Introduction
Molecular markers, e.g., microsatellites or simple sequence repeats (SSRs), are useful in order to generate linkage maps and so to locate polygenes which affects quantitative trait loci (QTL). Börner et al. (2002) described QTL as controlling agronomic traits due to the environment. Additionally, research has also been directed to detect QTL controlling the phenotypic responses to abiotic and biotic stresses or relationships between seedlings and microbes (Faris, Li, Liu, Chen and Gill, 1999; Díaz De León et al., 2011; Rojas, Castellanos and Díaz De León, 2013; Díaz De León, Castellanos, Ling, Rojas and Röder, 2015 ). At present, we know that an ample diversity of microorganisms continuously interacts and influences the growth and development of plants. Buerstmayr, Ban and Anderson (2009) reviewed a vast number of studies on the evaluation of Fusarium head blight and informed that all chromosomes, except 7D, presented QTLs at leaf or ear wheat level. Additionally, Faris et al. (1999) reported over 50 loci on the wheat genome, representing several classes of defense response (DR) genes responding to diverse rust infections on wheat leaf or stem level. At the root level, Williams et al. (2002) identified the Rlnn1gene conferring resistance to the nematode Pratylenchus neglectus in the Australian spring wheat variety ‘Excalibur’ using bulked segregant analysis and genetic mapping. Zwart, Thompson and Godwin (2005) reported QTLs on a polymorphic mapping population for Pratylenchus thornei and P. neglectus. So, at the time, the use of these markers in breeding programs increased wheat reservoirs against Pratylenchus spp. (Nicol and Ortiz-Monasterio, 2004). Also, at the root level, plant growth-promoting rhizobacteria (PGPR) positively affects growth. Among several bacteria, Azospirillum brasilense, Azotobacter vinelandii, and Pseudomonas stutzeri have been distinguished as PGPR, which exerts a positive effect when interacting with plant Azospirillum spp. is an important free-living bacteria due to its plant growth promotion and association with major economic cereals and other non-cereal plants such as tomato, pepper, cotton, soybean, and safflower (Nosheen et al., 2011). The growth response of diverse plants has tested positive after Azospirillumspp. inoculation at pot level, however, it is not the case on field experiments (Okon and Labandera, 1994; Díaz-Zorita and Fernandez-Canigia, 2009; Bashan and de-Bashan, 2010). However, the identity of QTL or plant genes enabling such PGPR-plant root associations and their role in supporting or enhancing these beneficial plant-microbe interactions are scarce (Remans et al., 2008; Díaz De León et al., 2015). Reports on QTL related to agronomic phenotyping affected by Azospirillum spp. presence had been reported in beans (Remans et al., 2008) and at the seedling stage on wheat (Díaz De León et al., 2015). Rojas et al. (2013) suggested that allelic state at various wheat host gene(s) influences Azospirillum spp. adhesion. In this way, controversy on the benefit of Azospirillum spp. inoculation in bread wheat (Millet, Avivi and Feldman, 1982; Okon and Labandera, 1994; Rodriguez-Sala, Nogueira, de Freitas and Parada 2007; Díaz-Zorita and Fernández-Canigia, 2009) reflected the genetically determined ability of cultivars to adhere Azospirillumspp. Thanks to the availability and use of plenty of molecular markers, it was possible to dissect the location of QTL on wheat chromosomes which control the expression of quantitative agronomic traits under the influence of A. brasilense inoculant using a highly dense molecular marker linkage map.
Materials and Methods
Bacterial strain, growth conditions, and preparation of inoculum
Azospirillum brasilense Cd strain (DSM 1843, Braunschweig, Germany) was grown in nutrient broth at 30 °C for 24 h at 120 rpm and harvested by centrifugation at 1000 g for 15 min. The cell button was resuspended in 0.75% saline solution and brought to a 106 CFU mL-1 concentration.
Plant material and field experiments
The mapping population SCUBA1+ consisted of 110 doubled-haploid (DH) lines derived from the cross of Opata × WSHD67.2(257), including the two progenitors, Triticum aestivum cv. Opata and synthetic hexaploid WSHD67.2 (257). The cultivar Opata is high-yielding bread wheat selected from the hybridization Blue Jay (SIB)/Jupateco 73 released by CIMMYT. A. brasilense, besides classified as salt tolerant (Díaz De León et al., 2011), adheres to Opata roots. In contrast, the synthetic hexaploid WSHD67.2 (257), also produced by CIMMYT, is a doubled haploid derived from the cross D67.2/P66270//Ae. squarrosa (257) and A. brasilense Cd does not adhere to its roots (Rojas et al., 2013).
We conducted two field experiments for two consecutive years in a sandy loam soil at CIBNOR experimental station located at La Paz, Baja California Sur, Mexico, where the climate is arid and hot. A total of 120 seeds per DH lineswere planted distributed over three 1 m rows (inter-seed distance 2 cm, inter-row distance 30 cm, and 40 seed per meter), arranged in triplicated 0.9 m × 1.6 m randomized plots. Control plots were irrigated with water pumped from a well of 1.0 dS m-1 EC) twice weekly for 3 h. The plots inoculated with A. brasilense Cd contained in 400 L of bacteria solution (106 A. brasilense CFUmL-1) also were irrigated with water pumped from a well, but for 2.5 h, afterward, 400 L of bacterial solution were pumped through the irrigation lines for 30 min. We took five years from the central row of each repetition and measured seven agronomic traits; Ear length (El). Spikelet number (Spkl). Grain number (Gn), calculated % of fertility (%F) = ((Gn)*100)/(Spkl*3)) (assuming that each spikelet yields three seeds)- Grain weight per ear (Gw). Tiller number (Tn) and total yield (Yld) from the cultivated central row.
