each block were at a close-enough distance that allowed for simultaneous observation. By manually removing inflorescence panicles, their number per plant was standardized to avoid differential insect attraction due to different flower number. Four treatments were conducted: (1) without spider, (2) with spiders, (3) with modified spider (carapace painted red to make it evident to the flower visitor), and (4) with decoy spider (breadcrumb painted the same light green as the living spider). We used a four-day period to apply one of the four treatments to every plant in each block (i.e. one day per treatment), so that at the end of the experiment, all treatments had been applied to all plants. The experiment was repeated every month from June to October 1998). We counted floral visitors in each of the three blocks from 11:00 to 13:00 (15 min per block) at each sampling period. We defined a visit as the direct contact of an insect with any flower of the target plant. The analyses were performed using the JMP software suit (SAS) on three blocks of four plants in four days per treatment per month (n = 3x4x4x5 = 960).
To search for differences in seed production in plants with and without spiders, we selected 60 C. aconitifolius individuals with comparably similar cover, height and number of panicles. This experiment was conducted once a month from June to October 1998 using the same 60 plants. Two treatments were assigned to these plants: half (n = 30) were plants with spiders present, and the other half without spiders (spiders were removed when they visited). The day prior to observations six virgin panicles (before anthesis) were selected, marked and enclosed with cheese cloth bags. The bags were removed the following day at 06:00 h, leaving the flowers exposed for 24 h, and again bagged until flowers lost receptivity. To avoid damage due to excess humidity, bags were removed when fruits started development then seeds were removed and counted.
A log-linear model was fitted using the GLIM-4 statistical system package (Francis et al., 1993) to test the hypothesis that the median rate of flower visits among treatments varied depending on spider or decoy presence. We used count data and a log-linear model with Poisson link errors, where the change in deviance could be compared directly with χ2 tables to assess significance (Crawley, 1993). In order to determine differences between months and treatments we used a Tukey HSD test after data were square-root transformed (√(y-1) (Zar, 1999), verifying for normality and after computing a four-way ANOVA for the visual effect experiment and a three-way ANOVA for seed production experiments. The results obtained with the ANOVAs were identical to those obtained using GLIM.
RESULTS
The diet of P. viridans was composed exclusively of arthropod species (Table 1). Thirty-two per cent of prey items were spiders (of which 4.3% were P. viridans). Among the insects recovered (68% of total prey items), the most abundant were Hymenoptera (28.4%), especially Apis mellifera (21.1% of total items captured), and Lepidoptera (26.0%), Prescisevarate zonalis (5.3% of total items captured). Other insect orders (Diptera, Heteroptera, Neuroptera, Odonata, and Orthoptera) represented less than 15% of the total of prey items recovered.

Individuals of P. viridans observed feeding had a mean (± SE) body length (including leg span) of 11.0 ± 0.4 mm (range 8.3-12.7 mm). Early instars of this spider were also found on C. multilobus plants, but were not included in the observations. Mean (± SE) body length of prey items was 5.9 ± 1.0 mm (range 1.6-16.5 mm). Thus, prey items of P. viridans were from 0.14 to 1.3 times the size of the spider.
The proportions of spiders with prey items at different times of the day suggest that this species mostly feeds during the day and evening (range 0500-2200). On any given observation day, there were less than 15% of the observed spiders feeding, but always with at least one individual ingesting a prey item. Based on the values of feeding frequency (ω) and the time available for prey capture and feeding in the field (Tf) (Table 2), one spider may capture a maximum of 2.4 prey items per day in June, 3.9 prey items per day in August and 2.9 prey items/per day in October.
