Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Terra Latinoamericana
versão On-line ISSN 2395-8030versão impressa ISSN 0187-5779
Terra Latinoam vol.38 no.3 Chapingo Jul./Set. 2020 Epub 12-Jan-2021
https://doi.org/10.28940/terra.v38i3.664
Special Number
Trichoderma atroviride as a biocontrol agent of Fusarium head blight by reducing the inoculum of the pathogen in wheat straw
1Área Microbiología. Departamento de Biociencias, Facultad de Química. Universidad de la República. Gral. Flores 2124. 11800 Montevideo, Uruguay.
2Alimentos y Nutrición, Instituto Polo Tecnológico, Facultad de Química, Universidad de la República. By Pass Ruta 8 s/n. 91000 Pando, Canelones, Uruguay.
3Instituto Nacional de Investigación Agropecuaria (INIA). INIA La Estanzuela. 70000 Colonia, Uruguay.
Fusarium head blight (FHB) is the principal disease affecting wheat worldwide, decreasing grain quality, and production. This disease is mainly caused by members of the Fusarium graminearum species complex (FGSC), which have the capability to produce mycotoxins in the contaminated grains. The pathogen overwinters on crop residues (wheat straw). Under adequate climate conditions, the pathogen conidia or ascospores infect wheat flowers, which are the susceptible part of the plant. To minimize disease incidence, cultural practices and fungicide treatments are recommended, but none of them are effective by themselves. In that sense, biological control appears to be an alternative strategy. Therefore, the aim of this study was to isolate and select native Trichoderma spp. strains for FHB control in wheat. Sixteen isolates were identified at species level. All of them showed at least one characteristic that could be associated to their potential ability as biological control agents. An isolate identified as T. atroviride was selected according to its ability to inhibit F. graminearum perithecium development on wheat straw and produce enzymes associated to wheat straw degradation, which is the pathogen physical support.
Index words: biocontrol; Fusarium graminearum
La fusariosis de la espiga (FE) es una enfermedad que causa grandes pérdidas de rendimiento y calidad en granos de trigo a nivel mundial. Es producida principalmente por hongos del complejo Fusarium graminearum, los cuales en su mayoría producen micotoxinas que contaminan los granos afectados. El inóculo primario de la enfermedad proviene de los rastrojos del cultivo del año anterior. En condiciones ambientales adecuadas ascosporas o conidias del patógeno infectan las flores que constituyen el órgano susceptible de la planta. Las medidas de control incluyen prácticas culturales y aplicación de fungicidas. Sin embargo, ninguna práctica es por sí sola totalmente efectiva. En este contexto el uso de control biológico surge como una medida complementaria. El objetivo de este trabajo consistió en aislar y seleccionar cepas nativas de Trichoderma spp. para el control del inóculo primario de FE en trigo. Se trabajó con 16 cepas de Trichoderma identificadas a nivel de especie las cuales exhibieron una o varias actividades que podrían relacionarse con su potencial como agente de biocontrol. Una de las cepas identificada como T. atroviride, fue seleccionada con base en su habilidad para inhibir la formación de peritecios por parte del patógeno sobre rastrojo de trigo y su capacidad de producción de enzimas asociadas a la degradación del rastrojo que sirve de soporte al patógeno.
Palabras clave: biocontrol; Fusarium graminearum
Introduction
Fusarium head blight (FHB) is a disease that has caused great losses in wheat grain yield and quality at world level (Casa et al., 2004). It is produced mainly by fungi that belong to the Fusarium graminearum species complex, formed by 16 species. In Uruguay, same as in other wheat-producing countries in the region, Fusarium graminearum sensu stricto is the predominant species (Umpiérrez et al., 2013). The fungi of this species are capable of producing mycotoxins in grains, of which deoxynivalenol (DON) is the most frequently produced in wheat (Edwards et al., 2001). Consumption of grains contaminated with DON may cause digestive disorders (vomiting, diarrhea), alterations in protein synthesis or deficiencies on the immunological system in animals, leading to weight loss (Pestka and Smolinski, 2005). Thus, efforts to control FHB in cereals should be maximized to decrease productive losses and avoid health problems caused by the consumption of contaminated grains or grain-derived products.
The FHB cycle in wheat starts with the primary pathogen inoculum that develops on wheat straw of the previous harvest. In the appropriate temperature and humidity conditions, the ascospores coming from perithecia - and eventually the pathogen conidia produced on wheat straw - may reach the wheat flower anthers (susceptible organ) of the new crop and cause infection. Sowing without tilling - a common practice in the region - favors the pathogen saprophytic survival on wheat straw, thus, favoring disease development (Inch and Gilbert, 2003).
The FHB is currently one of the diseases that has shown more challenges for its control. No practice is effective on its own, so it is important to adopt all the available control measurements both cultural, chemical or biological that may contribute to decrease disease incidence (Pereyra, 2003). One cultural practice that contributes to disease prevention is alternating in time cereal cultivations with other not susceptible crops, such as oilseed or leguminous plants to minimize the inoculum (Dill-Macky and Jones, 2000). At the same time, the use of wheat varieties with less sensitivity to FHB is another control option since no completely resistant variety exists to date (Mazzilli et al., 2011). Disease management also includes chemical control by applying fungicide, for example, triazole, at the start of flowering to avoid plant infection (Amarasinghe et al., 2013). Nonetheless, the effectiveness of chemical control depends on the moment and method of application, as well as the susceptibility of the pathogen population to be controlled. In this context, biological control comes to be a supplementary option to reduce disease incidence and severity. Even though no commercial product has been found until now for FHB, research development at world level has been extensive and shown the possibility of using microbial agents to supplement this disease control (Schisler et al., 2002). In function of the disease biology, two biocontrol strategies exist to decrease infection risks. The first one has as its objective to decrease the primary inoculum by applying a biocontrol agent on wheat straw of the previous crop. The second one focuses on protecting the infection site (the flower), and in this case, the biocontrol agent should be applied at the start of flowering to avoid or minimize plant infection (Palazzini et al., 2018). In the first case, the antagonists should colonize effectively wheat straw, in such a way that they can develop in the site of action, avoiding pathogen colonization and the propagation of the primary inoculum (Villar et al., 2019). In this sense, studies on the use of Trichoderma harzianum (Inch and Gilbert, 2007), Trichoderma gamsii (Matarese et al., 2012) or Microsphaeropsis spp. (Bujold et al., 2001) as biocontrol agents of F. graminearum on wheat straw have been previously published. The capacity of the biocontrol agent to survive and develop in wheat straw in the local environmental conditions would be fundamental to perform the control strategy. In that sense, the development of local antagonist strains have acquired relevance.
Therefore, the objective of this research study consisted of isolating and selecting native strains of Trichoderma spp. for the control of the primary FHB inoculum in wheat.
