Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Atmósfera
versão impressa ISSN 0187-6236
Atmósfera vol.20 no.3 Ciudad de México Jul. 2007
Total peroxides and sulfate in rainwater in the Mexican eastern
Pacific Ocean during the Climatic Experiment in the Americas
Warm Water Pools
H. PADILLA, M. C. TORRES, R. BÉLMONT, R. GARCÍA and A. BÁEZ
Laboratorio de Química Atmosférica, Centro de Ciencias de la Atmósfera,
Universidad Nacional Autónoma de México,
Circuito Exterior, Ciudad Universitaria, México D. F. 04510, México
Corresponding author: H. Padilla; e–mail: hppg@atmosfera.unam.mx
Received January 24, 2006; accepted January 8, 2007
RESUMEN
Se analizaron peróxidos totales y el ion sulfato en 26 muestras de agua de lluvia colectadas del 23 al 25 de mayo de 2001 a bordo del B/O "El Puma", propiedad de la Universidad Nacional Autónoma de México, durante la campaña denominada Experimento Climático en las Albercas de Agua Caliente de las Américas (ECAC–1) en la zona este del Océano Pacífico entre los 12 y 14° latitud norte, y entre los 98 y 100° longitud oeste. Las medias ponderadas por volumen de las concentraciones de peróxidos totales, sulfato y de sulfato proveniente de la sal marina fueron de 13.1 ± 1.94 μM, 0.76 ± 0.59 y 0.04 ± 0.06 mg L–1, respectivamente. El sulfato en exceso (sulfato que no proviene de la sal marina) representó el 95% del sulfato total. Las concentraciones de peróxidos totales correlacionaron significativamente con sulfato total y sulfato en exceso (0.560 y 0.586 a una p < 0.05, respectivamente) posiblemente debido a que la mayor parte del sulfato en exceso y del peróxido de hidrógeno observados en el Océano Pacífico oriental frente a las costas de México, pudo haber sido producida a partir de la oxidación de compuestos biogénicos de azufre. La correlación no significante entre sulfato de la sal marina y peróxidos totales se debió al hecho de que el H2O2 no reacciona con los sulfatos presentes en los aerosoles de la sal marina. También, la concentración de peróxidos totales no tuvo una correlación significativa con la intensidad de la lluvia, debido a que una fracción del H2O2 pudo haber sido generada dentro de las gotitas de la nube.
ABSTRACT
Total peroxides were analyzed in 26 rainwater samples collected on board of the R/V El Puma of the National Autonomous University of México (UNAM) during the Climatic Experiment in the Americas Warm Water Pools (ECAC–1) over the eastern Pacific Ocean between 12 and 14° N, and between 98 and 100° W, from May 23 to 25, 2001. The volume weighted mean concentration of total peroxides was 13.1 ± 1.94 μM. Also, sulfate ion was analyzed. The volume weighted mean concentrations for sulfate and sea–salt sulfate were 0.76 + 0.59 and 0.04 ± 0.06 mg L–1, respectively. Excess sulfate (non–sea–salt sulfate) represented 95% of the total sulfate. The total peroxides concentration in rainwater correlated significantly with total sulfate and excess sulfate (0.560 and 0.586 at a p < 0.05, respectively) possibly because most of the excess sulfate and hydrogen peroxide, observed in the Mexican eastern Pacific Ocean, could have been produced from the oxidation of biogenic sulfur compounds. The non–significant correlation between sea–salt sulfate and total peroxides was due to the fact that H2O2 does not react with the sulfate already present in sea salt aerosols. Also, the concentration of total peroxides did not correlate with rain intensity probably because a significant fraction of H2O2 could have been generated within cloud droplets.
Keywords: Total peroxides, sulfates, rainwater.
1. Introduction
The number of research projects to study the total peroxides concentration in rainwater over tropical seas and shorelines is very low, let alone over the Mexican eastern Pacific Ocean. The Climatic Experiment in the Americas Warm Water Pools (ECAC–1) was a unique opportunity to measure the concentration of total peroxides in oceanic rains over the open ocean in front of the Mexican Pacific Coast for the first time. Moreover, all the rain samples were collected during the early stages of the formation of hurricane Adolph, which became the strongest hurricane on record for the month of May in the eastern Pacific Ocean. Previous studies over tropical marine environments were made by Zika et al. (1982) in Florida and Bahamas Islands, Cooper et al. (1987) over the Gulf of México and western Atlantic Ocean, Yuan and Shiller (2000) over the South and Central Atlantic Ocean, and Deng and Zuo (1999) in Florida. Avery et al. (2001) also investigated major components at a coastal site in North Caroline, USA. Studies on hydrogen peroxide on continental areas have been accomplished by Kok (1980) in Claremont, California, Zika et al. (1982) in Miami, Florida, Yoshizumi et al. (1984) in Tokyo, Japan, Kelly et al. (1985) in the eastern United States, and Olszyna et al. (1988) at the Whitetop Mountain in Virginia, USA.