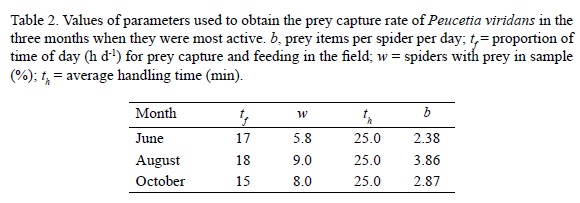
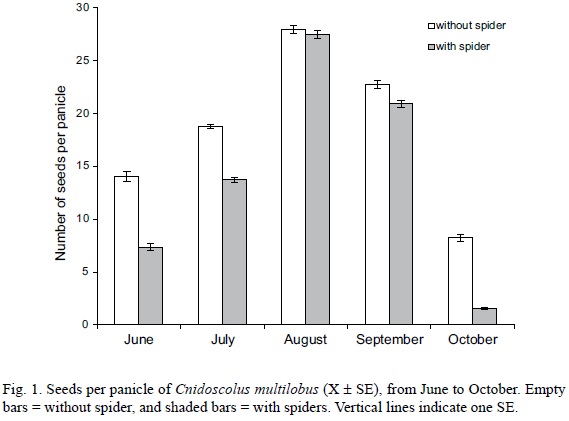
The abundance of floral visitors was significantly associated with treatment and month, and the generalized linear model explained 83% of the total variance (Table 3). Treatment alone explained 60% and month 21% of the total variance; the interaction between these variables explained an additional 2% (Table 3). Also, seeds per panicle were high in August and September because this is the result of pollinations (insect pollinators) in June and July, and indeed in August the insects are satiated, then the number of insect visitors declines as well as of spiders as a result of season change. Seed set in October was due to the drop of pollinators and spiders between August and September.

Seed set was significantly associated with treatment and month. The generalized linear model explained 77.1% of the total variance (Fig. 1, Table 4). Month alone explained 65% and the treatment 4% of the total variance; the interaction between these variates explained an additional 8.1% (Table 4). Seed set in months with lower abundance of floral visitors was different, whereas in August and September, the months with the highest number of floral visitors, we found no significant differences between treatments (Tukey HSD, P = 0.992 and 0.066, respectively).

DISCUSSION
Our results show that the diet of Peucetia viridans was composed exclusively of arthropods (spiders, hymenopterans and lepidopterans). Most of the two insect groups, were floral visitors, and considered as potential pollinators (e.g., bees represented 22% of total prey items). This is similar to observations in a Texas cotton field, where pollinating bees attracted to wild flowers and cotton plants during bloom were frequently overpowered by P. viridans, and constituted 23% of their prey (Nyffeler et al., 1992). The daily maximum prey capture rate of P. viridans (ca. 3 ind/day) is higher than for most studied spiders, which usually feed on 1-1.5 individuals per day. Our observations suggest that the number of spiders with prey is associated with the abundance of floral visitors, and not as a function of time of day, suggesting that P. viridans, as is the case for many other spiders, adjusts prey intake depending on differences in food supply (Foelix, 1982; Turnbull, 1962; 1965).
Our results also suggest that prey, when approaching an inflorescence of C. multilobus, do not detect the difference between a live spider and the absence of spiders, but do detect and avoid strange objects (red-painted spiders, decoys). Thus, there were more visits to inflorescences with live unpainted spiders or no spiders, and less to inflorescences with painted spiders or decoys. Results suggest that at the beginning of the cycle in June (or maybe late in May) the number of spiders is low (insect visitors high), reach a peak and decline. So, low number of spiders, more seed set at the beginning and end of the season. It may be worth considering that possibly painted spiders and decoys may have little or no effect on seed set. The body color and hairy legs of P. viridans mimic the surrounding vegetation when stalking within the base of the panicle or among the floral pedicels, which are also bright-green and covered with hairs. Many spiders, including P. viridans, have the ability to change their body color as a response to substrate color (Gertsch, 1949; Neck, 1978; Théry & Casas, 2002). The use of color signals varies greatly, and is usually associated with the diversity of rewards (Willson, 1983). Bees and butterflies are in general reported unable to distinguish red (Borror et al., 1981) or shades of green (Neck, 1978), and thus P. viridans. On the other hand, it has been hypothesized that red marks on the spider body absorb certain wavelengths making them invisible to insects, although they may function as a warning or deterrent device for predators of the spider, like birds and other vertebrates (Hinton, 1976; Neck, 1978).