Materials and Methods
Pathogen
The Fusarium graminearum F224a used in this study belongs to the Microbial Culture Collection of the Área Microbiología, Facultad de Química, at Universidad de la República (UDELAR), Montevideo, Uruguay. It was selected based on the elevated levels of deoxynivalenol (DON) produced in culture, its capacity of generating perithecia in wheat straw in controlled (humidity and temperature) conditions, and the high aggressiveness level when inoculated in wheat flower anthers in greenhouse assays (Umpiérrez et al., 2013).
Antagonist isolation
Trichoderma spp. strains utilized in this study were isolated from 20 wheat straw samples from the main production zone in our country located eastward from Rio Uruguay in the departments of Río Negro, Soriano, and Colonia. For this purpose, 10 g of wheat straw were placed in 100 mL of sterile physiological serum and homogenized in Stomacher 400® (Stomacher, UK) for two min at maximum speed. Dilutions of this suspension were sown on surface Potato Dextrose Agar (PDA, Oxoid) Petri dishes containing neomycin (50 mg mL-1). Typical colonies of Trichoderma spp. were cultured in PDA Petri dishes; subsequently, monosporic cultures were performed, and the isolates obtained were preserved in PDA test tubes at 5 °C until use.
Antagonist identification
Trichoderma strains were identified by studying the sequences of two genomic regions, ITS1-ITS2 and one part of the gene codifying for the translational elongation factor 1 alpha (tef1α) comprising the totality of intron 4 and part of the exons 4 and 5 of the gene (Jaklitsch and Voglmayr, 2015). DNA extraction was carried out according to Garmendia and Vero (2016). The amplification reactions were performed in a total volume of 25 μL utilizing 0.2 μL of each primer 25 μM, 0.15 U of Taq polymerase (Invitrogen, Thermo Fisher Scientific, Waltham MA, USA), 0.75 μL of MgCl2 50 mM, 1 µL of each dNTP 5 mM solution (Invitrogen, Thermo Fisher Scientific, Waltham MA, USA), 2.5 µL of amplification buffer (Invitrogen, Thermo Fisher Scientific, Waltham MA, USA), and approximately 20 ng of DNA, completing the reaction volume with sterile distilled water. In the case of the ITS1-ITS2 region amplification, the primers used were ITS1 (5´-TCCCGGTTCGCTCGCCGTTACTA-3´) and ITS4 (5´-TCCTCCCGCTTATTGATATGC-3´) (White et al., 1990). The reaction mixture was subjected to 5 min denaturation at 95 °C, followed by 35 cycles of one-min denaturation at 95 °C; 30 sec alignment at 55 °C; one min extension at 72 °C, ending with a 10 min extension at 72 °C.
In the case of the tef1α gene, the primers used were EF1-728F (5´-CATCGAGAAGTTCGAGAAGG-3´) (Carbone and Kohn, 1999) and Tef1 rev (5´-GCCATCCTTGGAGATACCAGC-3´) (Samuels et al., 2006). The PCR cycle was similar to the previous one except for the annealing temperature, which was 51 °C. The primers used in both cases were synthetized by Byo Synthesis Company (Lewisville, TX, USA). The PCR products were analyzed in agarose gel at 0.8% containing ethidium bromide (5 μg mL-1). A band of around 600 base pairs was obtained. The amplification products of both regions were purified and sequenced by Macrogen Inc. (Seoul, Korea). The sequences of both regions were compared with those deposited in Genbank (https://www.ncbi.nlm.nih.gov/genbank/) database using the BLAST tool.
Phylogenetic analyses
The phylogenetic analyses of the concatenated sequences (ITS1-ITS2 and tef1α gene) were aligned with the concatenated sequences of type strains obtained by GenBankusing MEGA program version 6 (Tamura et al., 2013). The phylogenetic trees were constructed by the Neighbor-Joining (Saitou and Nei, 1987) method, and the evolutionary distances were computed by the Jukes-Cantor (Jukes and Cantor, 1969) model. All the positions containing gaps or missing data were eliminated with the option of paired-sequence comparison (Pairwise deletion option). Group stability was determined using a bootstrap of 1000 replicates (Felsenstein, 1985).
Characterization of Trichoderma strains
Sensitivity to tebuconazole. The minimum inhibitory concentration (MIC) of the fungicide - defined as the lowest concentration that prevented visible growth of each Trichoderma strain - was determined. For this purpose, the fungicide Folicur 450( (Bayer Crop Science, Monheim am Rhein, DE) was diluted in sterile water and incorporated into PDA to achieve concentrations of 0, 2, 4, 8, 16, 32, and 64 mg L-1 of tebuconazole (Umpiérrez et al., 2013). In timely manner, 5 μL of conidial suspension adjusted to a concentration of 1 × 105 conidium mL-1 of each Trichoderma strain were inoculated on the surface of fungicide amended PDA plates Eight strains were inoculated per dish, and two replicates per treatment were performed. The Petri dishes were incubated at 25 °C for five days. Fungal growth was evaluated visually.
Determination of xylanase production capacity. For each Trichoderma spp. strain, the capacity of producing xylanase was determined in minimal medium containing xylan as carbon source. Cultures were performed in liquid Yeast Nitrogen Base (YNB) (BioChemica, Sigma Chemical, St. Louis MO, USA) medium amended with Xylan (Sigma Chemical, St. Louis, MO, USA) at 0.5% as the only carbon source. Each 40 mL Erlenmeyer flasks with culture medium were inoculated with 100 μL of conidial suspension 2 ×104 conidia mL-1) of one of the strains and incubated at 25 °C.
Enzymatic activity was determined by mixing 100 μL of citrate-phosphate buffer (pH 5.6), 50 μL of culture supernatant (centrifuged and filtered through a 0.45 μm pore filter), and 50 μL of xylan at 1% in distilled water. The mixture was incubated in water bath at 40 °C for one hour; subsequently, 300 μL of dinitrosalicylic acid (DNS) were added and heated in water bath to boil for five min. Finally, 500 μL of distilled water were added, and absorbance was measured at 540 nm against each blank, which was prepared similarly to the reaction mixture but adding the DNS and heating immediately after the mixture was prepared. Each reaction was performed in triplicate. One unit of enzyme was defined as the amount released by 1 μmol of reducing sugar (expressed in glucose equivalent) per minute in the reaction conditions.
Production of chitinases associated to fungal cell wall degradation. The capacity of producing chitinases by Trichoderma spp. strains was evaluated in YNB amended with fungal cell walls of F. graminearum in a concentration of 0.5% (m/v) as the only carbon source (Vero et al., 2013). The cultures were performed individually by inoculating 100 μL of a suspension of 2 × 104 conidia mL-1 of each Trichoderma spp. strain in Erlenmeyer 200 mL flasks containing 40 mL of the medium. Incubation was performed at 25 °C without agitation for 10 days. Each culture was performed in triplicate. Once incubation ended, each culture was centrifuged, and the supernatant was filtered through a 0.45-μm pore cellulose acetate filter.