2. Experimental
2.1 Sampling area
Rainwater samples were collected from May 23 to 25, 2001, during the ECAC–1 on board of the R/V El Puma of the National Autonomous University of México (UNAM) in the eastern Pacific Ocean between 12 and 14° N, and between 98 and 100° W (Fig. 1).
2.2 Rainwater sampling method
Rainwater samples were collected using a 25 cm in diameter high–density polyethylene funnel, placed 1 m above the floor on the second level of the ship to prevent contamination from splashing. The funnel was uncovered just before the onset of a rain event. The funnel was connected to a 2–L high–density polyethylene bottle. The ship was moving during all rain water samplings.
2. 3 Sample field treatment
Because of the rapid H2O2 decomposition during the time elapsed from sample collection and analysis (Ortiz et al., 2000), rain samples were treated to analyze total peroxides (H2O2 plus organic peroxides) as soon as the rain event ceased, to form the dimer of the p–hydroxiphenilacetic acid.
This dimer is obtained by the reaction of the p–hydroxiphenilacetic acid with peroxides in the presence of peroxidase as a catalyzer (Guilbault et al., 1968; Lazrus et al., 1985). Aldrich Chemical Co. Inc. p–hydroxiphenilacetic acid and Sigma Aldrich Co. peroxidase were used. Treated samples are stable for several days under ambient conditions (Kelly et al., 1985; Lazrus et al., 1985; Kok et al., 1986; Olszyna et al., 1988). Chemical reagents and analytical procedures used in this work are described by Lazrus et al. (1985).
2.4 Chemical analysis
Treated rainwater samples were analyzed by fluorescence at 320–410 nm with the spectra system FL3000 (thermoseparation products) fluorescence meter to determine the concentration of total peroxides. Merck 30% v/v hydrogen peroxide (perhydrol) reagent grade with a stabilizer was used for preparing calibration standards from 2 to 30 (iM. The standard solutions were also treated as described in section 2.3. Only total peroxides were determined because of reagent limitations, however, some tests indicated that H2O2 accounts for 95% of the total peroxides.
Sulfate was also analyzed because the oxidation of SO2 by H2O2 is the main source of this ion in rainwater. It was analyzed by non–suppressed ion chromatography using a Perkin Elmer instrument equipped with an isocratic LC pump 250 and a conductivity detector conductoMonitor III. Calibration curve was prepared from standard solutions prepared from 1000 mg L–1 High–Purity Standards (Charleston, SC).
2.5 Detection limit
The method detection limit (MDL) was determined using the procedure described by Glaser et al. (1981). Measurements were taken on seven samples of deionized water containing the analyte.
The standard deviation was calculated from the obtained data. Using the degrees of freedom from the data set and the 99% confidence level, the critical Student's t value was obtained from tables. The MDL was computed as the product of the standard deviation and the critical t–value. The computed MDL for H2O2 was 0.4 µM and 0.22 mg L–1 for SO42– .
2.6 Statistical analysis
The Shapiro–Wilk W test was applied to determine whether data of total peroxides, SO42–, excess sulfate (non sea–salt sulfate) SO42–xs, sea–salt sulfate (SO42–ss), rain amount, and rain rate, fitted a normal distribution. The null hypothesis establishing that the data corresponded to a normal distribution was rejected at the 5% significance level for SO42, SO42–xs, SO42–ss concentrations, and rain rate. Therefore, the Spearman's rank correlation was applied to compute the correlation between the studied variables.
3. Results and discussion
Twenty–six rainwater samples were collected from May 23 to 25, 2001 during the early stages of the formation of hurricane Adolph, which was a tropical depression located at 13.6° N and 101.1° W on May 25, 2001.
