Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Terra Latinoamericana
versión On-line ISSN 2395-8030versión impresa ISSN 0187-5779
Terra Latinoam vol.38 no.3 Chapingo jul./sep. 2020 Epub 12-Ene-2021
https://doi.org/10.28940/terra.v38i3.651
Special number
Morpho-productive response of bell pepper plants biofertilized with Pseudomonas putida and reduced dosage of synthetic fertilizers in greenhouse
1Centro de Investigaciones Biológicas del Noroeste S.C. Instituto Politécnico Nacional No. 195, Colonia Playa Palo de Santa Rita Sur. 23096 La Paz, Baja California Sur, México.
2Facultad de Ciencias Agrícolas-Xalapa, Universidad Veracruzana. Circuito Universitario Gonzalo Aguirre Beltrán, Colonia Zona Universitaria. 91090 Xalapa, Veracruz, México.
Human populations generate high food consumption, so this situation causes an increase in the application of synthetic fertilizers that are expensive and contaminate the environment. Rhizosphere bacteria are an alternative to synthetic fertilizer application because they stimulate plant growth and productivity, do not contaminate the environment, and their application is low cost. Thus the objective of this study was to determine the inoculation effect of three rhizobacterial strains of Pseudomonas putida and the application of two synthetic fertilization concentrations on the morphological parameters and fruit yield in bell pepper plants variety ‘California Wonder’ in greenhouse conditions. The plants were inoculated with the rhizobacteria P. putida cataloged as FA-8, FA-56, and FA-60, individually and in combination; two concentrations of synthetic fertilization were applied at 100 and 75% to determine plant height, stem diameter, root length and volume, fresh and dry biomass, yield and total soluble solid content of fruit and bacterial population. The results indicated that bacteria and synthetic fertilization dosage increased all the morphological parameters and productivity of bell pepper. The use of P. putida as a bio-fertilizer can be important in the sustainable production of horticultural crops such as C. annuum.
Index words: Capsicum annuum; rhizobacteria; inorganic fertilization; growth; fruit
La población mundial genera un alto consumo de alimentos, situación que provoca el incremento del uso de fertilizantes sintéticos, los cuales, son costosos y contaminan al medio ambiente. Las bacterias rizosféricas son una alternativa a la aplicación de fertilizantes sintéticos, debido a que estimulan el crecimiento y la productividad de las plantas, no contaminan al medio ambiente y su aplicación es de bajo costo. El objetivo de este estudio fue determinar el efecto de la inoculación de tres cepas rizobacterianas de Pseudomonas putida y aplicación de dos concentraciones de fertilización sintética sobre parámetros morfológicos y rendimiento de fruto en plantas de pimiento morrón variedad ‘California Wonder’ en condiciones de invernadero. Las plantas fueron inoculadas con tres rizobacterias de P. putida catalogadas como FA-8, FA-56 y FA-60 de manera individual y combinada. La concentración de la fertilización sintética fue del 100 y 75%. Se determinó la altura, el diámetro de tallo, longitud y volumen de raíz, biomasa fresca y seca, rendimiento y contenido de solidos solubles totales de fruto y población bacteriana. Los resultados indican que las bacterias y las dosis de fertilización sintética incrementaron todos los parámetros morfológicos y de productividad del pimiento morrón. El uso de P. putida como un bio-fertilizante puede ser importante dentro de la producción sustentable de cultivos hortícolas como el pimiento morrón.
Palabras clave: Capsicum annuum; rizobacterias; fertilización inorgánica; crecimiento; fruto
Introduction
In Mexico bell pepper (Capsicum annuum L.), also known as green pepper, is considered an important economic crop in agricultural exports because of its production volume; more than 80% of the fruit produced in Mexican soil is exported mainly to the United States and Canada with an economic value higher than MX 520 million pesos (SIAP, 2017). Currently, in the horticultural sector intensive production strategies have been implemented to increase cultivation surface and plant productivity, which have promoted the constant and excessive use of synthetic fertilizers, costly agricultural supplies, and to an extreme, contaminants both in soil and water, linked to human and animal health problems (Schulz and Glaser, 2012; Watanabe et al., 2015).
Facing the excessive use of synthetic nutritional supplies, sustainable and innocuous agriculture production alternatives for natural resources have been designed, among others, a better use of biofertilizers based on different types of beneficial microorganisms, for example, those formulated with rhizobacterial strains capable of promoting plant growth and development (Noh-Medina et al., 2014; Hernández-Montiel et al., 2017; Naili et al., 2018); among the rhizobacterial communities, numerous species that stand out are in the genera: Azotobacter, Pseudomonas, Bacillus, Enterobacter, Rhizobium, Azospirillum, among others of agricultural importance (Kamou et al., 2015; Espinosa et al., 2017; Pérez-Velasco et al., 2019).