Molecular marker analysis
Genomic DNA was isolated from pooled leaves of six plants of 8-days-old seedlings of the 110 DH lines and the parents using a modified CTAB method described by Doyle and Doyle (1990). A total of 96 DH lines (including four controls) were analysed with the Illumina wheat 9K iSelect Beadchip assay (Cavanagh et al., 2013) according to the manufacturer’s recommendations and protocols (Illumina), by TraitGenetics GmbH. Before use, DNA sample quality and quantity were assessed using fluorometry and agarose gels. Data analysis was carried out with the Genome Studio software. Allele calling was performed using a cluster file developed previously at Trait Genetics based on wheat lines that mainly represent European breeding material. No manual modifications were done following the evaluation via cluster file. The genotype data was entered into a MySQL database for quality control and downstream analysis. Altogether, out of 8632 markers on the array, 7627 (88%) were functional and could be scored. Following marker analysis, the data were assembled into a genotype table containing 1006 failed markers, 1922 monomorphic markers, and 5704 polymorphic markers.
Complementary, we isolated genomic DNA from 4 grains, manually crushed and powdered, of each of the 110 DH lines and the parents using a modified CTAB method described by Doyle and Doyle (1990). We diluted DNA in distilled water to a concentration of 5-10 ng μL-1 before use in SSR analysis. PCR reactions and amplification of SSR markers, Gatersleben wheat microsatellite (Xgwm), were performed as described by Röder et al. (1998). Fragments were detected by an Automated Laser Fluorescence (ALFexpres) sequencer (Amersham Biosciences Europe GmbH, Freiburg, Germany) using a polyacrylamide gel. The fragment sizes were calculated using the computer program Fragment Analyser Version 1.02 (Amersham Biosciences) using internal and external size standards.
Genetic map construction
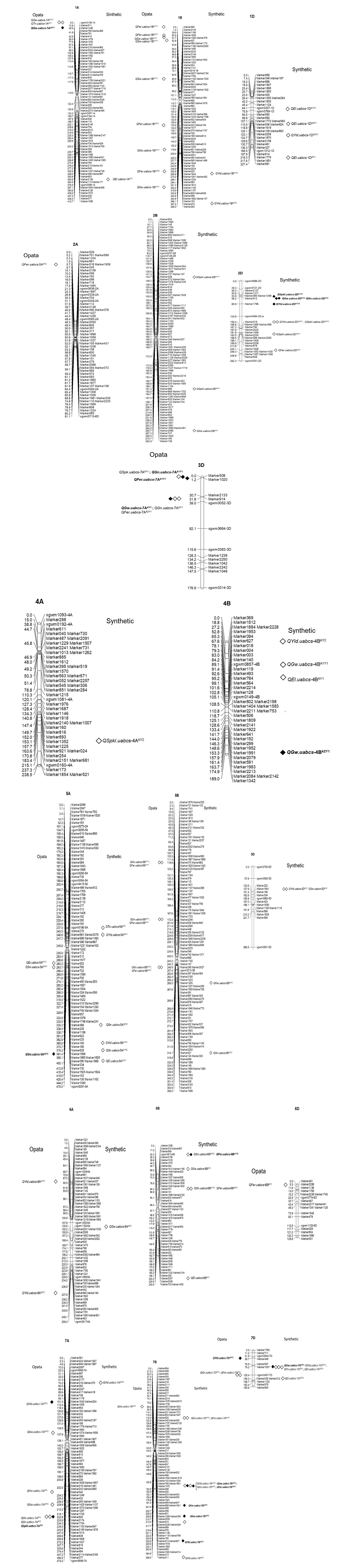
We used 157 polymorphic SSR and 5704 polymorphic SNP markers to make a linkage map. JoinMap 4.0 (van Ooijen, 2006) was used to determine the linkage groups and the approximate positions of centromeres. All microsatellite marker loci on the linkage groups of the 21 chromosomes were assigned using information from the genetic map for the ITMI population (Röder et al.,1998; Ganal and Röder, 2007).
Correlation and QTL analysis
We carried out Pearson’s correlations using Statsoft (2001). We performed QTL analysis using the software package GenStat 14th edition [VSN International, Hemel Hempstead, Hertfordshire, UK], the ‘QTL analysis’ module, and the ‘single trait linkage analysis’ function. The QTL analysis included all the mapped molecular markers. QTLs were identified via simple interval mapping using the default parameters. For selected QTLs, the marker loci within distances of 25 cM were combined. QTLs were classified ‘’major’’ or ‘’minor’’ according to whether the associated Logarithm of Odds (LOD) was greater or less than 3; those with a LOD of less than two were not considered. The notation for individual QTL followed the recommended format: Qphenotype.institution-chromosome location (e.g., QAdh.uabcs-5A, where Adh refers to Adh+ trait, uabcs the Universidad Autonoma de Baja California Sur, and 5A a place of QTL on chromosome 5A). Additionally, to distinguish those QTL under A. brasilense inoculation and those from control plants, designations were complemented as follows: QAdh.uabcs-5A followed by an A or C,nQTL,Y; A = inoculation with A. brasilense; C = control; nQTL = number of QTL in the same chromosome, Y = year detected: 1 or 2, e.g., QAdh.uabcs-5AA2Y1 means a QTL on chromosome 5A, detected under A. brasilense inoculation, it is the second QTL on the same chromosome and identified in the year 1 (Annex 3).