The predatory activity of Peucetiaviridans on the floral visitors of Cnidoscolus multilobus indirectly decreased its seed set; C. multilobus individuals without spiders exhibited a significantly higher seed set than plants with spiders. Seed set differed between plants with and without spiders in months with lower abundance of floral visitors, whereas in August, the month with the highest number of floral visitors, we found no significant differences between treatments. Probably the latter is the effect of spider satiation due to prey abundance consequently pollination was not affected in the month of peak pollinator visitation. Similarly, inflorescences of Haploppapusvenetus (Asteraceae) inhabited by P. viridans produced fewer seeds than inflorescences in plants without spiders (Louda, 1982, see also Gonçalves-Souza et al. 2008). Moreover, the viability of seeds from plants with spiders increased 17%, suggesting a positive net effect for the plant between the detrimental effect of spider predation on flower visitors and the beneficial effect of spider interference or predation on seed predators (Gonçalves-Souza et al., 2008; Louda, 1982). However, Louda (1982) used the tallest flowering branch for her study, and our results for Cnidoscolusaconitifolius have shown that P. viridans selects the most suitable places to hunt (e.g., the most attractive inflorescence for pollinators, Arango et al., 2000), which is not necessarily the tallest inflorescence, so a comparison should be conducted between inflorescences of different characteristics in order to assess the effect of the spider on a plant individual.
Even though we did not evaluate the effect of spiders on herbivore activity, which may balance their detrimental effect on pollination and seed set, it is clear that predators of flower visitors in general, such as spiders, can influence plant fitness by determining the balance between pollination and seed predation by insects (Louda, 1982). Moreover, we found spiders with pollen on their body, so they may affect limited pollination and somehow balance their detrimental effect on potential pollinators. On the other hand, it has been reported that spiders feed on pollen to complement their diet, and some species feed on it exclusively (Nyffeler et al., 1994). To fully comprehend this system and to evaluate the effect of changes in predator numbers on the success of the plant ('species-level cascade', Polis, 1999; Polis et al., 2000), future studies should consider the effect of spiders on herbivores and their potential role as pollinators.
ACKNOWLEDGMENTS
We appreciate the help of A. Capistrán, and the suggestions by M. L. Jiménez, L. Cervantes and J. G. Palacios-Vargas. Field work was supported in part by CONACYT (95-0137 to VRG) and Instituto de Ecología, A. C. (902-16), AMA was supported by a CONACYT (90679) graduate student scholarship.
LITERATURE CITED
Abdala-Roberts, L., V. Parra-Tabla, C. Díaz-Castelazo, L. Salinas-Peba & G. H. Delfín. 2010. Spatial variation in the strength of a trophic cascade involving Ruellia nudiflora (Acanthaceae), an insect seed predator and associated parasitoid fauna in Mexico. Biotropica 42: 180-187. [ Links ]
Arango, A. M. 2001. Ecología de Peucetia viridans (Araneae: Oxyopidae) y sus interacciones con Cnidoscolus aconitifolius (Euphorbiaceae). Ph.D. Dissertation. Instituto de Ecología, A.C. Xalapa, México. 96 pp. [ Links ]
