Introduction
Plant diseases caused by microorganisms are one of the limitations during production, storage, and marketing of cereals, fruits, and vegetables. Loss in annual crop production at world level due to phytopathogen microorganisms has been estimated around 12% (Oerke, 2006; Pimentel, 2007). The use of agrochemicals as the only measure of plant disease control is not an alternative anymore. Phytopathogens have generated resistance to these products and their excessive use has resulted harmful for the environment and human health (Aktar et al., 2009; Areas et al., 2018). Antagonist microorganisms are an alternative to the use of agrochemicals for disease control because they are specific for certain phytopathogens, harmless for non-target species, innocuous for man, and eco-friendly (O’Brien, 2017).
Soil is a reservoir for a complex microbiome of prokaryotic organisms (eubacteria and archaebacteria) and eukaryotes (fungi and algae) that play an important role in biogeochemical processes (Kaviya et al., 2019). In soil, actinobacteria, commonly called actinomycetes, belong to the phylum Actinobacteria; they participate actively in biological processes, such as nitrogen fixation, phosphate solubilization, and dead organic material degradation (Bhatti et al., 2017). Additionally, as part of the rhizosphere microbiome, they promote plant growth and protect the crop against phytopathogens (Sharma and Salwan, 2018). This bacterial group is found widely distributed in land and aquatic environments. It is also a prominent source of natural bioactive products of importance to biotechnology, medicine, and agriculture (Bérdy, 2005; Goodfellow, 2015).
Actinobacteria are an alternative to the use of agrochemicals for phytopathogen control. Some commercial products contain Streptomyces lydicus WYEC 108 (Actinovate®; Valent USA) and Streptomyces sp. K61 (Mycostop®; Verdera, Oy, FI) spores as active ingredient, which have demonstrated the capacity to control some diseases caused by phytopathogen fungus and oomycetes (Lahdenperä, 1987; Crawford et al., 1993). Some actinobacterial species associated to the rhizosphere have demonstrated their role as antagonists against phytopathogens, such as Alternaria solani, Xanthomonas oryzae pv. oryzae and Bacillus pumilus (Dicko, 2013). Similar studies Intra et al. (2011) have demonstrated the antagonistic activity of actinobacterial strains isolated from the rhizosphere soil of different fruit trees against C. gloeosporioides and C. capsici. Recently, Suárez-Moreno et al. (2019) isolated actinobacterial strains from rhizosphere soil samples of rice crops (Oryza sativa), some of which showed very important antibacterial and antifungal activity against Burkholderia glumae one of the most important phytopathogens in rice crops. Similarly, Dai et al. (2019) isolated actinobacteria with antagonistic activity against the phytopathogen fungus Valsa mali from rhizosphere soil of wild apple trees.
Colletotrichum gloeosporioides is a phytopathogen fungus widely distributed that causes anthracnosis in avocado in pre- and post-harvest stages, as well as in other economically important crops (Dean et al., 2012). On the other hand, Xanthomonas species causes bacterial leaf spot of pepper, leading to severe damage in crop production (EFSA-PLH, 2014). Therefore, the objective of this study was to characterize the antagonistic in vitro activity of rhizosphere actinobacteria from avocado trees (Persea americana) against Colletotrichum gloeosporioides and Xanthomonas sp.
Materials and Methods
Soil sampling in an avocado orchard in Michoacán
Rhizosphere soil samples were collected during 2017 in the avocado orchard “El Zarco” in the Municipality of Ziracuaretiro, Michoacán, México (19° 23’ 26.5” N; 101° 51’ 42.8” W). The orchard of avocado var. Hass had a combined conventional and organic agronomic management. Four healthy avocado trees were selected randomly, and two soil samples per tree were taken in the drip irrigation area at a depth no greater than 15 cm. Soil samples were stored in high density polyethylene bags, taken to the laboratory at room temperature, and then air-dried for one week. Soil samples were sieved in a 1-mm mesh; those samples coming from the same tree were mixed in a 1:1 (w/w) ratio to obtain a compound sample and stored at 4 ºC to be used later.
