Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Atmósfera
Print version ISSN 0187-6236
Atmósfera vol.25 n.4 Ciudad de México Oct. 2012
Evaluation of atmospheric corrosion in Orizaba, Mexico
J. L. Ramírez-Reyes
Instituto de Ingeniería, Universidad Veracruzana, SS Juan Pablo II s/n Zona Universitaria, Fracc. Costa Verde, 94294 Boca del Río, Veracruz, México. Corresponding author; e-mail: luiramirez@uv.mx
J. Uruchurtu-Chavarín
CIICAP, Universidad Autónoma del Estado de Morelos, 62209 Cuernavaca, Morelos, México
J. Genescá
Departamento de Ingeniería Metalúrgica, Facultad de Química, UNAM, Ciudad Universitaria, 04510 México, D.F.
R. Longoria-Ramírez
Instituto Tecnológico de Nuevo León, Av. Eloy Cavazos 2001, Col. Tolteca, 67170 Guadalupe, Nuevo León, México
RESUMEN
En este estudio se evaluó la agresividad atmosférica en la ciudad de Orizaba, Veracruz, México, dentro del proyecto "Mapa de corrosividad atmosférica del Estado de Veracruz" desarrollado por la Universidad Veracruzana en el periodo 2007-2008. Se determinaron los índices de corrosividad marino, industrial y rural utilizando el método estándar del alambre sobre tornillo, así como los niveles de corrosividad por medio de probetas planas metálicas de acero al carbón, acero galvanizado, cobre y aluminio, de acuerdo con la norma ISO 9223. Se realizaron estudios de morfología en los productos de corrosión formados en las muestras planas después de un año de exposición atmosférica, aplicando microscopia electrónica de barrido y análisis químico elemental por dispersión de rayos X.
ABSTRACT
This study evaluated the atmospheric aggressiveness in the city of Orizaba, Veracruz, Mexico, as part of the "Atmospheric corrosiveness map of the state of Veracruz" project, developed by Universidad Veracruzana from 2007 to 2008. The corrosiveness in marine, industrial and rural environments was determined with the standard method of bolt and wire, and the corrosion levels throughout flat samples of mild steel, galvanized steel, copper and aluminum in accordance to ISO 9223. The corrosion products of flat samples were submitted to morphologic studies, using scanning electron microscopy (SEM) and elemental analysis with energy-dispersive X-ray (EDX) spectroscopy after a year of atmospheric exposure in Orizaba.
Keywords: Atmospheric corrosion, corrosiveness index, air pollution, aggressive climatic conditions.
1. Introduction
Atmospheric corrosion produces extensive damage to structural materials, with a 40% impact on the total cost of corrosion (Genescá, 1994). In settings where motor cars, bridges or buildings (which contain high amounts of metal,) are exposed to atmospheric conditions, more sectors are affected by climatic conditions (Corvo et al., 2010). It has been observed also that in the Northern Hemisphere, the effects of corrosion are less evident at the southern and eastern sections of an exposed structure, while the northern and western sections suffer more corrosion caused by rain and by the magnitude and direction of the wind (Morcillo et al., 1999). At industrial zones, the main pollution agent is sulphur dioxide (SO2), as a result of fossil fuel burning; its concentration is higher in cold seasons due to the use of central heating or from power generation emissions. Thus the corrosiveness will be higher in winter and spring, during the dry season of the year. A 0.1-100 μg/m3 level of SO2 is typical of industrial zones, but the effects of chlorides in marine zones are more aggressive than the sulphur compounds, and they are two or three times higher when these environments are combined (Echeverría et al., 2009).
Atmospheric corrosion has been classified according to its aggressiveness in the environment as industrial, marine or rural. The environment is highly influenced by the presence of microclimates associated with specific pollutants and climatic conditions regulated by topography, (e.g., in a mountainous region, arid desert or sea bottom of a coastal zone, etc.) (van Orden and Cook, 1997; Cook et al., 1998, Morcillo et al., 1998; Mariaca et al., 2000; Correa Bedolla et al., 2007).