Chitinolytic activity from the culture filtrates was measured according to the method of Mahadevan and Crawford (1997) with some modifications. Briefly, 90μL of culture filtrate and 10 μL of a solution 0.18 mM of p-nitrophenyl-N-acetyl-β-D-glucosaminide (Sigma, St. Louis, MO, USA) dissolved in sodium phosphate buffer 50 mM, pH 6.1 were placed in wells of a flat-bottom microtitre plate. The plate was incubated at 25 °C for 24 h without agitation, and the reaction ended by the addition of 10 μL of NaOH 1 M. Absorbance was read at 405 nm in an automatic Microplate Autoreader (Bio-tek Instruments, Winooski, VT, USA) against a blank of each sample, in which NaOH solution was added previous to incubation. The para-nitrophenol (pNP) concentration obtained in each case was determined using a standard curve, one unit of enzyme was defined as the amount needed to release 1 μmole of pNP in one min. The assay was performed in triplicate per each strain.
Production of Volatile Antifungal Compounds. The production of volatile antifungal compounds was analyzed according to Arrarte et al. (2017) with modifications. Petri dishes containing PDA were inoculated centrally with a 5 mm mycelial agar plug from a non-sporulating, two-day-old PDA culture of each Trichoderma strain. At the same time, other plates containing PDA were inoculated in the same manner with a mycelial agar plug of the pathogen.
The lids of the dishes were removed, and the bases containing the pathogen were placed over those with the antagonist, in such a way that the cultures would be confronted. Each set of confronted dishes was sealed with Parafilm and incubated at 25 °C. As control, pairs with F. graminearum in both Petri dishes were used. The F. graminearum colony diameter was measured at day 5, at the moment in which the pathogen covered all the control dish. Inhibition was expressed as the reduction percentage of the colony diameter compared with the control. The assay was performed in triplicate.
Production of soluble antifungal compounds in liquid medium. This assay followed the methodology of Marques et al. (2018) with some modifications. The different Trichoderma strains were inoculated in flasks containing 50 mL of YES medium (yeast extract, 2%; sacarose, 15%, pH = 6) adding 1 mL of 1 × 106 conidia mL-1 suspension. The cultures were cultivated at 25 °C, without agitation for seven days. At the end of this interval, the culture supernatant was filtered with 0.45-μm pore filters to obtain a cell-free solution, which was incorporated to an equal volume of PDA at double concentration and poured into Petri dishes previously adjusted to pH = 6. As control, PDA plates were prepared in the same manner but adding non-inoculated YES medium. On these dishes, a 5 mm mycelial agar plug of the pathogen was placed and incubated at 25 °C. At the end of day 4 of incubation, F. graminearum colony diameters were measured and compared with those in control plates. Inhibition was expressed as colony growth reduction percentage in the Petri dishes containing different filtrates compared with the average growth in control plates. The assay was performed in triplicate.
Dual pathogen-antagonist cultures
The capacity of the different Trichoderma spp. strains of inhibiting Fusarium graminearum F224a growth was determined by means of dual cultures in PDA Petri dishes at 25 °C. Disks of 5-mm from a three-day-old culture of antagonists and pathogen were cut from the border of each colony with a hole.puncher. Both pathogen and antagonist disks in Petri dishes with PDA were confronted, each one at 2.5 cm from the dish center. As control, a pathogen disk was sown in a Petri dish with PDA. The Petri dishes were incubated at 25 °C, and the diameter of each colony was measured after five days, moment in which the pathogen colony had covered the control dish. The antagonistic capacity of each species was established according to the following scale: (1) the F. graminearum colony radius was greater than 2.5 cm; (2) the F. graminearum radius was less than 2.5 cm; (3) the Trichoderma spp. strain grew over all the Petri dish covering the F. graminearum colony. The assay was performed in triplicate for each case.
Production inhibition of the pathogen perithecia in the pathogen-antagonist culture on wheat straw
Five Trichoderma spp. strains of the Viride clade were selected to perform this assay. The selection was based on the results obtained in the dual culture study, in such a way to have representatives of all the defined categories in such assay. This study assessed the inhibition in perithecium production by the pathogen on wheat straw caused by Trichoderma spp. strains (Bujold et al., 2001). Ten pieces of wheat straw of approximately 7 cm in length - each one with a knot - were previously inoculated by 1-min immersion in 50 mL of a suspension of conidia from the pathogen and one antagonist (1 × 104 conidia mL-1). The conidial suspensions were prepared from the cultures in the leaned PDA tubes using physiological serum with Tween 80 at 0.1% to extract conidia. The inoculated wheat straw was placed on Petri dishes containing humid sand previously sterilized. Each pathogen-antagonist treatment was performed in triplicate. Both wheat straw and sand used in this assay were sterilized by two autoclave sessions at 121 °C for 30 min in two consecutive days. As control, wheat straw inoculated with a suspension containing only pathogen conidia was used. The Petri dishes containing treated wheat straw were incubated at a temperature of 25 °C with light-darkness cycles of 12 h to promote perithecia development. Sand humidity of each Petri dish was controlled each five days, and sterile distilled water was added when necessary to maintain initial weight of each Petri dish. At 40 days, all perithecia in the upper face of the wheat straw of each Petri dish were counted under a magnifying glass. The results were expressed as inhibition percentage with respect to the average of the perithecium amount found in the control Petri dishes.
Correlation between antagonism characteristics
The correlation between chitinase and antifungal compounds (volatile and soluble) production, pathogen growth in dual cultures in Petri dishes and perithecia development on wheat straw, was determined by means of Spearman correlation coefficient, using the statistical program Infostat( (Di Rienzo, 2015).
Statistical analyses
The results were analyzed with one-way Analysis of Variance (ANOVA) using Infostat( (Di Rienzo, 2015). Previous to the analysis, the percentage values were divided by 100 and transformed to arcsine square root. The comparison between averages was performed with Fisher’s least significant difference (LSD) (P < 0.05).
Results and Discussion
Antagonist isolation and identification
A total of 16 isolates of Trichoderma spp. from 20 samples of wheat straw from the different cultivation areas were identified at species level by the corresponding sequence analysis of the ITS1-ITS2 region and part of the tef1α gene. By comparing with the corresponding sequences of the type strains deposited in the GenBank and by phylogenetic analysis, the strains were determined to belong to five different species. The identification of each one of the strains is shown in Table 1. Nine strains were identified as T. koningiopsis (57%), three as T. atroviride (19%), two as T. longibrachiatum (12%), one as T. afroharzianum and another one as T. rifaii.