Results and Discussion
Phenotypic characterization
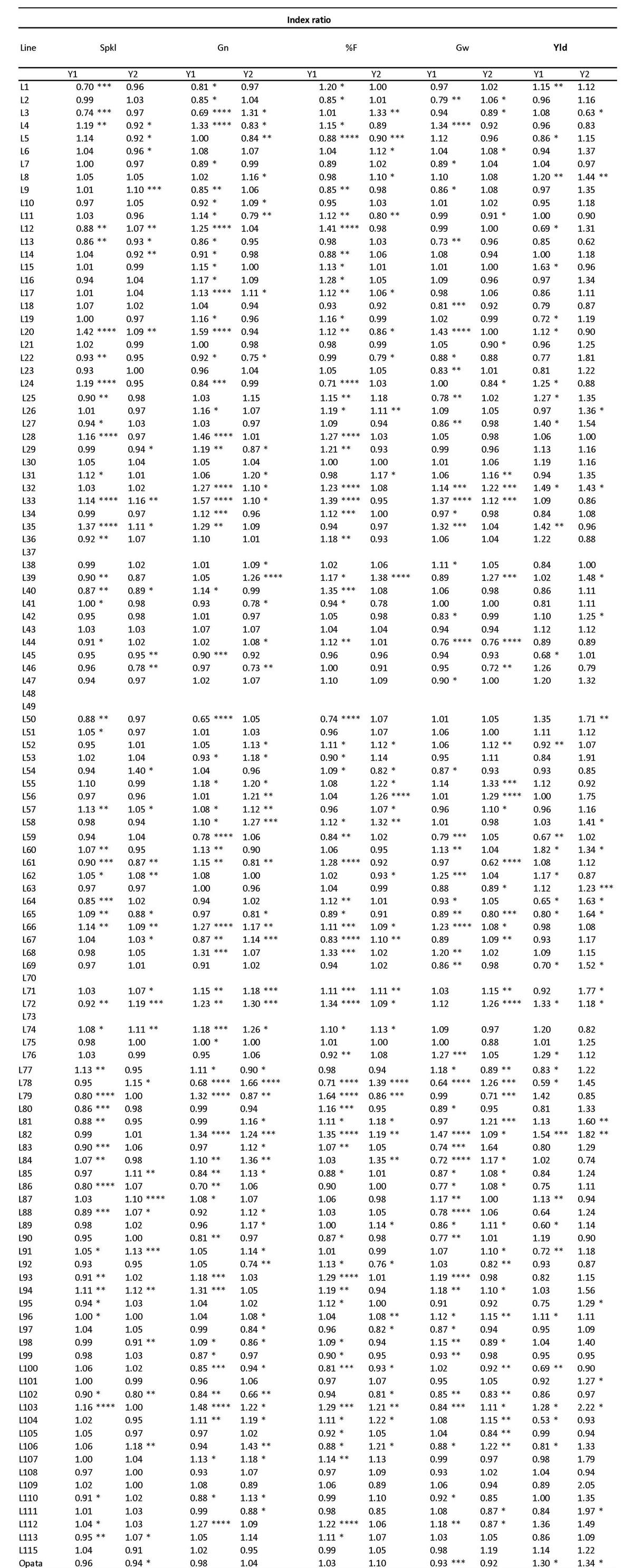
Performance of the DH lines and correlations among yield trials. The values of simple correlations among the seven tested traits in both years kept the same correlations. The correlations were positive except for trait Tn (Annex 1). From one year to another, the differences in measured values for the traits of the cultivar Opata showed that Spkl and Gn decreased significantly while Gw and %F had an increase, and Yld did not vary significantly. The average of the DH lines presented the same significant decrement for Spkl but increment for Gn, Gw, %F, and Yld (Table 1). The same results were observed in control and A. brasilense inoculated DH lines.
Table 1: Phenotypic characterization of the SCUBA 1+ mapping population inoculated with Azospirillum brasilense.
| El | Spkl | Gn E-1 | F | Gw E-1 | Tn | Yld | ||
| Control | g | % | g | g | ||||
| Opata | ||||||||
| Year 1 | 10.69a | 20.80a | 42.93a | 68.80a | 2.21a | 48.33b | 76.68b | |
| Year 2 | n.d. | 14.93a | 36.60a | 82.44a | 2.91a | n.d. | 76.81b | |
| Average All lines | ||||||||
| Year 1 | 9.21 | 19.10 | 38.25 | 66.88 | 1.95 | 51.65* | 72.84 | |
| Year 2 | n.d. | 15.78 | 41.57 | 87.57 | 3.31 | 51.65* | 86.21 | |
| +Az | ||||||||
| Opata | ||||||||
| Year 1 | 10.21a | 19.87b | 42.13a | 70.69a | 2.06b | 61.00a | 99.66a | |
| Year 2 | n.d. | 14.07b | 38.13a | 90.30a | 2.67a | n.d. | 102.70a | |
| Average All lines | ||||||||
| Year 1 | 9.50* | 18.95 | 39.60* | 69.48* | 1.93 | 49.78 | 71.09 | |
| Year 2 | 15.89 | 43.08* | 90.07* | 3.33 | n.d. | 100.65* |
Under T-student test analysis: a.b = significant difference (P < 0.05) between Opata -Az and Opata +Az; *= significant difference (P < 0.05) between lines -Az and +Az.
One of our goals was to check out if the detected adhesion phenotype (Adh+) in 37 Adh+ lines of the SCUBA1+ mapping population (Rojas et al., 2013) was associated with an impact on yield trait. We found that the 37 Adh+ DH lines spliced in three subsets in the first year. The first subset consisted of 24.3% of lines where the Adh+ phenotype correlates with a positive effect on yield. The second subset consisted of 59% of lines where A. brasilense had no impact on yield, though it could positively or negatively impact other traits. Furthermore, the third subset consisted of 13.5% of lines where the Adh+ phenotype correlates negatively with yield. Furthermore, we detected a set of lines (7 lines) that did not present the Adh+ phenotype because the presence of A. brasilense had a positive effect on yield and other traits tested (Annex 2). For the second year, the set of 37 Adh+ DH lines was split into two groups. The first subset consisted of 16.2% of lines where the Adh+ phenotype correlates with a positive effect on yield. Furthermore, the second subset consisted of 81.1% of lines where A. brasilense had no impact on yield, though it could positively or negatively impact other traits. We detected 14 DH lines that did not present the Adh+ phenotype but because the presence of A. brasilense presented a positive effect on yield and other traits tested (Annex 2).