Actinobacteria isolation
Actinobacteria were isolated with the spread plate technique starting from a compound sample of rhizosphere soil for each tree. From each tree, 10 g of soil were taken, deposited independently in dilution bottles with 90 mL of sterile water, and mixed for five min. Subsequently, serial dilutions were prepared in sterile distilled water until a 10-4 dilution was obtained for each soil suspension. A 100 µL aliquot of soil suspension of 10-2 up to 10-4 dilutions were inoculated in the isolation medium and disseminated with a Digralsky metal handle. The culture medium for isolation was potato-dextrose-agar (PDA); 2 g L-1 yeast extract (YE) were added, and the pH was adjusted to 7.0 with NaOH 3 M. The culture medium (PDA-YE) was supplemented with 12.5 mg L-1 of nalidixic acid and 50 mg L-1 of cycloheximide (Intra et al., 2011). Petri plates were incubated at 28 ºC from 7 to 14 days. The emerging actinobacterial type colonies with different morphological characteristics (Kämpfer, 2015) were selected. Spores and/or mycelia of the isolated actinobacteria were preserved with 25% glycerol at -80 ºC (Shepherd et al., 20210).
Phytopathogens: culture media and growth conditions
Colletotrichum gloeosporioides isolated from papaya fruit with typical anthracnosis symptoms was cultured in a routine manner in PDA medium at 28 ºC for 12 days in darkness. A fungal mycelial disk of 7 mm in diameter was used for the antagonism assay. Xanthomonas sp. BV801 previously isolated from a poblano Capsicum annuum pepper with typical symptoms of the bacterial leaf spot (López-Vielma et al., 2016) was cultured routinely in nutrient-yeast extract glycerol (NYG) liquid or solidified agar (NYGA) medium (5 g L-1 bacto peptone, 3 g L-1 yeast extract and 20 mL L-1 glycerol; the same solid medium contained 15 g L-1 agar) at 28 ºC for 16 or 48 h, respectively (Daniels et al., 1984).
Bioassays of in vitro antagonistic actinobacterial selection
Antagonistic activity against Colletotrichum gloeosporioides. The selection of actinobacteria with antagonistic activity against C. gloeosporioides was done by spot inoculation method (Shomura et al., 1979) with slight modifications. The actinobacteria strains isolated were streaked on Petri plates (45 mm in diameter) with PDA-YE medium, incubated at 28 ºC for seven days and obtained mycelium disks (7‑mm in diameter) from each strain using a cork borer. Of the four strains randomly selected, one mycelium disk was inoculated in each one of the four points equidistant to the center of the Petri plate onto PDA medium to then inoculate one active mycelium disk of C. gloeosporioides in the center (Figure 1). The same procedure was repeated with all the isolated strains. As growth control, one fungus mycelium disk was inoculated in the center of the Petri plates with PDA medium. All cultures were incubated at 28 ºC for 12 days. The antagonistic activity was determined by measuring the inhibition zone radius starting from the center of the disk of each actinobacteria until the phytopathogen fungus growth border was reached. All the actinobacterial strains and the control were performed in triplicate.
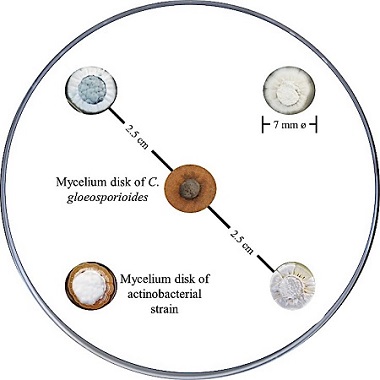
Figure 1: Graphic representation of the in vitro antagonistic activity assay for actinobacterial strain selection against Colletotrichum gloeosporioides.
Antagonistic activity against Xanthomonas sp. BV801. The antagonistic actinobacterial selection was performed by the double-layer agar method previously described by Salamoni et al. (2010) with slight modifications. The disks of each strain were obtained and inoculated as described previously in Petri plates (90 mm in diameter) in PDA medium and incubated at 28 ºC for five days before confrontation. Xanthomonas sp. BV801 grew 20 mL of the NYG medium at 30 ºC in agitation at 200 rpm for 16 h. The bacterial culture was adjusted to a OD600nm = 1 with the fresh NYG medium. The overlay agar (top layer) was prepared by mixing 400 µL of the bacterial culture in 4 mL of soft NYGA (0.6% agar) tempered in a thermo-water bath at 48 ºC and poured over the Petri plates that contained the actinobacterial mycelium disks. The same procedure was performed for the control group in Petri plates with PDA without actinobacteria. All the cultures were incubated for two days at 28 ºC. The antagonistic activity was determined by measuring the radius of inhibition zone of Xanthomonas sp. BV801 starting from the center of the disk of each actinobacteria until the inhibition border was reached. The data were expressed as inhibition zone diameter. All the actinobacterial strains and the control were performed in triplicate.
Experimental design and statistical analysis
A complete randomized experimental design was used where each actinobacteria corresponded to one treatment. The inhibition data of each actinobacterial strain were obtained from three independent biological replicates. The response variables of inhibition diameter and radius were analyzed by a one-way analysis of variance (ANOVA) and a comparison of means with Tukey’s test (P = 0.05) utilizing the statistical package StatGraphics Centurion XV (StatPoint Inc., 2005).