Table 1: Identification of Trichoderma spp. strains isolated from wheat straw.
|
Strain |
Species |
|
T1, T4, TR7, TR8, TR9, T10, T11, T12, T251a |
T. koningiopsis |
|
TmE, TmB, To |
T. atroviride |
|
TR4 |
T. rifaii |
|
T35 |
T. afroharzianum |
|
T2, T3 |
T. longibrachiatum |
The phylogenetic tree obtained in such analysis included type strain sequences that showed similarity (98-100%) with both sequences of the strains in study (Figure 1). The presence of three great groups with bootstrap values of 100% could be established. One of them corresponded to Viride clade where 12 of the isolated strains were included, of which nine were consistently grouped with T. koningiopsis GJS 93-20 (100% bootstrap) type strain and three of them with T. atroviride CBS 142.95 (100% bootstrap) which was also type strain.
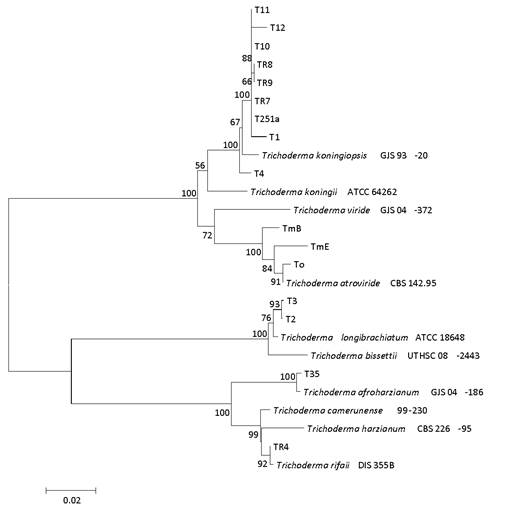
Sequences from type strains retrieved from GenBank were included. The tree was constructed by Neighbour-Joining and evolutive distances were calculated by Jukes-Cantor method. Bootstrap values were calculated from 1000 replications. Phylogenetic analysis was carried out with MEGA6.
Figure 1: Phylogenetic tree based on concatenated ITS1-ITS2 y tef1α gene sequences of uruguayan Trichoderma spp. strains used in this work.
Another group, in which the sequences corresponding to two strains (T35 and T4) were located, corresponded to the Harzianum clade. One of the sequences was grouped with T. afroharzianum GJS 04-186 (type strain) and the other one with T. rifaii DIS 355B (type strain), clearly separated from the type strains of the other species in the same clade. The group formed by the T3 and T2 strains corresponded to the Longibrachiatum clade. The Uruguayan strains grouped with T. longibrachiatum ATCC 18648 type strain and were separated from T. bissettii UTHSC 08‑2443 type strain, which was located in the same clade.
Most of the strains were identified as T. koningiopsis within the Viride clade. The presence of this species has already been described in warm regions of South America (Brazil and Peru) and in colder regions of North America in Canada and eastern United States (Samuels, 2006). Additionally, within the same clade, other three strains were identified as T. atroviride - species that has also been reported as cosmopolitan (du Plessis et al., 2018). Two isolates were identified as T. longibrachiatum, a species of wide geographic distribution which has been reported as a causal agent of localized and systemic infections in immunodepressed or dialyzed patients (Druzhinina et al., 2008). These characteristics preclude the use of this species as a biocontrol agent, especially in extensive crops, and consequently, the strains of these species were not considered in further studies. The remaining isolates were identified as T. afroharzianum and T. rifaii, which belong to the species complex T. harzianum within the Harzianum clade (Chaverri et al., 2015). This complex gathers at least 14 species, including T. harzianum sensu stricto which gives its name to the complex. A recent work about species reclassification within such complex has determined that T. harzianum could only be found in the northern hemisphere (Chaverri et al., 2015). On the other hand, other species of the clade have different geographic distribution patterns. For example, T. rifaii, has only been found in South America, while T. afroharzianum has a worldwide distribution. Thus, finding these two species in Uruguay was not strange. To our knowledge, this is the first report of T. afroharzianum and T. rifaii in Uruguay.
Characterization of Trichoderma spp. strains
Sensitivity to Tebuconazol. The sensitivity of Trichoderma spp. native strains to this fungicide was studied to determine the possibility of a joint application in an integrated disease management. The MIC values obtained for each strain were concordant between replicates. The strains of the Harzianum and Longibrachiatum clades, which represented 25% of the total, showed a MIC of 16 mg L-1 while those of the Viride clade were inhibited with 32 mg L-1 of the fungicide. The sensitivity to tebuconazole of Trichoderma spp. strains was very similar to that found for F. graminearum strains in a previous study (Umpiérrez et al., 2013), In turn, the Trichoderma strains isolated in this study resulted more sensitive to the fungicide than those of European origin described by Hatvani et al. (2006), which showed MIC values of 100 mg L-1 for tebuconazole. According to the results, a joint application of the fungicide with the biocontrol agent would not be effective since it could compromise its own viability.
Xylanase production. The capacity of Trichoderma spp. strains to produce xylanases was determined. Xylanases are enzymes associated to plant tissue degradation, which would facilitate the degradation of wheat straw that acts as support for the pathogen. All the strains were capable of producing xylanases in the assayed conditions (Figure 2). Significant differences (P ≤ 0.05) were found between strains of the Viride clade species, while the strains identified as T. longibrachiatum showed a very similar and relatively low activity. At the same time, the strain with less activity was TR4 identified as T. rifaii, which belongs to the Harzianum clade.
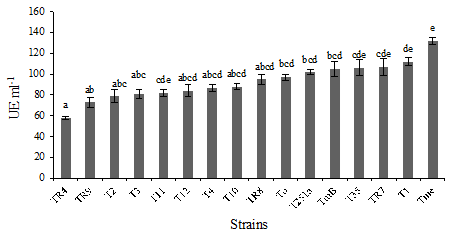
Error bars represntatandard deviation of 3 repetitions. Distinct letters in the same column indicate significant differences (LSD P 0.05).
Figure 2: Xylanases (UE mL-1) produced by Trichoderma spp. strains in presence of xylan a carbon source.
Chitinase production. The capacity of all Trichoderma strains to produce chitinases and glucanases, enzymes associated to fungal cell wall polymers degradation was evaluated in study. The extracellular production of such enzymes has been related to the capacity of mycoparasitism, mechanism associated to Trichoderma spp. with demonstrated biocontrol activity against fungal plant diseases (Matarese et al., 2012). The chitinolytic capacity of the different Trichoderma spp. strains are shown in Figure 3 and represents the average of three independent determinations performed at 25 °C. In such conditions, all the strains produced extracellular chitinases. However, when the activity assay was performed at 40 °C for 30 min (protocol developed for xylanases), the activity values were very low in average and not detectable in some cases.