In summary, regarding the phenotypic characterization in the field, the mapping population SCUBA 1+ was segregated into three subpopulations when tested in the presence of A. brasilense: neutral, negatively, and positively affected. Out of the 110 lines, 19 lines practically presented stimulation in all its traits, including yield, and so they become part of a collection of wheat genotypes that interact positively with A. brasilense (Annex 2).
Effect of environmental conditions and A. brasilense inoculation on yield and yield components are traits of primary importance, and a precise phenotyping of these phenotypes is made difficult by the interactions between the environment and the genotype (Robert, Berard and Hennequet, 2001). The observed agronomic variations in yield components seem to be explainable by GE, as it has also been documented in other wheat mapping populations when tested in different regions (Groos, Robert, Bervas and Charmet, 2003). Our experimental station is located in an environment with average solar irradiation of 230-240 W/m2 and climate type Bwh (very warm and dry) (Díaz De León et al., 2011). Nonetheless, the trend observed pointed out that A. brasilense favored an increment of Yld significantly, as it has reported earlier for Opata or other wheat varieties (Saubidet, Fatta and Barneix, 2002; Díaz-Zorita and Fernández-Canigia, 2009; Díaz De León et al., 2011). The variation of the effect of A. brasilense, comparing line per line or trait, confirms that the association of A. brasilense and its effects are genotype-dependent, as published elsewhere (Millet et al., 1984; Baldani, Baldani and Döbereiner, 1987; Kapulnik, Okon and Henis, 1987; Rojas et al., 2013). The segregation of the population in a neutral, positive and negative effect under the influence of A. brasilense on the tested traits is not surprising. The benefits of A. brasilense on earlier stages of growth and development were reported not to be consistent at final stages, e.g., yield or yield components (Díaz-Zorita and Fernández-Canigia, 2009) or if tested at a different location over different years as observed in the majority of T. aestivum cultivars tested (Kapulnik et al., 1987). Hungria, Campo, Souza and Pedrosa (2010) reported that the choice of Azospirillum strain is of great importance to have consistent results on the benefits of Azospirillum spp. on yield. However, other reports pointed out that the action of Azospirillum spp. is wheat genotype dependent (Kapulnik et al., 1987; Rojas et al., 2013). It seems wise to conclude that both are complementary and of utmost importance to optimize a successful interaction and that the SCUBA1+ population segregation results represent both criteria.
Genotypic characterization
Our analysis showed that 49 major QTL and 150 minor QTL controlled the agronomic traits distributed on all chromosome groups of genomes A, B, and D (Annex 3). We found very few constitutive QTL from one year to another, and some were expressed on control and inoculated plants in the same year. Twenty-four major QTL and 67 minor QTL controlled the quantitative trait expression on control plants. In the presence of A. brasilense, 18 major QTL and 83 minor QTL controlled the expression of measured phenotypes (Annex 3).
This manuscript is the first report on QTL controlling agronomic traits under A. brasilense fertilization for entire seasons. We found pleiotropic QTL on chromosomes 1A, 2D, 3D, group 5, and group7. The most notorious was group 5 and group 7, where QTL controlled 4 of 6 traits tested (Table 2). Previously, it has been reported that QTL controls traits under several abiotic stressors in the same span region of chromosome 7A (Quarrie et al., 2006) and QTL for yield components associated with wheat chromosome 5A and 2D (Kumar, Kulwal, Balyan and Gupta, 2007).
Table 2: Pleiotropic QTL detected for yield and yield components in mapping population SCUBA1+
| TRAITS | Chromosome arm | Marker interval |
| Tn. Gw | 1AS | Xgwm136-1A – marker511 |
| Spkl. Gw. Yld | 2DS | Xgwm0721-2D – marker1789 |
| Spkl. Yld | 2DL | marker019 – marker2023 |
| Spkl. Fer. Gn | 3DS | marker508 – marker1020 |
| Fer. Gn. Gw | 3DS | marker2133 – Xgwm0052-3D |
| Tn. El. Gw. Yld | 5AL | marker282 – marker702 |
| El. Gn. Gw. Yld | 5AL | marker1188 – marker135 |
| Fer. Gn | 5BS | marker1168 – marker1164 |
| Fer. Gn | 5BS | marker175 – marker2246 |
| Fer. Gn | 5BL | marker669 – marker2027 |
| Fer. Gn | 5BL | marker650 – marker649 |
| Gw. Yld | 5DS | marker222 – marker146 |
| Fer. Gn | 6BS | marker568 – marker2155 |
| Fer. Gn | 6BS | marker1305 – marker667 |
| Spkl. Fer. Gn. Gw | 7AL | marker2190 – marker1137 |
| Fer. Gn. Gw. Yld | 7BL | marker1244-marker005 |
| Fer. El. Gw. Yld | 7BL | marker005 – marker165 |
| Tn. El. Gw. Yld | 7DS | markerXgwm0044-7D – marker1037 |
| El. Fer. Gn | 7DL | markerxgwm0437-7D – marker575 |
Yld trait
Under the absence of A. brasilense, three major QTL located on chromosomes 7A, 7B, and 2D controlled Yld (Annex 3). Six minor QTL located on chromosomes 5A, 6A, 3B, 4B, and 7B accompanied the major QTL. The major QTL QYld.uabcs-2DCY2 explained 28.9% of the variation in the population (Annex 3). None of the QTL showed to be constitutive between the years tested. On the other hand, under A. brasilense, four major QTL controlled this trait and were located on chromosomes 7A, 7B, 2D, and 7D. Fifteen minor QTLs located on chromosomes 5A, 6A, 7A, 1B, 4B, 7B, 2D, 5D, 6D, and 7D accompanied these major QTLs (Annex 3). The major QTL QYld.uabcs-7AA2Y1 explained 11.9% of the variation, while Qld.uabcs-2DA1Y2 and Qld.uabcs-2DCY2 explained 28.2% and 28.9%, respectively, of the variation of the population (Annex 3). The rest of QTLs explained the population in low proportion.