Rhizobacteria as bio-fertilizer agents are capable of directly and indirectly stimulating plant growth, development and productivity by different metabolic routes, such as mineral phosphorus solubilization, biological fixation of the nitrogen found in the atmosphere (Zaidi et al., 2015; Coy et al., 2019) and plant hormone production, such as auxins and gibberellins and cytokinins (Hernández-Montiel et al., 2017; Puente et al., 2018). Because of the effects that rhizobacteria have in plants, they are considered natural elicitor microorganisms with the ability of improving crop growth and productivity; thus, they are a biotechnological alternative as feasible biofertilizers with low cost and easy application, which can be used in sustainable and non-contaminant production of agricultural crops established in field or greenhouse, favoring the reduction of synthetic fertilizers that harm the environment (Yang et al., 2009; Sunar et al., 2015; Dar et al., 2018).
Studies on rhizobacteria and synthetic fertilizers have reported plant increase as a result of greater nutrient absorbance and assimilation capacity promoted by population increase and rhizobacterial metabolic action (Dinesh et al., 2013; Díaz et al., 2018; Tahir et al., 2018). In that respect Chiquito-Contreras et al. (2017) pointed out that the application of rhizobacteria of the species Pseudomonas putida with synthetic fertilization reduced at 75% of macro and micronutrients increased fruit yield and plant growth of bell pepper in greenhouse. On the other hand, Naseri and Mirzaei (2010) indicated in a similar study that the application of rhizobacteria of the genus Azotobacter and Azospirillum with synthetic fertilization reduced at 50% of inorganic N in field conditions increased safflower plant growth. In a study performed in field, Yousefi and Barzegar (2014) reported similar yields in wheat plants treated with phosphorus fertilizer at 100% and plants inoculated with Azotobacter chrocooccum and Pseudomonas fluorescens with the application of 50% of the same synthetic fertilizer. Even though the positive effect of combining rhizobacteria with reduced doses of synthetic fertilizers in plants is consistent, it is essential to know and determine the best combination that facilitates the synergic process between the biological agents and synthetic nutritional supplies that contribute to obtain the greatest growth and productivity of economically important plants (Hernández-Montiel et al., 2017; Tripti et al., 2017; Cordero et al., 2018). Therefore, the objective of this study was to determine the effects of inoculating three rhizobacterial strains of Pseudomonas putida and applying two synthetic fertilization concentrations on the morphological parameters and fruit yield in bell pepper plants in greenhouse conditions.
Materials and Methods
Study area
This research study was implemented in a greenhouse type tunnel of 160 m2 and 3 m in height in the central part with lateral ventilation, in the experimental field of the Facultad de Ciencias Agrícolas-Xalapa at Universidad Veracruzana (UV) located at 1450 m above the sea level, 19° 30’ N and 96° 55’ W in the city of Xalapa, Veracruz, México.
Obtaining rhizobacteria and growth
Three Pseudomonas putida strains, classified as FA-8, FA-56, and FA-60, provided by the Chemical Agriculture Laboratory from the Facultad de Ciencias Agrícolas-Xalapa were used. The rhizobacteria were cultivated individually in King’s B medium (composed of: peptone 15 g L-1, magnesium sulfate at 1.0 M 1 mL L-1, dipotassium phosphate 1.5 g L-1 and glycerol 10 mL L-1), subsequently placed in an incubator (BinderTM model BF 400, Tuttlingen, DE) at 26 °C with orbital agitation at 180 rpm for 48 h. The concentration of each rhizobacteria was adjusted to 1 ( 109 cells mL-1 (by dissolution with sterile solution of NaCl at 0.85% p/v) utilizing a digital spectrophotometer (Thermo Spectronic Genesys 20, Fisher Scientific, Inc., Waltham, MA, USA) calibrated at a wavelength of 660 nm until an absorbance of 1.0 was obtained in the concentration of each bacterial culture.
Bell pepper (Capsicum annuum L.) seedling production
To obtain bell pepper plants, the seeds used were of the variety ‘California Wonder’ cultivar of the company Hortaflor-Rancho Los Molinos( (Tepoztlán, Morelos, México), characterized by having square, thick and sweet fruit with determined plant growth and semi-precocious cultivation cycle. For seed germination, a 2.5 × 2.5 × 6 cm rigid polystyrene germinator 200-well tray was used, previously disinfected with sodium hypochlorite at 5%; the germinator cavities were filled with substrate based on pumice, sand and vermi-compost in a ratio of 2:1:2 (v/v), disinfected and sanitized with Anibac 580( liquid solution (Promotora Técnica Industrial, S.A. de C.V. Jiutepec, Morelos, México, active ingredient: double-chain quaternary ammonium salts at 3.7% and first generation quaternary ammonium at 8.6%) in a dose of 10 mL L-1. One seed per tray well was placed at depth of 1 cm and subsequently left at greenhouse temperature conditions of 25 ( 3 (C and relative humidity of 55 ( 5% for 40 days; irrigation with tap water was applied daily until a plant height of 18 cm in average was obtained at the moment of transplant.