Results and Discussion
Actinobacterial antagonistic activity against Colletotrichum gloeosporioides and Xanthomonas sp. BV801
A total of 41 actinobacterial strains of different phenotypes were isolated from avocado tree soil. Of all the strains isolated, 44% resulted antagonistic against C. gloeosporioides or Xanthomonas sp. BV801 (Figures 2 and 3). Of this percentage, 15% (six isolates) corresponded to actinobacteria that only antagonized C. gloeosporioides growth; 22% (nine isolates) showed antimicrobial activity against Xanthomonas sp. BV801; 7% (three isolates) showed inhibition activity against both phytopathogen microorganisms.
The actinobacterial strains showed C. gloeosporioides growth inhibition radii, which fluctuated from 6.6 to 15.5 mm (Figure 2). Several strains showed outstanding activities; BVEZA1 02 strain recorded the greatest C. gloeosporioides mycelial growth inhibition radius, with significant differences with respect to the other strains (Tukey’s, P = 0.05). Moreover, the BVEZA2 17, BVEZA2 27, and BVEZA3 30 strains stood out because of their antifungal and antibacterial activities (Figures 2 and 3). With respect to the strains that showed antibacterial activities against Xanthomonas sp. BV801, the results indicated that the inhibition zones fluctuated from 15.5 to 62.7 mm in diameter (Figure 3). Three strains called BVEZA2 24, BVEZA4 34, and BVEZA4 38 stood out showing significant differences with respect to the inhibition diameters of the rest of the actinobacteria (Tukey’s, P = 0.05).
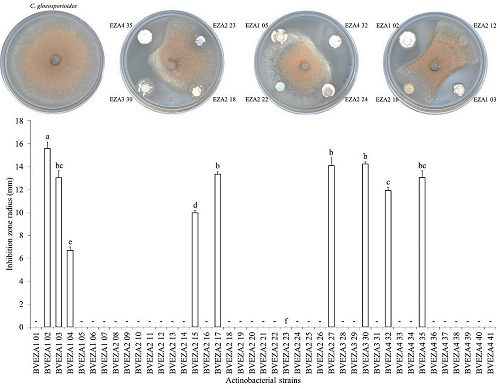
Figure 2: In vitro antagonistic activity of actinobacterial strains isolated from the rhizosphere of avocado trees on the growth of Colletotrichum gloeosporioides. The bars above the rectangle represent the standard deviation (n = 3). - = no inhibition. Different letters indicate significant differences according to Tukey’s test (P = 0.05).
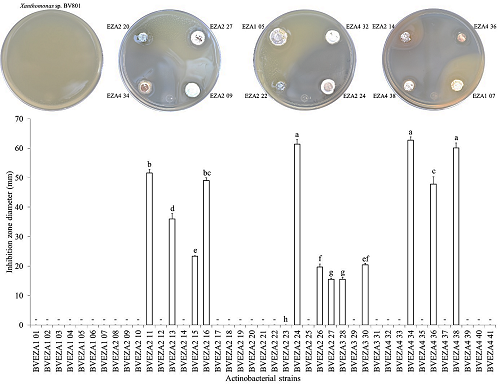
Figure 3: In vitro antagonistic activity of actinobacterial strains isolated from the rhizosphere of avocado trees on Xanthomonas sp. BV801 growth. The bars above the rectangle represent the standard deviation (n = 3). - = no inhibition. Different letters indicate significant differences according to Tukey’s test (P = 0.05).
Avocado rhizosphere is a potential source of antagonistic microorganisms against phytopathogen fungi. Ramírez et al. (2015) reported the antagonistic activity of 76 endophyte bacteria from the root and the rhizoplane of healthy avocado trees, of which 36.8, 32.8, and 68.4% showed in vitro growth inhibition in different degrees (inhibition halos from 1 to 40 mm) of Phytophthora cinnamomi, Colletotrichum sp. and Phomopsis sp., respectively. In another study, Méndez-Bravo et al. (2018) isolated avocado tree rhizobacteria that demonstrated to promote plant growth (seven isolates) and when they characterized the antagonistic activity of these isolates, A4d and A8a inhibited P. cinnamomi mycelial growth in direct confrontation. Guevara-Avendaño et al. (2018) demonstrated the antagonistic potential of bacteria that belonged to the genus Bacillus, isolated from avocado tree rhizosphere against the phytopathogen fungus Fusarium euwallaceae. Of the 168 rhizobacteria isolated, 42.8% inhibited F. euwallaceae radial mycelial growth from 15 to 46% in direct confrontation.