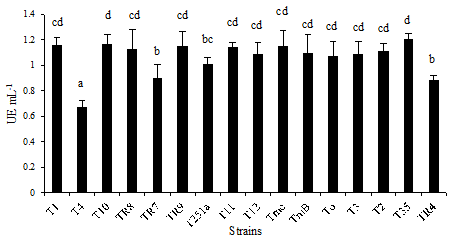
Error bars represent standard deviation of 3 repetitions. Distinct letters in the same column indicate significant differences (LSD P ≤ 0.05).
Figure 3: Chitinolytic activity (UE mL-1) produced of different Trichoderma spp. strains.
The strains belonging to the Viride clade showed different levels of activity with significant differences (P ≤ 0.05) in activity for strains of the same species, such as in the case of those of T. koningiopsis species. The strains of the Longibrachitum clade developed very similar activities with no significant differences (P ≤ 0.05) between them or with those that belonged to T. atroviride species of the Viride clade. The chitinolytic activity within the Harzianum clade was variable. The activity associated to the strain identified as T. afroharzianum was significantly (P ≤ 0.05) greater than that of the T. rifaii strain.
The results evidenced that all the strains in study can produce enzymes capable of degrading chitin, a fundamental activity to act as mycoparasite.
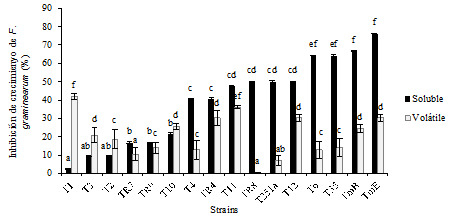
Pathogen growth inhibition due to volatile and soluble compounds in the culture medium
Most of the Trichoderma spp. strains in the assay were capable of producing volatile and soluble metabolites with antifungal activity against the pathogen of study. The greatest pathogen inhibition percentages (around 40%) caused by volatile compounds were achieved with T1 and T11 strains identified as T. koningiopsis, which resulted significantly different (P ≤ 0.05) to the rest (Figure 4). Nevertheless, the inhibition achieved by other strains of the same species (TR8, TR7, and T251) was lower, which shows that the production of volatile antifungal metabolites is a strain dependent characteristic. Several volatile compounds have been characterized as responsible for the antifungal activity of the Trichoderma strains. Among them, simple aromatic compounds, pyrones, volatile terpenes, and isocyanates have been reported (Stoppacher et al., 2010). The 6-pentyl-2H-pyran-2-ona (one pyrone) - a volatile compound associated to coconut aroma, characteristic of T. atroviride (Garnica-Vergara et al., 2016) - has antifungal activity. Its capacity of inhibiting in vitro growth of different fungal pathogens, such as Fusarium oxysporum f. sp. lycopersici and Rhizoctonia solani, has been demonstrated (Reino et al., 2008). According to these authors, the volatile compounds act at a distance empowering the localized action of the enzymes and the non-volatile antifungal compounds. The strains that inhibited the pathogen at a greater degree by producing soluble antifungal compounds were those that belonged to T. atroviride (TmE, TmB, and To) and T35 (T. afroharzianum). They produced more than 60% of inhibition, showing significant differences (P ≤ 0.05) with the rest of the strains. On the other hand, T1 (T. koningiopsis), T2, T3 (T. longibrachiatum) practically did not inhibit pathogen growth in this assay, showing values lower than 15%. The strains of the genus Trichoderma produced a variety of secondary metabolites with antibacterial, antifungal, and antiviral activities. Among those that stand out are some non-ribosomal peptide synthesis formed from five to 20 amino acids, some of which do not form part of proteins, such as alpha aminoisobutyric acid and isovaline (Marik et al., 2018). These compounds receive the name of peptaibols and act at membrane level. Some of them are characteristic of determined species (Oh et al., 2005). For example, koninginins are produced by T. koninigiopsis (McMullin et al., 2017) while T. atroviride produces atroviridis and neoatroviridins (Komon-Zelazowska et al., 2007). Since these compounds are secondary metabolites, the production levels depend on the medium where the producing fungus develops, so the real role of these compounds in a specific pathosystem should be verified in situ. This study considered the production of soluble antimicrobial substances in YES medium, which was recommended by Paterson and Bridge (1994) to promote secondary metabolite production. Therefore, the results obtained in this study should be considered only as indicative of the antifungal activity of each strain. It would be important to corroborate its relationship with the antagonistic activity on wheat straw.
Antagonist-pathogen dual cultures
Pathogen growth inhibition was evaluated confronting the different Trichoderma spp. strains in dual culture in PDA Petri dishes at 25 °C. The pathogen colony radius in the confrontation zone with the antagonist was determined on day 5 of culture, moment at which the F. graminearum mycelium covered all the surface of the medium in the control Petri dishes. TmE, TmB, and To (T. atroviride) and TR8 (T. koningiopsis) produced the total inhibition of the pathogen, growing and sporulating over its mycelium. T2, T3, T4, TR4, T12, TR9, and T251 inhibited slightly the pathogen growth in the assayed conditions, and the rest of the strains showed an intermediate behavior. Figure 5 shows the radius of the pathogen colony in the confrontation zone of dual cultures.
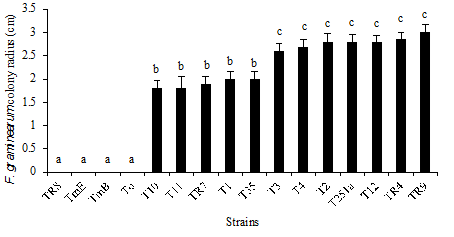
Error bars represent standard deviation of 3 repetitions. Distinct letters in the same column indicate significant differences (LSD P ≤ 0.05).
Figure 5: Radius of F. graminearum colony facing Trichoderma spp. strains in dual cultures.
Figure 6 shows examples of dual cultures. In (a) the antagonist (T. atroviride TmE) grew over the pathogen, and in (b) the pathogen grew beyond the confrontation zone with T. koningiopsis 251a, which is a weak antagonist in the assayed conditions.
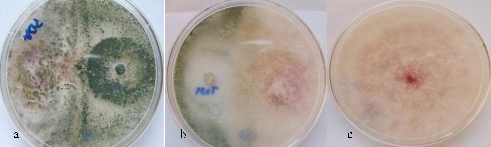
Figure 6: Inhibition of F. graminearum in dual cultures against Trichoderma spp.; a) TmE - F. graminearum; b) T251a - F. graminearum y c) control.