We found that for the Yld trait, under control conditions or inoculated with A. brasilense, 3 QTL located on chromosome 7A and 6 QTL in chromosome 7B controlled this trait. Quarrie et al. (2006) identified major QTL in homologous locations on 7AL and 7BL, respectively, under stressed and non-stressed conditions. The 7AL yield QTL was associated with biomass at maturity and tiller and ear weight, significantly higher flag leaf chlorophyll content, and broader flag leaves (Quarrie et al., 2006). Besides those QTL described by Quarrie et al. (2006) for controlling yield trait under nutrient, nitrogen, drought, or ozone stress, reported linking maps showed that major QTL QYld.uabcs-7AA2Y1 and minor QYld.uabcs-7AA1Y1 (Annex 4 Group 7), located in the same region of that described controlling the growth of seedling leaf QTL QLls8.uabcs-7A under salt stress (García-Suárez, Röder and Díaz De León, 2010). Also, the major and minor QTL QYld.uabcs-7BC3Y2 and Qld.uabcs-7BA3Y2 (Annex 4, Group 7) are located in the same chromosome region as the minor QTL QYld.uabcs-7B of a wheat population tested under salt stress (Díaz De León et al., 2011).
Gw trait
Under the absence of A. brasilense, the Gw trait was controlled by six major QTL located on chromosomes 5A, 1B, 7B, and 2D (Table 2). Eight minor QTL located on chromosomes 1A, 2A, 5A, 7A, 2B, 7B, and 7D were present also. The major QTL QGw.uabcs-2DCY2 explained 11.71% of the variation in the population (Annex 3). None of the QTL showed to be constitutive between the years tested. On the other hand, under A. brasilense, ten major QTL controlled this trait and were located on chromosomes 1A, 5A, 4B, 7B, 2D, 3D, and 7D. Also, 15 minor QTL located on chromosomes 1A, 5A, 6A, 7A, 1B, 2B, 4B, 6B, 7B and 5D (Annex 3). The major QTL QGw.uabcs-3DAY1 explained 11.9% of the variation, while GQ.uabcs-2DAY2 explained 8.4% and QGw.uabcs-5AA2Y1 explained 7.7% of the variation of the population (Annex 3). However, the minor QTL QGw.uabcs-1AA1Y1 explained 15.92% of the variation in the population (Annex 3). The rest of the major QTL explained the variation in minor proportion.
Gn trait
Irrigation without A. brasilense showed that Gn phenotype was under the control of 5 major QTL located on chromosomes 4A, 5A, 7A, 2D, and 7D and 15 minor QTL located on chromosomes 1A, 2A, 3A, 4A, 5A, 7A, 2B, 3B, 5B, 6B, 7B, 3D, and 7D (Annex 3). The major QTL QGn.uabcs-7AC2Y2 and QGn.uabcs-2DCY2 explained 7.32% and 7.88% of the population variation, respectively (Annex 3). Under the control experiment, the QTL QGn.uabcs-4AC1Y1 and QGn.uabcs-4AC1Y2 presented as constitutive minor QTL inter-years. On the other hand, under A. brasilense, two major QTL controlled this trait and were located in chromosomes 2D and 3D, accompanied by 15 minor QTL located on chromosomes 5A, 7A, 5B, 6B, 7B, 3D, and 7D (Annex 3). The major QTL QGn.uabcs-2DAY2 explained 7.33% of the variation, while GQ.uabcs-3DA11Y1 explained 15.4% of the variation in the population (Annex 3). The rest of the major QTL explained them in a lower proportion.
%f trait
Under the absence of A. brasilense, the %F was controlled by six major QTL located on chromosomes 1A, 3A, 4A, 7A, and 7D, accompanied by 18 minor QTL located on chromosomes 1A, 4A, 7A, and 7D (Annex 3). The major QTL QFer.uabcs-4AC2Y2 explained 7.05% of the variation in the population (Annex 3). Under the control experiment, the QTL QFer.uabcs-5AC2Y1 and QFer.uabcs-5AC2Y2 presented as constitutive minor QTL inter-years. On the other hand, A. brasilense induced four major QTL controlling this trait located on chromosomes 6B, 7B, and 3D, accompanied by 17 minor QTL located on chromosomes 2A, 7A, 1B, 5B, 6B, 7B, 2D, 3D, 6D and 7D (Annex 3). The major QTL QFer.uabcs-6BA1Y2 explained 6.61% of the variation as minor QTL QFer.uabcs-7BA4Y2 and QFer.uabcs-3DA2Y1. However, minor QTL QFer.uabcs-1BA1Y2 explained 8.44% of the variation in the population (Annex 3). The rest of the major and minor QTL explained them in low proportion.
Spkl trait
Under the absence of A. brasilense, the Spkl phenotype was controlled by three major QTL located on chromosomes 2D and 3D, accompanying nine minor QTL located on chromosomes 1A, 5A, 7A, 2B, 5B, 1D, 2D, and 3D. The major QTL QSpkl.uabcs-2DC1Y1 and QSpkl.uabcs-2DC2Y2 explained 7.3% and 16.8% of the population variation, respectively; minor QSpkl.uabcs-2DC3Y1 explained 10.8% of the variation in the population (Annex 3). On the other hand, under A. brasilense, two major QTLs controlled this trait and were located on chromosomes 7A and 2D, with seven minor QTLs located on chromosomes 4A, 2B, 7B, 2D, and 3D (Annex 3). The major QTL QSpkl.uabcs-2DA1Y2 and QSpkl.uabcs-7AAY2 explained 19.6% and 9.24% of the variation in the mapping population, respectively (Annex 3). The rest of the major and minor QTL explained the variation of the population in low proportion.