Recently, studies have reported activity of antagonistic microorganisms associated to avocado tree rhizosphere, which belong to the genus Bacillus spp., Streptomyces spp. and Trichoderma spp. against C. gloeosporioides and F. oxysporum (Vega-Torres et al., 2019; Guerrero-Barajas et al., 2020). The results in this study indicated that the rhizosphere of avocado trees is a source of actinobacteria capable of inhibiting phytopathogen microorganisms’ growth, such as C. gloeosporioides and Xanthomonas sp. Further studies are necessary to determine the mechanism by which actinobacteria antagonize growth of these phytopathogens. Different species of Streptomyces, isolated from the rhizosphere of agricultural crop have been reported as potential biocontrol agents against Colletotrichum spp., antagonizing their growth in vitro and decreasing disease severity (Palaniyandi et al., 2011; Sadeghian et al., 2016; Shu et al., 2017; Thilagam and Hemalatha, 2019). Thus, antagonistic actinobacteria BVEZA1 02, isolated from the rhizosphere of avocado trees - could be useful as a potential biocontrol agent of C. gloeosporioides.
On the other hand, plant rhizosphere is a source of antagonistic actinobacteria against phytopathogen bacteria. Streptomyces spp. antagonists isolated from the rhizosphere of healthy potato plants have been reported against soft rot caused by Pectobacterium spp. (Mansour et al., 2008; Baz et al., 2012). Rincón-Enríquez et al. (2014) isolated 80 actinobacteria from agave rhizosphere, of which 17.5% inhibited growth in vitro of Pseudomonas syringae pv. phaseolicola, which causes halo blight in bean. Suárez-Moreno et al. (2019) reported that 60 actinobacteria, isolated from the rhizosphere of rice crops, only 5% showed antibacterial activity against B. glumae and Pseudomonas fuscovaginae rice phytopathogen bacteria. Muangham et al. (2015) reported a great proportion of rice and rubber tree rhizosphere actinobacteria; of the 210 isolates, 57.1% showed antagonistic activity against X. oryzae pv. oryzae with inhibition zones from 1 to 18.5 mm. In another study, Mingma et al. (2014) demonstrated that rhizosphere and roots of leguminous plants were a source of antagonistic actinobacteria of the genera Streptomyces and Amycolatopsis against X. campestris pv. glycine, which causes the bacterial pustule of soybean. In this study, the actinobacterial strains BVEZA2 24, BVEZA4 34, and BVBZA4 38, isolated from avocado tree rhizosphere, showed the greatest antagonistic activity against Xanthomonas sp. These strains showed the potential as biocontrol agents of the bacterial spot. Further studies are needed to determine the identity of the actinobacterial strains, as well as perform studies on secondary metabolite production and their role as growth inhibitors.
Conclusions
This study characterized the antagonistic activity of 41 actinobacterial strains isolated from avocado tree rhizosphere against C. gloeosporioides and Xanthomonas sp. BV801; some of them; BVEZA2 15, BVEZA2 27, and BVEZA3 30, showed antifungal and antibacterial activity. These results showed the importance of rhizosphere actinobacteria as antagonists of phytopathogen microorganisms and suggest that the strains BVEZA1 02, BVEZA2 24, BVEZA4 34, and BVEZA4 38 could be used in a near future as biological control agents of anthracnosis and the bacterial spot diseases caused by C. gloeosporioides and Xanthomonas sp., respectively.
Data availability
The sets of data used or analyzed during this study are available through the corresponding author upon reasonable request.
Funding
The authors are grateful to the Fondo Mixto Aguascalientes-CONACYT for financing the project: Desarrollo de una tecnología para el control biológico de la marchitez del chile por medio de actinomicetos nativos del Estado de Aguascalientes. Clave CONACYT AGS-2011-C02-181930.
Authors’ contribution
Conceptualization: J.R.T.C. and E.E.Q.A. Methodology: J.R.T.C., E.E.Q.A., Z.E.M., and G.R.E. Formal analyses: J.R.T.C. and G.R.E. Research: J.R.T.C. Resources: E.E.Q.A. and G.R.E. Data curation: J.R.T.C. Original draft preparation and translation: J.R.T.C. and E.E.Q.A. Review and edition: J.R.T.C., E.E.Q.A., G.R.E., and Z.E.M. Supervision: E.E.Q.A., G.R.E., Z.E.M., C.G.G., J.N.E.V. and L.L.P. Project administration and funding acquisition: E.E.Q.A.











 nueva página del texto (beta)
nueva página del texto (beta)