3.1 Total peroxides in rainwater
The volume weighted mean concentration of total peroxides was 13.1 ± 1.9 μM. This value is noticeably lower than those observed by Cooper et al. (1987) in the Gulf of México (40 ±21 μM), Florida Keys (28 ± 4 μM), but equal to the mean value observed in the western Atlantic Ocean (13 ± 5 μM). The rains present over large areas and the absence of direct sunlight maintained a low concentration of total peroxides. Also, the low concentrations possibly indicated that there was no advection of air pollutants over the sampling area during the ECAC–1. As far as we know, the results of this study on total peroxides concentrations were the first obtained during the early stages of formation of a category 4 hurricane over the open ocean in the eastern Pacific Ocean, therefore, they are not representative of the rains over the Mexican eastern Pacific Ocean, obviously because there are more atmospheric phenomena that favor deep convection.
3.2 Variations of H2O2 with rain rate
Figure 2 shows the variation of the total peroxides concentration and rain rate from May 23 to 25, 2001. The concentration of total peroxides rapidly decreased from the first to the second rain fraction on May 23, although the rain rate remained fairly constant during the first three rain fractions. This was possibly due to a build up of peroxides before the beginning of rains. On May 24, the concentration of total peroxides was high, corresponding to the low rain rate, as it was expected. On May 25, high concentrations of peroxides also were observed during periods of relatively low rain rates. However, the relationship between the concentration of peroxides and rain rate is much more complex. As examples, the concentration of peroxides increased sharply from 13:30 to 13:35 h despite the rain rate increased slowly from 11:17 to 13:35 h, and the concentration of peroxides presented small variations from 13:35 to 13:51 h, whereas the rain rate alternately increased and decreased. Also, it is not easy to explain why the highest rain rates observed from May 23 to 25 corresponded to very different total peroxides concentrations. It must be realized that the main component of the total peroxides (hydrogen peroxide) decomposes rapidly, adding uncertainty in the variation of its concentration in rain water between the moment when raindrops fall on the funnel and the time when rain water samples are treated for chemical analyses.
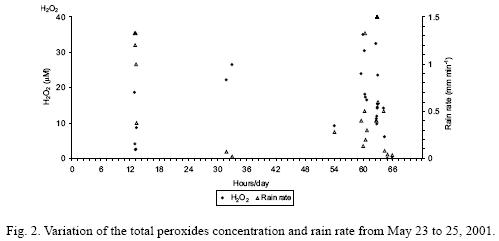
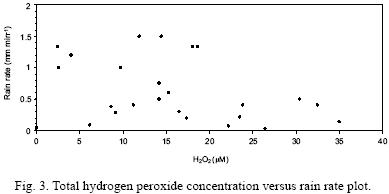
Figure 3 shows a rain rate versus hydrogen peroxide concentration plot. It is observed that only the concentrations of hydrogen peroxide higher than about 20 μM are related to low rain rates. For concentrations below 20 μM the relationship between these parameters is not clear.
3.3 Sulfate concentrations
The volume weighted mean concentrations for SO42– and SO42–ss were 0.76 ± 0.59 and 0.04 ± 0.06 mg L-1, respectively. The quantification of sea salt contribution was based on sodium. Excess sulfate (non sea–salt sulfate) SO42–xs represented 92% of the total sulfate.
3.4 Correlations
The total peroxides concentrations in rainwater correlated significantly with SO42– and SO42–xs (0.560 and 0.586 at a p < 0.05, respectively) but not with SO42–SS. The significant and positive correlation with SO42–xs differs from that obtained by other authors such as Deng and Zuo (1999) in Florida, where the maximum H2O2 concentration was observed when the non–sea–salt sulfate concentration was low. It is possible that most of the non–sea–salt sulfate they observed in Florida came from SO2 emitted by anthropogenic sources, which reacted with H2O2. In contrast, in the eastern Pacific Ocean far away from polluting anthropogenic sources, it is possible that H2O2 oxidized the sulfur dioxide formed by photochemical reactions of biogenic sulfur compounds. According to Becker et al. (1993), the non–sea–salt sulfate is produced by oxidation of biogenic sulfur compounds in the presence of water vapor. The positive correlation between H2O2 and non–sea–salt sulfate (SO42–xs) could simply indicate that higher H2O2 concentrations can oxidize higher amounts of sulfur dioxide producing higher concentrations of (SO42–xs).