Pathogen inhibition in dual cultures is one of the most utilized assays to study the biocontrol capacity of a microorganism. The extended use of this method is due to the simplicity of the assay. Nevertheless, it should be considered that it only provides indicative - non-definitive - results. In the first place, growth velocity of the two confronted microorganisms in the culture medium depended on the culture conditions, which are not always similar to those where the interaction would be expected to occur. In second place, the capacity of the biocontrol agent to inhibit the pathogen mostly depends on the inducible compound production or secondary metabolism products, so inhibition in the culture medium would not necessarily correspond to inhibition in the field (Mondino and Vero, 2006).
Inhibition of perithecia production on wheat straw
The production inhibition of perithecia by the pathogen on wheat straw was the definitive assay of this study since it reproduced - in the most faithful manner - what was expected to occur in field. The previous assays were only indicative of the potential biocontrol of the antagonists. All the Trichoderma spp. strains could significantly inhibit the production of perithecia on wheat straw (Figure 7).
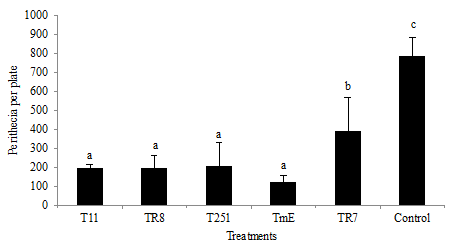
Error bars represent standard deviation of 3 repetitions. Distinct letters in the same column indicate significant differences (LSD P ≤ 0.05).
Figure 7: Perithecia produced by the pathogen on wheat straw y absence (a) and presence (b) of Trichoderma spp. from Viride clade.
An effective colonization of wheat straw by the different Trichoderma strains could also be observed (Figure 8). Of the strains selected, TmE and T11 produced higher levels of volatile antifungal compounds, which could contribute to control at distance. TmE, in turn, showed a significantly greater production of antifungal soluble compounds and also an elevated production of chitinases. The synergic action between the antimicrobial compounds and the hydrolytic enzymes have already been reported for Trichoderma spp. strains (Saravanakumar et al., 2017) and linked with the capacity of acting by mycoparasitism. In turn, TmE was the strain that produced greater xylanase levels, which could degrade the xylans in wheat straw, favoring its degradation. According to the results of this study, TmE identified as T. atroviride could be a good antagonist capable of colonizing wheat straw and inhibiting the development of F. graminearum mycelium and perithecia, thus contributing to Fusarium Head Blight control by decreasing the primary inoculum.
Correlation between antagonism characteristics
A Spearman correlation analysis was performed with the results obtained for the strains from the Viride clade in the assays for chitinolytic activity, pathogen inhibition by volatile or soluble antifungal compounds, pathogen growth in dual cultures in plates, and the production of perithecia by the pathogen in dual cultures on wheat straw. Table 2 shows the results of the analysis. The main diagonal shows the correlation of variables with themselves (Value 1). The values above the main diagonal correspond to the probabilities associated to the null correlation hypothesis between the variables. The values located beneath the main diagonal show the correlation coefficients between the variables. Through these analyses, a significantly (P ≤ 0.05) strong positive correlation (0.85) was determined between the pathogen growth in dual culture against strains from the Viride clade and the production of perithecia by the pathogen on wheat straw in the presence of those antagonists. Moreover, this study confirmed that the more an antagonist affected pathogen growth in the dual culture in Petri dish, the greater results in inhibition of perithecia production by the pathogen in wheat straw were observed in the presence of such antagonist. Likewise, a good negative correlation (-0.78) with a lower level of significance (P = 0.12) was obtained between chitinase production by the antagonists and the production of perithecia by the pathogen in wheat straw in presence of such antagonists. The chitinase production also showed a negative correlation (-0.80) with pathogen growth in the dual culture in the presence of the different Trichoderma spp. strains. In this case a greater chitinase production was correlated with lower pathogen growth in the dual culture (P = 0.10). The inhibition of the pathogen by volatile and soluble antifungal compounds produced by the Trichoderma spp. strains did not show a significant correlation with the inhibition of perithecium production by the pathogen or the antagonist activity in the dual culture. According to these results, only would the antagonism study in dual culture in Petri dish and the chitinase production in presence of the pathogen walls be necessary to determine the potential of the Trichoderma strains as biocontrol agents of F. graminearum production of perithecia on wheat straw, making it unnecessary to perform the rest of the assays to estimate such potential.
Table 2: Correlation between different activities of Trichoderma spp. strains associated to F. graminearum control.
|
Activities |
Activities |
||||
|
a |
1 |
0.10 |
0.93 |
0.31 |
0.05 |
|
b |
-0.80 |
1 |
0.72 |
0.14 |
0.12 |
|
c |
-0.05 |
0-22 |
1 |
0.72 |
0.23 |
|
d |
-0.57 |
0.75 |
0.22 |
1 |
0.22 |
|
e |
0.85 |
-0.78 |
-0.60 |
-0.67 |
1 |
a = F. graminearum growth in dual cultures; b = chitinases production; c = inhibition of F. graminearum growth by volatile antifungal compounds; d = inhibition of F. graminearum growth by soluble antifungal compounds; e = production of perithecia by F. graminearum.
Conclusions
This study obtained native Trichoderma spp. strains capable of colonizing wheat straw and inhibiting the development of perithecia by the pathogen. Their use could be effective in controlling the primary inoculum and thus decrease the risks of wheat fusarium head bright appearance. All the strains obtained exhibited one or several activities that could be related to their potential as biocontrol agents. However, the correlation studies among the different activities and perithecium inhibition in wheat straw evidenced that dual culture and chitinase production performance are characteristics related clearly with the antagonist performance. The selected T. atroviride TmE strain stood out for its antagonist potential and its capacity to produce xylanases linked to the colonization and degradation of wheat straw that serves as pathogen support.