The last published reports on the economic impact of corrosion in the US covered the years 1999 through 2001, and they showed a total cost per year of USD 276 billions, equivalent to 3.1% of the gross domestic product (GDP) (Koch et al., 2002). In Peru, by the year 2000, the loss caused by corrosion reached 8% of the GDP, representing an approximate cost of USD 1.2 billion (Romero et al., 2005). In spite of its importance, a complete study of the corrosion problem in Mexico, with estimated costs of its effects, has yet to be done (Genescá, 1994; Ramírez and Uruchurtu, 2008) since Mexican authorities are too busy with other priorities. However, it is necessary to adopt appropriate measures in order to determine a protection strategy against corrosion. The application of ISO regulations in developed countries is compulsory. At the same time, these countries are building scenarios for the 21st century, which anticipate rapid and severe changes in the corrosion processes (Cole and Patterson, 2010) and degradation of materials exposed to the atmosphere in undeveloped countries, where no preventive methods are applied (Graedel and Leygraff, 2001).
2. Methodology
Orizaba is a highland region in the state of Veracruz, Mexico, located 1259 m above the sea level at 18º 5' north latitude and 97º west longitude. Its main climate is dry winter (Cw) according to the Koppen Index (Ocampo, 1986, Fig. 1). In the past, the city of Orizaba was called "the Mexican Manchester" due to the establishment of fabric manufacturers without modification of the rural atmosphere (van Orden and Cook, 1997). However, in the last 10 years a variety of industries (e.g., cement industries, breweries, and metallurgical and chemical manufacturers) have been incorporated into the local economy, producing changes in the atmospheric aggressiveness (Ramírez and Uruchurtu, 2008).
Wire-on-bolt samples were used to determine the corrosiveness index, according to ASTM G116-99 and ISO TC/156/WG3/1979 standard. They were exposed to each weather season, and then used to classify the different types of atmosphere in Orizaba. To determine the marine corrosiveness index we used the galvanic system with bolt of mild steel and aluminum wire; for the industrial corrosiveness index, we chose copper bolt with aluminum wire; and for the rural corrosiveness index, the bolt was made of nylon and wrapped in aluminum wire. The corrosion rate level was evaluated according to ISO 9223 with 4" × 6" flat sheet samples subjected to one year of exposure. Three different surface conditions were studied in the mild steel samples: (a) AC-R was cleaned with an oxide removal substance, (b) AC-P was cleaned with HCl pickling solution and (c) AC-S was cleaned by sandblasting.
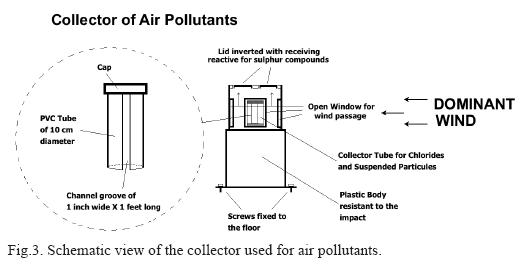
A second type of samples consisted of galvanized steel prepared with different surface conditions: (d) commercial galvanization (Gal-A) with 0.030" thicknes, (e) industrial galvanization (Gal-B) with 0.050" thickness, and (f) industrial galvanization plus chroming with 0.06" film (Gal-C). To complement the experimental set, flat sheet samples of copper and aluminum were prepared for field testing in frames, according to ISO 8565 (as seen in Fig. 2). The amounts of pollutants were determined by means of a specially designed collector (Fig. 3), based on the British Standard (BS) 1747-1969 (Mariaca et al., 2000). The analytical determinations were made according to ISO 922, and ASTM-G91 specifications, as well as the Manual of Standard Methods procedures. The historical data of weather in the Orizaba region throughout the four seasons of the year were averaged as shown in Table I.
3. Results
Weather characteristics of the Orizaba region were taken from the normal parameters collected by the weather observatory of the Servicio Meteorológico Nacional of Mexico for the period 1980 to 2000. The evaluation of the corrosiveness indices began in the early fall of 2007 and lasted until the summer of 2008, with the respective changes of samples on each season of the year. The results are shown in Table II, arranged according to the type of atmosphere and marked with colour codes (as seen below) that represent the established levels of aggressiveness for each season.