On the other hand, the lack of significant correlation between sea–salt sulfate and total peroxides (–0.007, at a p < 0.05) could be due to the fact that H2O2 does not react with the SO42– already present in sea salt aerosols. Also, the concentration of total peroxides in rainwater samples collected in this study did not correlate with rain rate. The reason could be the significant fraction of H2O2 that is generated within cloud droplets (Zika et al., 1982).
4. Conclusions
Most of the non–sea–salt sulfate and total peroxides, observed in the eastern Pacific Ocean, could have been produced by the oxidation of biogenic sulfur compounds.
The significant correlation between total peroxides concentrations and excess sulfate observed in rainwater in the eastern Pacific Ocean, could simply indicate that higher H2O2 concentrations can oxidize higher amounts of sulfur dioxide, produced by oxidation of biogenic sulfur compounds, resulting in higher concentrations of (SO42–xs).
Total peroxides did not correlate with sea–salt sulfate because H2O2 does not react with the sulfate already present in sea salt aerosols.
The concentration of total peroxides in rainwater samples collected in this study did not correlate with rain intensity, suggesting that a significant fraction of H2O2 is generated within cloud droplets.
Acknowledgements
The authors wish to thank Dr. Rosa Cerón and Calixto Cuevas for the rainwater sampling, and Dr. Ernesto Caetano for providing navigation information of the R/V El Puma.
References
Avery Jr. G. B., J. D. Willey and R. J. Kieber, 2001. Diurnal variations in major rainwater components at a coastal site in North Caroline. Atmos. Environ. 35, 3927–3933. [ Links ]
Becker K. H., J. Bechara and K. J. Brockmann, 1993. Studies on the formation of H2O2 in the ozonolysis of alkenes. Atmos. Environ. 27A, 57–61. [ Links ]
Cooper W. J., E. S. Saltzman and R. G. Zika, 1987. The contribution of rainwater to variability in surface ocean hydrogen peroxide. J. Geophys. Res. 92, 2970–2980. [ Links ]
Deng Y. and Y. Zuo, 1999. Factors affecting the levels of hydrogen peroxide in rainwater. Atmos. Environ. 33, 1469–1478. [ Links ]
Glaser J. A., D. L. Foerst, G. D. McKee, S. A. Quave and W. L. Budde, 1981. Trace analyses for wastewaters. Environ. Sci. Technol. 15, 1426–1435. [ Links ]
Guilbault G. G., P. J. Brignac Jr. and M. Juneau, 1968. New substrate for the fluorometric determination of oxidative enzyme. Anal. Chem. 40, 1256–1263. [ Links ]
Kelly T. J., P. H. Daum and S. E. Schwartz, 1985. Measurement of peroxides in cloudwater and rain. J. Geophys. Res. 90 (D5), 7861–7871. [ Links ]
Kok G. L., 1980. Measurements of hydrogen peroxide in rainwater. Atmos. Environ. 14, 653–656. [ Links ]
Kok G. L., K. Thompson, A. L. Lazrus and S. E. McLaren, 1986. Derivatization technique forthe determination of peroxides in precipitation. Anal. Chem. 58, 1192–1194. [ Links ]
Lazrus A. L., G. L. Kok, S. N. Gitlin, J. A. Lind and S. E. McLaren, 1985. Automated fluorometric method for hydrogen peroxide in atmospheric precipitation. Anal. Chem. 57, 917–922. [ Links ]
Olszyna K. J., J. F. Meagher and E. M. Bailey, 1988. Gas–phase, cloud and rain–water measurements of hydrogen peroxide at a high–elevation site. Atmos. Environ. 22, 1699–1706. [ Links ]
Ortiz V., M. A. Rubio and E. A Lissi, 2000. Hydrogen peroxide deposition and decomposition in rain and dew waters. Atmos. Environ. 34, 1139–1146. [ Links ]
Yoshizumi K., K. Aoki, I. Nouchi, T. Okita, T. Kobayashi, S. Kamakura and M. Tajima, 1984. Measurements of the concentration in rainwater and of the Henry's law constant of hydrogen peroxide. Atmos. Environ. 18, 395–401. [ Links ]
Yuan J. and A. M. Shiller, 2000. The variation of hydrogen peroxide in rainwater over the South and Central Atlantic Ocean. Atmos. Environ. 34, 3973–3980. [ Links ]
Zika R., E. Saltzman, W. L. Chameides and D. D. Davis, 1982. H2O2 levels in rainwater collected in South Florida and the Bahama Islands. J. Geophys. Res. 87, 5015–5017. [ Links ]