Tn trait
This trait was only evaluated in the first year, and in the absence of A. brasilense, the Tn trait was controlled by four minor QTL located on chromosomes 5A, 6A, 2B, and 7D (Annex 3). The minor QTL QTn.uabcs-2BCY1 and QTn.uabcs-6ACY1 explained 6.8% and 7.1% of the variation in the mapping population, respectively (Annex 3). On the other hand, under A. brasilense, three minor QTL controlled this trait and were located on chromosomes 1A, 5A, and 7D. The minor QTL QTL QTn.uabcs-1AAY1, QTn.uabcs-5AAY1 and QTn.uabcs-7DAY1 explained 7.68, 6.43% and 7.78% of the variation of the mapping population, respectively (Annex 3).
El trait
In the first year, without A. brasilense, the El was controlled by one major QTL located on chromosomes 5A and seven minor QTL distributed on chromosomes 2A, 2B, 2D, 4A, 5A, and 7B (Annex 3). Although major QTL QEl.uabcs-5ACY1 explained 6.8% of the variation in the mapping population, the minor QTL exhibited similar percentages (Annex 3). On the other hand, under A. brasilense, two major QTLs that controlled this trait were located on chromosomes 2D and 11 minor QTLs distributed on chromosomes 1A, 1D, 2D, 3A, 4B, 5A, 6B, and 7D (Annex 3). The major QTL QEl.uabcs-2DAY1 explained 8.4% of the variation in the population. However, other minor QTL presented similar or higher values, e.g., minor QEl.uabcs-1DA1Y1 (Annex 3).
Synteny of wheat marker-QTL associated sequences with rice gene sequences
The QYld.uabcs-7AA2Y1 and QYld.uabcs-7AA1Y1 locate under a contiguous area of ca. 40 cm wide (Annex 4 Group 7) and have associated at least 12 molecular markers whose sequences were syntenic with rice sequences (Table 3). The marker 2177, the nearest to associated- marker 316 minor QYld.uabcs-7AA1Y1, is syntenic with a rice sequence that codifies for 69867 (Mohler, Klahr, Wenzel and Schwarz, 2002; Shuang-He, Ping and Xiang, 2004) The A G-protein subunit gene was found in homologous location to the wheat 7AL yield QTL (Quarrie et al., 2006). The marker 316 matched a rice sequence codifying for a coatomer subunit beta implicated in multiple physiological processes giving place to multi-ovary in wheat (Li et al., 2011). The marker 1542 linked to major QYld.uabcs-7AA2Y1 (Annex 4 Group 7) is homologous to a rice sequence codifying for a PRP8 splicing-type protein which has been involved in the regulation of gene expression by removal of introns from pre-mRNA transcripts which are a critical process in the maturation of mRNA (Simpson et al., 1992). The surrounding markers between QTLs QYld.uabcs-7AA2Y1 and Qld.uabcs-7AA1Y1 (Annex 4 Group 7) were syntenic to rice sequences codifying for the SEC14 cytosolic factor involved with signal transduction related proteins (Ndimba, Chivasa, Simon and Slabas, 2005) (Table 3), and peptidases of the T1 family that participate in the inhibition of alfa-amylases in the wheat kernel (Maeda, Kakabayashi and Matsubara, 1985). The ABC transporter family gene mediates transport in biological membranes, e.g. complex carbohydrates, or as proton-pumps or involved in the structure of ion channels and detoxification processes (Martinoia et al., 2002). The cinnamoyl Co-A reductase is involved in the lignin pathway biosynthesis, particularly in stem development in wheat (Ma, 2007). The AP-1 complex subunit gamma mediates cargo trafficking, continuous addition, and retrieval of proteins and lipids. The membrane coats contain clathrin which is a protein associated to the AP-1 and the AP-2 (Neubrand et al., 2005). The protein phosphatase 2C regulates phosphorylation/dephosphorylation processes and is found as a soluble cytosolic enzyme in wheat leaves (Mackintosh, Coggins and Cohen, 1991). A retrotransposon protein Ty3-gypsy is related to the plant genome evolution and its use as a genetic tool in plant biology in the Tritaceae family (Todorovska, 2007) (Table 3). The major QTL Qyld.uabcs-7BA2Y2 (Annex 4 Group 7) contains markers presenting synteny with rice chromosome related to a leucoanthocyanidin dioxygenase or anthocyanidin synthase. Both contribute to physiological functions such as seed colour, maturation, and dormancy (Shirley, 1998; Gu et al., 2011) (Table 3).
Table 3: Proteins associated to rice loci with high synteny with sequences of wheat markers linked to field QTL under A. brasilense biofertilization.