Arango, A. M., V. Rico-Gray & V. Parra-Tabla. 2000. Population structure, seasonality, and habitat use by the green lynx spider Peucetia viridans (Oxyopidae) inhabiting Cnidoscolus aconitifolius (Euphorbiaceae). J. Arachnol. 28: 185-194. [ Links ]
Borror, D. J., D. M. DeLong & C. A. Triplehorn. 1981. An introduction to the study of insects. Saunders College Publishing. Philadelphia, USA. 827 pp. [ Links ]
Brady, A. R. 1964. The lynx spiders of North America, north of Mexico (Araneae, Oxyopidae). Bull. Mus. Comp. Zool. 131: 506-518. [ Links ]
Carbajal-Rodríguez, M. 1998. Biología reproductiva y efectos de la hebivoría foliar en la expresión sexual y el éxito reproductivo en la planta ginomonóica Cnidoscolusaconitifolius (Mill.) I. M. Johnstone (Euphorbiaceae). B.Sc. thesis. Universidad Autónoma de Yucatán. Mérida, México. 44 pp. [ Links ]
Crawley, M. J. 1993. GLIM for ecologists. Blackwell Scientific Publications. Oxford, UK. 379 pp. [ Links ]
Edgar, W. D. 1970. Prey and feeding behaviour of adult females of the wolf spider Pardosaamentata (Clerk). Netherlands J. Zool. 20: 487-491. [ Links ]
Exline, H. & W. H. Whitcomb. 1965. Clarification of the mating procedure of Peucetiaviridans (Araneidae: Oxyopidae) by a microscopic examination of the epigynal plug. Florida Entomol. 48: 169-171. [ Links ]
Foelix, R. 1982. Biology of spiders. Harvard University Press. Cambridge, Massachusetts, USA. 274 pp. [ Links ]
Francis, B., M. Green & C. Payn (eds.). 1993. The GLIM system, release 4 manual. Clarendon Press. Oxford, UK. 821 pp. [ Links ]
Freitas, A. V. L. & P. S. Oliveira. 1996. Ants as selective agents on herbivore biology: effects on the behaviour of a non-myrmecophilous butterfly. J. An. Ecol. 65: 205-210. [ Links ]
Gertsch, W. J. 1949. American spiders. D. van Nostrand Co. Inc., New Jersey, USA. 274 pp. [ Links ]
Gonçalves-Souza, T., P. M. Omena, J. C. Souza & G. Q. Romero. 2008. Trait-mediated effects on flowers: artificial spiders deceive pollinators and decrease plant fitness. Ecology 89: 2407-2413. [ Links ]
Hinton, H. E. 1976. Possible significance of the red patches of the female crab spider, Misumenavatia. J. Zool. 180: 35-39. [ Links ]
Louda, S. M. 1982. Inflorescence spiders: a cost/benefit analysis for the host plant, Haplopappus venetus Blake (Asteraceae). Oecologia 55: 185-191. [ Links ]
Nahas, L., M. O. Gonzaga & K. del Claro. 2012. Emergent impacts of ant and spider interactions: herbivory reduction in savanna tree. Biotropica 44: in press (doi: 10.1111/j.1744-7429.2011.00850.x). [ Links ]
Neck, R. W. 1978. Reddish coloration in a green spider: evolutionary origin and subsequent adaptation. J. Zool. 184: 267-269. [ Links ]
Nyffeler, M., D. A. Dean & W. L. Sterling. 1987a. Evaluation of the importance of the striped lynx spider, Oxyopes salticus (Araneae: Oxyopidae), as a predator in Texas cotton. Department of Entomology, Texas A&M University. College Station, Texas, USA. pp. 1114-1123. [ Links ]
Nyffeler, M., D. A. Dean & W. L. Sterling. 1987b. Predation by green lynx spider, Peucetiaviridans (Araneae: Oxyopidae), inhabiting cotton and woolly Croton plants in east Texas. Environ. Entomol. 16: 355-359. [ Links ]
Nyffeler, M., D. A. Dean & W. L. Sterling. 1992. Diets, feeding specialization, and predatory role of two lynx spiders, Oxyopes salticus and Peucetia viridans (Araneae, Oxyopidae), in a Texas cotton agroecosystem. Environ. Entomol. 21: 1457-1465. [ Links ]
Nyffeler, M., W. L. Sterling & D. A. Dean. 1994. How spiders make a living. Environ. Entomol. 23: 1357-1367. [ Links ]
Parra-Tabla, V. & C. M. Herrera. 2010. Spatially inconstant direct and indirect effects of herbivory on floral traits and pollination success in a tropical shrub. Oikos 119: 1344-1354. [ Links ]