Table III shows the atmospheric pollution agents, and their average values during the weather seasons. The results of corrosion levels on the flat samples as established in the ISO 9223 standards are shown in Table IV.
The scanning electron microscopy (SEM) studies were carried out on the exposed samples and the chemical analysis by energy-dispersive X-ray (EDX) is included for each material evaluated.
4. Discussion
Weather characteristics have direct influence on the atmospheric corrosion effects, including the aggressiveness impact of the pollution agents throughout the seasons (Prato et al., 1999; Reyes Trujeque, 1999; Ferm et al., 2006; Ramírez and Uruchurtu, 2008). Table II shows that marine corrosiveness index increases during spring and summer, but remains below the standard level. The industrial corrosiveness indices were also high and above the standard levels during the same seasons. The rural corrosiveness indices confirm the presence of aggressiveness during spring and summer, but remain below the standard levels. In sum, the Orizaba atmosphere is defined as industrial, since its characteristics no longer correspond to the rural type during the warm and dry seasons.
Table III shows the behavior of the pollutant agents. The time-of-wetness (TOW) is low in dry seasons, but maintains a high level when the season data is compared with the yearly average. We didn't observe a direct relationship between TOW and the corrosiveness index. This could be attributed to the short time of the wire-on-bolt test (Padilla, 1999; Correa et al., 2007). As for the uncertain impact of sulphur dioxide (SO2) within the corrosion behavior (in spite of its expected aggressiveness), it can be attributed to the rainfall recorded in that season of the year (see Table I); besides, it has been reported that sulphur compounds have only shown corrosive effects when combined with chlorides in marine environments (Corvo et al., 2008). The high concentrations of chlorides and suspended particles observed had a noticeable impact in the corrosive behavior of the atmosphere in the windy and dry seasons. Thus the rain is considered an attenuating factor of corrosiveness, since it drags and cleans the pollution agents accumulated over the metal surface in the dry seasons (Echeverría Lage et al., 2003).
The results from flat samples of the ISO 9223 type (Table IV) showed the following tendency in the corrosion rate: Cu > Al > galvanized steel > mild steel, which was unexpected for the traditionally rural atmosphere of Orizaba. It is considered that the attack was selective for the nonferrous metals due to the presence of chlorides and sulphur in the atmosphere during the years 2007-2008. The mild steel samples showed less corrosive impact on the surface with the sandblasting method applied on AC-S samples, and close to 20% less than the corrosion rate level observed on the samples treated with HCl. Likewise, in the Gal-C samples with chromate film, less than 50% of the corrosion rate level was observed in comparison with the simply galvanized samples.
The corrosion products of the AC-R steel sample with oxide removal treatment registered sulphur concentrations with less presence of chlorides, so it was concluded that lepidocrocite (gamma-FeOOH) formations are gradually converted into magnetite (Fe3O4) across the metal substrate at mixed atmospheres (Rosales et al., 1998), as shown in Figure 4.
The galvanized samples behaved in a slightly different way, accordingly with the presence of a zinc corrosion layer whose products are sufficiently impervious to protect the steel (Rosales et al., 1998). However, the presence of sulphur and chloride was detected with SEM in the corrosion products (Fig. 5). So, less corrosiveness means that films made of alkaline zinc chloride and sulphate compounds prevent the passage of aggressive ions toward the zinc-iron interface by blocking the corrosion zones between grains leaved by the run-off action of the rain (Veleva et al., 2010).
Copper samples develop a film with insoluble copper corrosion products of cuprite (Cu2O) (Rosales et al., 1998; Puentes et al., 2010). Due to its selectivity, copper reacts mainly with sulphur (Cu4(SO4)OH6•2H2O) and less with chlorides (Cu2Cl(OH)3) (Watanabe et al., 2006), as shown in Figure 6. The corrosiveness was significant and it could be attributed to the combined exfoliative action of wind and rain, and the reactivity of the generated copper sulphate layers, which allow the passage of aggressive ions through the copper substrate, mainly during spring and summer when weather conditions vary from low to high rain and wind from the southeast industrial zone.