| Marker accesion | Wheat marker | % Ident (P-value) | Rice locus (CDS 3´-5´) | Product/phenotype | |
| Marker316 | wsnp_Ex_rep_c69123_68034403 | 77.50 | LOC_Os02g11830.2 ( 6129217 – 6119039) | coatomer subunit beta. putative. expressed | |
| Marker2177 | wsnp_Ku_B4615_8326355 | 78.21 | LOC_Os11g03650 (1421232 – 1416436) | mla1. putative. expressed | |
| Marker1542 | wsnp_Ku_c12701_20446223 | 89.45 | LOC_Os05g07050 (3714513 – 3704179) | pre-mRNA-processing-splicing factor 8. putative. expressed | |
| Marker082 | wsnp_Ex_c13248_20898211 | 72.64 | LOC_Os05g18294 (10546650 – 10537386) | SEC14 cytosolic factor family protein. putative. expressed | |
| Marker417 | wsnp_Ku_c5938_10491100 | 84.34 | LOC_Os06g06440 (3009250 – 3001105) | ABC transporter. ATP-binding protein. putative. expressed | |
| 78.05 | LOC_Os11g05700 (2610544 – 2605745) | ABC transporter family protein. putative. expressed | |||
| Marker418 | wsnp_Ku_c5938_10491311 | 85.59 | LOC_Os06g06440.1 (3009250 - 3001105 | ABC transporter. ATP-binding protein. putative. expressed | |
| LOC_Os11g05700.1 (2610544 – 2605745) | ABC transporter family protein. putative. expressed | ||||
| Marker2247 | wsnp_RFL_BonAig2789_2553657 | 82.18 | LOC_Os06g06030 (2774076 – 2771069) | peptidase. T1 family. putative. expressed | |
| Marker708 | wsnp_Ex_A2017_3787478 | 61.33 | LOC_Os11g05700 (2610544 – 2605745) | ABC transporter family protein. putative. expressed | |
| 58.43 | LOC_Os01g21990 (12344460 – 12351233) | CRS2-associated factor 2. chloroplast precursor. putative. expressed | |||
| Marker1176 | wsnp_Ex_c41150_48040078 | 71.88 | LOC_Os02g30190 (17948296 – 17950941) | expressed protein | |
| 68.18 | LOC_Os01g45200 (25647224 – 25640719) | cinnamoyl-CoA reductase-related. putative. expressed | |||
| Marker204 | wsnp_Ex_c42653_49180485 | 87.50 | LOC_Os06g07090 (3376566 – 3386549) | AP-1 complex subunit gamma-1. putative. expressed | |
| Marker1006 | wsnp_Ra_rep_A69620_67130107 | 64.04 | LOC_Os02g13100 (6956581 – 6959847) | protein phosphatase 2C. putative. expressed | |
| 73.91 | LOC_Os05g51390 (29470011 – 29474254) | uncharacterized protein PA4923. putative. expressed | |||
| Marker954 | wsnp_Ku_A6065_10682531 | 65.04 | LOC_Os12g04910 (2108525 – 2113086) | retrotransposon protein. putative. Ty3-gypsy subclass. expressed | |
| Marker513 | wsnp_Ex_c11106_18003546 | 71.17 | LOC_Os06g08060 (3900488 – 3903056) | leucoanthocyanidin dioxygenase. putative. expressed | |
| 70.00 | LOC_Os06g08032 (3893672 – 3895400) | flavonol synthase/flavanone 3-hydroxylase. putative. expressed | |||
| Marker211 | wsnp_Ex_c46061_51675763 | 86.84 | LOC_Os06g03600 (1395804 – 1389013) | transcriptional corepressor SEUSS. putative. expressed | |
| Marker1789 | wsnp_BG275030D_Aa_2_2 | 78.18 | LOC_Os07g49320 (29534469 – 29545698) | HEAT repeat family protein. putative. expressed | |
| Marker449 | wsnp_Ra_c2930_5550811 | 74.75 | LOC_Os06g08740 (4376383 – 4370966) | expressed protein | |
| Marker1037 | wsnp_RFL_AonBig3557_3736656 | 85.15 | LOC_Os06g10430 (5359508 – 5367120) | protein of unknown function DUF1296 domain containing protein. expressed; Hydroxyproline-rich glycoprotein; Uridine kinase related. | |
| Marker937 | wsnp_Ku_A27286_37236472 | 88.56 | LOC_Os06g09880 (5035358 – 5029395) | EMB1270. putative. expressed; related to embryogenesis | |
| Marker1644 | wsnp_RFL_ContiA3951_4390396 | 73.12 | LOC_Os05g48800 (27971088 – 27967561) | drought induced 19 protein. putative. expressed. Protein DEHYDRATION-INDUCED 19; inn barley. Fiber protein Fb2 | |
| Marker2208 | wsnp_Ra_B17989_26960545 | 89.42 | LOC_Os01g01689 (335809 – 370910) | phosphatidylinositol 3- and 4-kinase family protein. expressed | |
| Marker1446 | wsnp_Ex_c7965_13520238 | 72.58 | LOC_Os02g43830 (26469280 – 26465591) | 3-isopropylmalate dehydratase small subunit 2. putative. expressed | |
| 69.50 | LOC_Os05g01780 (471400 – 476283) | STE_PAK_Ste20++TranslationKinase_Slob_Wnk.1 - STE kinases include homologs to sterile 7. sterile 11 and sterile 20 from yeast. expressed; orthologous gene serine/threonine-protein kinase WNK2 in maize. | |||
| 80.65 | LOC_Os08g33330 (20790427 – 20794816) | protein kinase PKN/PRK1. effector. putative. expressed | |||
| 79.37 | LOC_Os12g12470 ( | NADP-dependent oxidoreductase. putative. expressed | |||
| Marker1556 | wsnp_Ku_c28467_38394887 | 75.49 | LOC_Os02g02670 (988442 – 995506) | NBS-LRR disease resistance protein. putative. expressed | |
| Marker912 | wsnp_JD_rep_A63957_40798083 | 83.33 | LOC_Os04g12580 (6969039 – 6966603) | receptor-like protein kinase. putative. expressed | |
| Marker1841 | wsnp_Ex_B1278_2449191 | 80.65 | LOC_Os08g33330 (20790427 – 20794816) | protein kinase PKN/PRK1. effector. putative. expressed | |
| 79.37 | LOC_Os12g12470 (6869983 – 6872480) | NADP-dependent oxidoreductase. putative. expressed | |||
| Marker042 | wsnp_CAP11_c827_513472 | 74.58 | LOC_Os12g11410 (6159962 – 6157256) | retrotransposon protein. putative. LINE subclass. expressed | |
| 73.58 | LOC_Os02g03750 (1574900 – 1570082) | polygalacturonase. putative. expressed | |||
| 70.67 | LOC_Os07g29630 (17425456 – 17421301) | SNF7 domain containing protein. putative. expressed | |||
| Marker1248 | wsnp_bq170165B_TB_1_1 | 70.59 | LOC_Os01g03570 (1446849 – 1450473) | transcription factor X1. putative. expressed | |
| Marker432 | wsnp_Ku_rep_c110993_94857161 | 84.08 | LOC_Os06g38940 (23102037 – 23098857) | RMD5 homolog A. putative. expressed | |