REFERENCES
Amarasinghe, C. C., L. Tamburic-Ilincic, J. Gilbert, A. L. Brûlé-Babel, and W. G. Dilantha Fernando. 2013. Evaluation of different fungicides for control of Fusarium head blight in wheat inoculated with 3ADON and 15ADON chemotypes of Fusarium graminearum in Canada. Can. J. Plant Pathol. 35: 200-208. doi: https://doi.org/10.1080/07060661.2013.773942. [ Links ]
Arrarte, E., G. Garmendia, C. Rossini, M. Wisniewski, and S. Vero. 2017. Volatile organic compounds produced by Antarctic strains of Candida sake play a role in the control of postharvest pathogens of apples. Biol. Control. 109: 14-20. doi: https://doi.org/10.1016/j.biocontrol.2017.03.002. [ Links ]
Bujold, I., T. C. Paulitz, and O. Carisse. 2001. Effect of Microsphaeropsis sp. on the production of perithecia and ascospores of Gibberella zeae. Plant Dis. 85: 977-984. doi: https://doi.org/10.1094/PDIS.2001.85.9.977. [ Links ]
Carbone, I. and L. M. Kohn. 1999. A method for designing primer sets for speciation studies in filamentous ascomycetes. Mycologia 91: 553-556. doi: https://doi.org/10.2307/3761358. [ Links ]
Casa, R. T., E. M. Reis, M. M. C. Blum, A. Bogo, O. Scheer e T. Zanata. 2004. Danos causados pela infecção de Gibberella zeae em trigo. Fitopatol. Bras. 29: 289-293. doi: https://doi.org/10.1590/S0100-41582004000300008. [ Links ]
Chaverri, P., F. Branco-Rocha, W. Jaklitsch, R. Gazis, T. Degenkolb, and G. J. Samuels. 2015. Systematics of the Trichoderma harzianum species complex and the re-identification of commercial biocontrol strains. Mycologia 107: 558-590. doi: https://doi.org/10.3852/14-147. [ Links ]
Dill-Macky, R. and R. K. Jones. 2000. The effect of previous crop residues and tillage on Fusarium head blight of wheat. Plant Disease. 84: 71-76. doi: https://doi.org/10.1094/PDIS.2000.84.1.71. [ Links ]
Di Rienzo, J. A., F. Casanoves, M. G. Balzarini, L. Gonzalez, M. Tablada, and C. W. Robledo. 2015. Software para análisis estadístico InfoStat. Grupo InfoStat, FCA, Universidad Nacional de Córdoba. URL http://www.infostat.com.ar. Argentina. [ Links ]
Druzhinina, I. S., M. Komon-Zelazowska, L. Kredics, L. Hatvani, Z. Antal, T. Belayneh, and C. P. Kubicek. 2008. Alternative reproductive strategies of Hypocrea orientalis and genetically close but clonal Trichoderma longibrachiatum, both capable of causing invasive mycoses of humans. Microbiology 154: 3447-3459. doi: https://doi.org/10.1099/mic.0.2008/021196-0. [ Links ]
du Plessis, I. L., I. S. Druzhinina, L. Atanasova, O. Yarden, and K. Jacobs. 2018. The diversity of Trichoderma species from soil in South Africa, with five new additions. Mycologia 110: 559-583. doi: https://doi.org/10.1080/00275514.2018.1463059. [ Links ]
Edwards, S. G., S. R. Pirgozliev, M.C. Hare, and P. Jenkinson. 2001. Quantification of trichothecene-producing Fusarium species in harvested grain by competitive PCR to determine efficacies of fungicides against Fusarium head blight of winter wheat. Appl. Environ. Microbiol. 67: 1575-1580. doi: https://doi.org/10.1128/AEM.67.4.1575-1580.2001 [ Links ]
Felsenstein, J. 1985. Confidence limits on phylogenies: An approach using the bootstrap. Evolution 39: 783-791. doi: https://doi.org/10.2307/2408678. [ Links ]
Garnica-Vergara, A., S. Barrera-Ortiz, E. Muñoz-Parra, J. Raya‑González, A. Méndez-Bravo, L. Macías-Rodríguez, L. F. Ruiz-Herrera, and J. López-Bucio. 2016. The volatile 6-pentyl-2H-pyran-2-one from Trichoderma atroviride regulates Arabidopsis thaliana root morphogenesis via auxin signaling and ethylene insensitive 2 functioning. New Phytol. 209: 1496-1512. doi: https://doi.org/10.1111/nph.13725. [ Links ]
Garmendia, G. andS. Vero . 2016. Occurrence and biodiversity of Aspergillus section Nigri on ‘Tannat’grapes in Uruguay. Int. J. Food Microbiol. 216: 31-39. doi: https://doi.org/10.1016/j.ijfoodmicro.2015.08.020. [ Links ]
Hatvani, L., Z. Antal , L. Manczinger, A. Szekeres, I. S. Druzhinina , C. P. Kubicek , A. Nagy, E. Nagy, C. Vágvölgyi, andL. Kredics . 2007. Green mold diseases of Agaricus and Pleurotus spp. are caused by related but phylogenetically different Trichoderma species. Phytopathology 97: 532-537. doi: https://doi.org/10.1094/PHYTO-97-4-0532. [ Links ]
Inch, S. andJ. Gilbert 2007. Effect of Trichoderma harzianum on perithecial production of Gibberella zeae on wheat straw. Biocon. Sci. Technol. 17: 635-646. doi: https://doi.org/10.1080/09583150701408865. [ Links ]
Jaklitsch, W. M. and H. Voglmayr. 2015. Biodiversity of Trichoderma (Hypocreaceae) in Southern Europe and Macaronesia. Stud. Mycol. 80: 1-87. doi: https://doi.org/10.1016/j.simyco.2014.11.001. [ Links ]
Jukes, T. H. and C. R. Cantor. 1969. Evolution of protein molecules. pp. 21-132. In: H. N. Munro (ed.). Mammalian protein metabolism. Academic Press. New York, NY, USA. doi: https://doi.org/10.1016/B978-1-4832-3211-9.50009-7. [ Links ]
Komon-Zelazowska, M., T. Neuhof, R. Dieckmann, H. von Döhren, A. Herrera-Estrella, C. P. Kubicek , and I. S. Druzhinina . 2007. Formation of atroviridin by Hypocrea atroviridis is conidiation associated and positively regulated by blue light and the G protein GNA3. Eukaryotic Cell 6: 2332-2342. doi: https://doi.org/10.1128/EC.00143-07. [ Links ]
Mahadevan, B. and D. L.Crawford. 1997. Properties of the chitinase of the antifungal biocontrol agent Streptomyces lydicus WYEC108. Enz. Microbial Technol. 20: 489-493. doi: https://doi.org/10.1016/S0141-0229(96)00175-5. [ Links ]
Marik, T., C. Tyagi, G. Racić, D. Rakk, A. Szekeres , C. Vágvölgyi , and L. Kredics . 2018. New 19-residue peptaibols from Trichoderma clade Viride. Microorganisms 6:85. doi: https://doi.org/10.3390/microorganisms6030085. [ Links ]
Marques, E., I. Martins, and S. C. Marques de Mello. 2018. Antifungal potential of crude extracts of Trichoderma spp. Biota Neotrop. 18: e20170418. doi: https://doi.org/10.1590/1676-0611-bn-2017-0418. [ Links ]