Aluminum is highly reactive to atmospheric oxygen. When it comes into contact with this element, it forms Al2O3•3H2O, which is very protective in atmospheric conditions without aggressive contaminants (Rosales et al., 1998). The selective attack on aluminum is revealed through pitting with high chloride content at the bottom of the cracked alumina layers observed on wire-on-bolt samples during spring and summer, coincident with the lowest TOW values and the highest concentrations of chloride and sulphur observed in analytical determinations, which produce salt gradients with more aggressive effects (Syed, 2010) (Fig. 7).
5. Conclusions
The atmosphere in Orizaba is predominantly industrial. However, the influence of chlorides is quite significant due to the presence of north wind without rain, which produces greater aggressiveness in the copper and aluminum samples and contributes to the increasing corrosiveness index in these materials. This means that both metals suffered selective attack by the sulphur compounds from industrial emissions, which is increased by the presence of chlorides in certain weather conditions during spring and summer, in coincidence with the seasons of less dampness and more warmth in the environmental conditions of Orizaba.
The surface conditions of mild steel samples showed less attack from corrosion when sandblasted, in comparison with those subjected to chemical cleaning, and the galvanized plus chromate samples showed much lower corrosion rates than the simply galvanized samples due to the plugging effects of zinc, the inhibition action of chromates and the magnetite protective films formed over originally pollution-free steel samples.
Acknowledgments
Thanks to the Dirección General de Investigaciones of the Universidad Veracruzana for the financial support given to project 29101.
References
Cole I. S. and D. A. Paterson, 2010. Possible effects of climate change on atmospheric corrosion in Australia. Corros. Eng. Sci. Techn. 45, 19-26. [ Links ]
Cook D. C., A. C. van Orden, J. J. Carpio and S. J. Oh, 1998. Atmospheric corrosion in the Gulf of Mexico. Hyperfine Interact. 113, 319-329. [ Links ]
Correa Bedoya E., C. Botero Vega, A. H. Restrepo, L. Juan Delgado, J. G. Castaño and F. Echeverría E., 2007. Corrosión del acero al carbono, acero galvanizado y aluminio en diferentes atmósferas colombianas. Scientia et Technica 8, 7-12. [ Links ]
Corvo F., T. Pérez and L. R. Dzib, 2008. Outdoor-indoor corrosion of metals in tropical coastal atmospheres. Corros. Sci. 50, 220-230. [ Links ]
Corvo F., J. Reyes, C. Valdés, F. Villaseñor, O. Cuesta, D. Aguilar and P. Quintana, 2010. Influence of air pollution and humidity on limestone materials degradation in historical buildings located in cities with tropical coastal climates. Water Air Soil Pollut. 205, 359-375. [ Links ]
Echeverría Boán M., C. A. Echeverría Lage, M. Boán, C. A. Echeverría Boán, S. Benavides García, M. Peterson Roldán and S. M. Betancourt Miguel, 2009. Determinación de iones cloruro y sulfato en un mismo captador en investigaciones de corrosión atmosférica. Revista CENIC. Ciencias Químicas 40, 11-16. [ Links ]
Echeverría Lage C. A., J. Rodríguez Pérez, M. Echeverría Boán and A. González Betancourt, 2003. Corrosión atmosférica del acero en la Universidad de Matanzas Camilo Cienfuegos. Monografía. Matanzas, Cuba, 40 pp. [ Links ]
Ferm M., J. Watt, S. O'Hanlon, F. Santis and C. Varotsos, 2006. Deposition measurement of particulate matter in connection with corrosion studies. Anal. Bioanal. Chem. 384, 1320-1330. [ Links ]
Genescá J., 1994. Más allá de la herrumbre III. Corrosión y medio ambiente. Mexico: Fondo de Cultura Económica, 80 pp. [ Links ]