Chromosome 7D participates with several QTL on different traits whose linked markers are associated with Qld.uabcs-7DA2Y1 (Annex 4 Group 7), QTn.uabcs-7DAY1 (Annex 4 Group 7), and QEl.uabcs-7DA1Y1 (Annex 4 Group 7), had sequences in synteny with rice sequences codifying for hydroxyproline-rich glycoproteins involved in cell wall structures. These proteins are also critical in the plant reproductive process of pollination as an important constituent of the pollen tube and the pistil (Toppan, Roby and Esquerré-Tugayé, 1982; Sommer-Knudsen, Bacic and Clarke, 1998), in this way, a successful ovule fertilization takes place (Wu, De Graaf, Mariani and Cheung, 2001). The linked-marker sequence of QGw.uabcs-7DAY2 had synteny with a rice sequence codifying for the EMB1270 family of pentatricopeptide repeat (PPR) proteins involved in the processes of embryogenesis (Cushing, Forsthoefel, Gestaut and Vernon, 2005). Under A. brasilense inoculation, chromosome 2D contributed with a major QTL QYld.uabcs-2DA1Y2, with the largest LOD signal, explaining 28.2% of the variation yield. This chromosome has been characterized as one involved in different kernel traits such as width, length, weight, and flour yield, which are under the control of QTL with nearby markers as Xwmc111, Xgwm261, Xwmc112 (Börner et al., 2002; Breseghello and Sorrels, 2006), in some regions as the major QTL Qld.uabcs-2DA1Y2, QGw.uabcs-2DAY2, QGw.uabcs-2DAY22 and QSpkl.uabcs-2DA1Y2 locate (Annex 4 Group 2). In this region, the short arm of chromosome 2D was associated with the dwarfing gene Rht8 and the photoperiodic insensitivity pleiotropic gene Ppd-D1, which play an important role in determining the geographic adaptation of modern wheat varieties (Pestsova and Röder, 2002). The sequence of marker1789 linked to Qld.uabcs-2DA1Y2 is syntenic to a sequence of rice codifying for a heat repeat family protein, whose main functions are related to condensins cohesins, and other complexes involved in chromosome-related functions (Neuwald and Hirano, 2000) (Table 3). Minor QTL accompanying the yield control, as QYld.uabcs-1BA1Y2 (Annex 4 Group 1), had a linked marker1644 whose sequence matched LOC_Os05g48800 of rice for a putative dehydrin protein. This protein, a member of a superfamily of proteins, accumulates in response to dehydrative processes, e.g. seed maturation (Close, 1996). The accompanying minor QTL QYld.uabcs-1DAY1 is linked to a marker2208 which sequence is syntenic with rice and codifies for a phosphatidylinositol 3- and 4-kinase family protein which seems to distribute specifically within the plant nucleus and nucleolus at the transcriptional level (Bunney et al., 2000) (Table 3).
Under A. brasilense inoculation, the Gw trait was controlled by seven major QTL located on chromosomes 1A, 5A, 4B, 7B, 2D, 3D, and 7D (Annex 4 Groups 1, 2, 3, 4, 5, and 7). Group 1, 2, and 5 are in synteny with chromosomes 5, 4, and 9 of rice. The sequence of the marker of QGw.uabcs-1AA2Y1 matched the rice sequence for a 3-isopropyl malate dehydratase (S-IPMD) small subunit two and Serine/Threonine-20 kinase (Ste-20 kinase) (Table 3). The S-IPMD is related to purine and amino acid biosynthesis in Arabidopsis thaliana due to salt and osmotic stress (Ndimba et al., 2005). The Ste-20 kinases are related to cell proliferation and cell death (Wu, Huang, Dong and Pan, 2003). Under osmotic stress, in yeast and soybean, Ste-20 kinases phosphorylate a mitogen-activated protein kinase (MAPK) and activates the MAPK cascade (Raitt, Posas and Saito, 2000; Phang et al., 2011). Also, the sequence of marker1556 of QGw.uabcs-5AA2Y2 is syntenic to a rice sequence codifying for leucine-rich repeats (LRRs). The LRRs are proteins which have a specific response and involvement in the plant cell death process (Belkhadir, Subramaniam and Dangl, 2004). The marker912 of QGw.uabcs-2DAY2 is syntenic with rice sequence codifying for a receptor-like protein kinase. This class of kinases appears to be serine/threonine protein kinases involved in signal transduction pathways (Walker, 1994), e.g. the abscisic acid pathway involved in seed maturation and seed dormancy events in A. thaliana (Osakabe et al., 2005). Two major QTLs control the Gn trait and are found on chromosomes 2D and 3D. The major QTL QGn.uabcs-2DAY2 is orthologous to major QTL QGw.uabcs-2DAY2 and QEl.uabcs-2DA1Y1, and its associated marker sequence presented synteny to the same sequence of rice LOC_Os04g12580 which codified for a receptor-like protein kinase (Table 3).
Conclusions
We present the effect of A. brasilense on different phenotype stages of a wheat mapping population. We detected 101 QTL who’s some of the nearest markers sequences to some of them were syntenic to rice sequences which codified for at least 38 candidate genes involved in processes of embryogenesis, seed formation, tissue formation, seed colour, inhibition of alfa amylase at the kernel level, purine and amino acid biosynthesis. This work is the first step to attempting to understand the interaction of A. brasilense throughout the life span of wheat and the basis to search for the heritability of wheat alleles to recognize and interact with A. brasilense.
Availability of Supporting Data
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
Authors’ Contributions
Conceptualization: T.C.C. and J.L.D.L. Methodology and data curation: T.C.C. and J.L.D.L. Formal analysis: J.L.D.L. Investigation: T.C.C. and J.L.D.L. Resources: M.R. Writing-original draft preparation: J.L.D.L. Writing-review and Editing: T.C.C., J.L.D.L. and M.R. Supervision: T.C.C. and J.L.D.L. Project administration and funding acquisition: T.C.C.











 nueva página del texto (beta)
nueva página del texto (beta)