Parra-Tabla, V., V. Rico-Gray & M. Carbajal. 2003. Effect of herbivory on leaf growth, sexual expression and reproductive success of Cnidoscolus aconitifolius (Euphorbiaceae). Plant Ecol. 173: 153-160. [ Links ]
Polis, G. A. 1999. Why are parts of the world green? Multiple factors control productivity and the distribution of biomass. Oikos 86: 3-15. [ Links ]
Polis, G. A., A. L. W. Sears, G. R. Huxel, D. R. Strong & J. Maron. 2000. When is a trophic cascade a trophic cascade? Trends Ecol. Evol. Syst. 15: 473-475. [ Links ]
Pollard, S. D., M. W. Beck & G. N. Dodson. 1995. Why do male crab spiders drink nectar? An. Behav. 49: 1443-1448. [ Links ]
Randall, J. B. 1982. Prey records of the green lynx spider, Peucetia viridans (Hentz) (Araneae, Oxyopidae). J. Arachnol. 10: 19-22. [ Links ]
Romero, G. Q. & J. Vasconcelos-Neto. 2004. Beneficial effects of flower-dwelling predators on their host plant. Ecology 85: 446-457. [ Links ]
Ruhren, S. & S. N. Handel. 1999. Jumping spiders (Salticiade) enhance the seed production of a plant with extrafloral nectaries. Oecologia 119: 227-230. [ Links ]
Rzedowski, J. 1978. Vegetación de México. Editorial Limusa. México, D.F., México. 432 pp. [ Links ]
Simon, E. 1980. Études arachnologiques 22e Mémoire. 34 étude sur les Arachnides de'l Yemen. Ann. Soc. Entomol. France (Ser. 6)10: 77-124. [ Links ]
Simpson, M. R. 1995. Covariation of spider egg and clutch size: the influence of foraging and parental care. Ecology 76: 795-800. [ Links ]
Soto, M. & E. García. 1989. Atlas climático del estado de Veracruz. Instituto de Ecología, A.C. Xalapa,Veracurz, México. 125 pp. [ Links ]
Théry, M. & J. Casas. 2002. Predator and prey views of spider camouflage. Nature 415: 133. [ Links ]
Turnbull, A. L. 1962. Quantitative studies of the food of Linyphia triangularis Clerck (Araneae: Linyphidae). Can. Entomol. 94: 1233-1249. [ Links ]
Turnbull, A. L. 1965. Effects of prey abundance on the development of the spider Agelenopsispotteri (Blackwell) (Araneae:Agelenidae). Can. Entomol. 97: 141-147. [ Links ]
Van Niekerk, P. & A. S. Dippenaar-Schoeman. 1994. A revision of the Afrotropical species of Peucetia (Araneae: Oxyopidae). Entomology Memoir 89: 1-50. [ Links ]
Vasconcelos-Neto, J., G. Q. Romero, A. J. Santos & A. Dippenaar-Schoeman. 2006. Associations of spiders of the genus Peucetia (Oxyopidae) with plants bearing glandular hairs. Biotropica 39: 221-226. [ Links ]
Weems, H. V. & W. H. Whitcomb. 1977. The green lynx spider, Peucetiaviridans (Hentz) (Araneae: Oxyopidae). Entomology Circular No 181, Department of Agriculture and Consumer Services. Florida, USA. 4 pp. [ Links ]
Whitcomb, W. H. & R. Eason. 1965. The matting behavior of Peucetia viridans (Araneidae: Oxyopidae). Florida Entomol. 48: 163-167. [ Links ]
Whitcomb, W. H. & R. Eason. 1967. Life history and predatory importance of the striped lynx spider (Araneidae: Oxyopidae). Proc. Arkansas Acad. Sci. 21: 54-59. [ Links ]
Whitcomb, W. H., M. Hite & R. Eason. 1966. Life history of the green lynx spider Peucetiaviridans (Araneae: Oxyopidae). J. Kansas Entomol. Soc. 39: 259-267. [ Links ]
Willson, M. F. 1983. Plant reproductive ecology. John Wiley and Sons. New York, USA. 282 pp. [ Links ]
Wise, D. H. 1993. Spiders in ecological webs. Cambridge University Press. New York, USA. 328 pp. [ Links ]
Zar, J. H. 1999. Biostatistical analysis. Prentice-Hall, Inc., Upper Saddle River, New Jersey, USA. 663 pp. [ Links ]