Graedel T. E. and C. Lygraf, 2001. Scenarios for atmospheric corrosion in the 21st Century. In: Atmospheric Corrosion. London: Wiley and Sons, 24-30 (Electrochemical Society Series). [ Links ]
Koch G. H., M. P. H. Brongers, N. G. Thompson, Y. P. Birmani and J. H. Prayer, 2002. Corrosion costs and preventive strategies in the United States. Washington, DC: Federal Highway Administration, U. S. Department of Transportation, 156 pp. [ Links ]
Mariaca L., J. Genescá, J. Uruchurtu and L. S. Hernández, 2000. Corrosividad atmosférica (MICAT-Mexico). Mexico: Plaza y Valdés, 232 pp. [ Links ]
Morcillo M., E. Almeida, B. Rosales, J. Uruchurtu and M. Marrocos (eds.), 1998. Corrosión y protección de metales en las atmósferas de Iberoamérica. Parte I. Mapa de Iberoamérica de corrosividad atmosférica (Proyecto MICAT, XV.1). Madrid: Programa CYTED, 5-581. [ Links ]
Morcillo M., 1999. Fundamentos de la corrosión atmosférica de metales. In: Morcillo M., E. Almeida, B. Rosales, J. Uruchurtu and M. Marrocos (eds.). Corrosión y protección de metales en las atmósferas de Iberoamérica. Parte I. Mapa de Iberoamérica de corrosividad atmosférica (Proyecto MICAT, XV.1). Madrid: Programa CYTED, 1-52. [ Links ]
Ocampo M., 1986. Inversión térmica y contaminación. Información Científica y Tecnológica 8, 19-21. [ Links ]
Padilla, E. D., 1999. Estudio de la corrosión de materiales en la atmósfera de Lima. Método ALCAN. Rev. Inst. Inv. Fac. Minas. Metal. Cienc. Geograf. 2, 123-135. [ Links ]
Prato M. R., Rosales B. M. and J. Uruchurtu, 1999. Climatología de la región iberoamericana. In: Morcillo M., E. Almeida, B. Rosales, J. Uruchurtu and M. Marrocos (eds.). Corrosión y protección de metales en las atmósferas de Iberoamérica. Parte I. Mapa de Iberoamérica de corrosividad atmosférica (Proyecto MICAT, XV.1). Madrid: Programa CYTED, 141-201. [ Links ]
Puentes M., R. Vera, D. Delgado, R. Araya, S. Hidalgo, 2010. Corrosión atmosférica de cobre en ambiente marino: exterior e interior. Congreso IBEROMET XI, X CONAMET/SAM. Viña del Mar, Chile. [ Links ]
Ramírez Reyes J. L. and J. Uruchurtu, 2008. Mapa de corrosividad atmosférica del estado de Veracruz. Reporte 29101-01. Mexico: Instituto de Ingeniería, Universidad Veracruzana. [ Links ]
Reyes Trujeque J., 1999. Influencia de los principales factores climáticos y de la calidad del aire sobre la corrosión atmosférica de los metales en la costa del sureste del golfo de México. M. S. thesis in environmental engineering. Mexico: Universidad Veracruzana. [ Links ]
Romero M. E., B. Aponte, S. Arias, F. F. de García, O. T. de Rincón and O. Larreal, 2005. Diseño de un modelo matemático computarizado de costos por corrosión. Rev. Téc. Ing. Univ. Zulia, 28(1). [ Links ]
Rosales B., J. Uruchurtu and M. Marrocos, 1988. Corrosión y protección de metales en las atmósferas de Iberoamérica. Parte I. Mapa de Iberoamérica de corrosividad atmosférica (Proyecto MICAT, XV.1). Madrid: Programa CYTED, 55-243. [ Links ]
Syed S., 2010. Influence of the environment on atmospheric corrossion of aluminum. Corros. Eng. Sci. Techn. 45, 282-287. [ Links ]
Van Orden A. C. and Cook D. C., 1997. NSF funding planning visit for corrosion research in Mexico. NSF-INT 96-02990. [ Links ]
Veleva L., Meraz E. and Acosta M, 2010. Zinc precipitation runoff from galvanized steel in humid tropical climate. Corros. Eng. Sci. Techn. 45, 76-83. [ Links ]
Watanabe M., S. Shinozaki, E. Toyoda, K. Asakura, T. Ichino, N. Kuwaki, Y. Higashi, and T. Tanaka, 2006. Corrosion products formed on silver after a one-month exposure to urban atmospheres. Corrosion 62, 243-250. [ Links ]