Matarese, F., S. Sarrocco, S. Gruber, V. Seidl-Seiboth, and G. Vannacci. 2012. Biocontrol of Fusarium head blight: interactions between Trichoderma and mycotoxigenic Fusarium. Microbiology 158: 98-106. doi: https://doi.org/10.1099/mic.0.052639-0. [ Links ]
Mazzilli, S. R., C. M. Becerril-Pérez y O. Ernst. 2011. Una alternativa para optimizar el uso de fungicidas para controlar fusariosis de espiga en trigo. Agrociencia 15: 60-68. doi: https://doi.org/10.2477/vol15iss2pp60-68. [ Links ]
McMullin, D. R., J. B. Renaud, T. Barasubiye, M. W. Sumarah, and J. D. Miller. 2017. Metabolites of Trichoderma species isolated from damp building materials. Can. J. Microbiol. 63: 621-632. doi: https://doi.org/10.1139/cjm-2017-0083. [ Links ]
Mondino, P. y S. Vero . 2006. Control biológico de patógenos en plantas. Udelar. CSEP. Montevideo, Uruguay. ISBN: 997-40-035-98. [ Links ]
Oh, S. U., B. S. Yun, S. J. Lee, and I. D. Yoo. 2005. Structures and biological activities of novel antibiotic peptaibols neoatroviridins AD from Trichoderma atroviride F80317. J. Microbiol. Biotechnol. 15: 384-387. [ Links ]
Palazzini, J., P. Roncallo, R. Cantoro, M. Chiotta, N. Yerkovich, S. Palacios, V. Echenique, A. Torres, M. Ramírez, P. Karlovsky, and S. Chulze. 2018. Biocontrol of fusarium graminearum sensu stricto, reduction of deoxynivalenol accumulation and phytohormone induction by two selected antagonists. Toxins 10: 88-98. doi: https://doi.org/10.3390/toxins10020088. [ Links ]
Paterson R. R. M. and P. D. Bridge. 1994. Biochemical techniques for filamentous fungi. CaB International. Wallingford, UK. ISBN-13: 978-0851988993. [ Links ]
Pestka, J. J. and A. T. Smolinski. 2005. Deoxynivalenol: toxicology and potential effects on humans. J. Toxicol. Environ. Health Part B: Critical Rev. 8: 39-69. doi: https://doi.org/10.1080/10937400590889458. [ Links ]
Pereyra, S. 2003. Prácticas culturales para el manejo de la fusariosis de la espiga. pp. 1-9. In: Jornada técnica de cultivos de invierno. INIA. La Estanzuela, Colonia, Uruguay. [ Links ]
Reino, J. L., R. F. Guerrero, R. Hernández-Galán, and I. G. Collado. 2008. Secondary metabolites from species of the biocontrol agent Trichoderma.. Phytochem Rev. 7: 89-123. doi: https://doi.org/10.1007/s11101-006-9032-2. [ Links ]
Saitou, N. and M. Nei. 1987. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 4: 406-425. doi: https://doi.org/10.1093/oxfordjournals.molbev.a040454. [ Links ]
Samuels G. J. 2006. Trichoderma: Systematics, the sexual state, and ecology. Phytopathology 96: 195-206. doi: https://doi.org/10.1094/PHYTO-96-0195. [ Links ]
Samuels, G. J., C. Suarez, K. Solis, K. A. Holmes, S. E. Thomas, A. Ismaiel, and H. C. Evans. 2006. Trichoderma theobromicola and T. paucisporum: Two new species isolated from cacao in South America. Mycol. Res. 110: 381-392. doi: https://doi.org/10.1016/j.mycres.2006.01.009. [ Links ]
Saravanakumar, K., Y. Li, C. Yu, Q. Wang, M. Wang, J. Sun, J. Gao, and J. Chen. 2017. Effect of Trichoderma harzianum on maize rhizosphere microbiome and biocontrol of Fusarium stalk rot. Sci. Rep.7: 1771. doi: https://doi.org/10.1038/s41598-017-01680-w. [ Links ]
Schisler, D. A., N. I. Khan, and M. J. Boehm. 2002. Biological control of Fusarium head blight of wheat and deoxynivalenol levels in grain via use of microbial antagonists. pp. 53-69. In: J. W. DeVries, M. W. Trucksess, and L. S. Jackson (eds.). Mycotoxins and food safety. Advances in experimental medicine and biology, vol 504. Springer. Boston, MA, USA. Online ISBN: 978-1-4615-0629-4. doi: https://doi.org/10.1007/978-1-4615-0629-4_6. [ Links ]
Stoppacher, N., B. Kluger, S. Zeilinger, R. Krska, and R. Schuhmacher. 2010. Identification and profiling of volatile metabolites of the biocontrol fungus Trichoderma atroviride by HS-SPME-GC-MS. J. Microbiol. Methods 81: 187-193. doi: https://doi.org/10.1016/j.mimet.2010.03.011. [ Links ]
Tamura, K., G. Stecher, D. Peterson, A. Filipski, and S. Kumar. 2013. MEGA6: Molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 30: 2725-2729. doi: https://doi.org/10.1093/molbev/mst197. [ Links ]
Umpiérrez, M., G. Garmendia , M. Cabrera, S. Pereyra, andS. Vero . 2013. Diversity of pathogen populations causing Fusarium head blight of wheat in Uruguay. pp. 31-44. In: T. Alconada and S. Schulze (eds.) Fusarium head blight in Latin America. Springer. Dordrecht. Print ISBN: 978-94-007-7090-4. [ Links ]
Vero, S., G. Garmendia , M. B. Gonzalez, O. Bentancur, and M. Wisniewski . 2013. Evaluation of yeasts obtained from Antarctic soil samples as biocontrol agents for the management of postharvest diseases of apple (Malus ( domestica). FEMS Yeast Res. 13: 189-199. doi: https://doi.org/10.1111/1567-1364.12021. [ Links ]
Villar, A., O. Ernst, M. Cadenazzi, S. Vero , S. Pereyra , N. Altier, D. Chouhy, F. Langone y C. A. Pérez. 2019. Efecto de la secuencia de cultivos sobre la población nativa de Trichoderma spp. en agricultura sin laboreo. Agrociencia Uruguay 23: 18-27. doi: http://dx.doi.org/10.31285/agro.23.1.5. [ Links ]
White, T. J., T. Bruns, S. Lee, J. Taylor, M. A. Innis, D. H. Gelfand, and J. Sninsky. 1990. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. pp. 315-322. In: M. A. Innis , D. H. Gelfand , J. J. Snisky, and T. J. White (eds.). PCR protocols: A guide to methods and applications. Academic Press. San Diego, CA, USA. ISBN-13: 978-0123721815. [ Links ]
Recommended citation:
Cabrera, M., G. Garmendia, C. Rufo, S. Pereyra y S. Vero. 2020. Trichoderma atroviride como controlador biológico de fusariosis de espiga de trigo mediante la reducción del inóculo primario en rastrojo. Terra Latinoamericana Número Especial 38-3: 629-651. DOI: https://doi.org/10.28940/terra.v38i3.664
Received: October 21, 2019; Accepted: December 12, 2019











 texto em
texto em