Transplant and biofertilization of bell pepper plants with Pseudomonas putida
At the moment of transplanting, the root of each seedling was washed with distilled water; subsequently, 10 groups were established with eight seedlings in 250 mL beakers for inoculation by root immersion in 150 mL of bacterial suspension (1 ( 109 cells mL-1) individually and combined with the three rhizobacterial strains (MIXED treatment) for 20 min. At the end of immersion time, the seedlings were deposited in 10 kg polyethylene bags (40 ( 40 cm) containing 9 kg of pumice as inert substrate, previously washed with tap water, and disinfected by applying Anibac 580( liquid solution in a concentration of 5 mL L-1. The experiment was a randomized complete block design with 10 treatments and eight plants as replicate, of which two groups of plants without bacterial suspension application were provided a nutritive solution at 75 and 100% of concentration as control treatments. When the experiment was established, the plants were fertilized daily with the nutritive solution, dosed according to the plant development cultivation stage; two days after transplant (DAT), 0.3 L of nutritive solution were applied per plant; the volume was increased at 1 L day‑1 at 25 DAT and 1.8 L day-1 from 70 DAT until the end of the experiment. The concentrations of the nutritive solution provided to the plants were 75 and 100% (Table 1), both solutions with the pH adjusted to 6.0. All the bell pepper plants remained in greenhouse for a period of 135 DAT; during the experimental stage, an average temperature of 25 ( 2 (C and relative humidity of 55-60 % were maintained. When the experimental stage concluded, the following quantifications were performed: stem diameter (mm), height (cm), radicle volume (cm3), root length (cm), fresh biomass (g), dry biomass (g), colony forming units (CFU) in roots, fresh fruit weight (g) and percentage of total soluble solids content in fruit juice (three fruit per plant) by the refractometry method (Refracto 30PX, Mettler Toledo, Columbus, OH, USA) expressed as (Brix.
Table 1: Quantity of synthetic fertilizers used in the production of nutritive solution provided to bell pepper plants for their growth and production.
|
Commercial fertilizer |
Formula |
Quantity (Dose at 100%) |
Quantity (Dose at 75%) |
|
- - - - gL-1 - - - - |
|||
|
Calcium nitrate |
Ca(NO3)2∙4H2O |
1.43 |
1.07 |
|
Magnesium nitrate |
Mg(NO3)2 |
0.90 |
0.67 |
|
Potassium nitrate |
KNO3 |
0.35 |
0.26 |
|
Monopotassium phosphate Monopotassium |
KH2PO4 |
0.35 |
0.26 |
|
Micronutrients (TradecorpAZ)† |
Fe, Zn, Mn, B, Cu, Mo |
0.04 |
0.03 |
† Trade Corporation International, S.A.U. (Madrid, ES).
Quantification of rhizobacterial population in roots
The colony-forming unit (CFU) of the rhizobacteria in bell pepper plant roots was estimated at the end of the experimental stage. For such purpose, a sample composed of 3 g of fresh roots from bio-fertilized plants with rhizobacterial strains and without bio-fertilizer (control group) were collected; the root samples of the different treatments assessed were placed individually in Petri boxes, which contained a sterilized physiological solution of NaCl (0.85% p/v). Subsequently, following Holguin and Bashan (1996) methodology, the roots were macerated with a sterilized glass rod; from the mixture obtained, 1 mL was collected and deposited inside a test tube; then, 9 mL of sterile physiological solution of NaCl (0.85% p/v) were added to reach a final volume of 10 mL; from the final volume obtained, serial dilutions were performed to obtain CFU in triplicate for each treatment in Petri plates containing solid B-King culture medium; the Petri plates were incubated at a constant temperature of 26 °C for a period of 72 h. The rhizobacterial population obtained in each treatment was expressed as CFU log 107 g-1 of root.
Results and Discussion
According to the results obtained, the statistical analysis showed significant (P ≤ 0.05) differences for the agronomic variables of bio-fertilized bell pepper plant growth and productivity, individually and combined, with the rhizobacterial strains of P. putida and the application of two concentrations of synthetic fertilization (Table 2). The plants inoculated with the combination of rhizobacterial strains FA-8, FA-56, and FA-60 (MIX Treatment) with the fertilization dosage at 100% increased height and fresh biomass compared with the control (synthetic fertilization at 100%) group in 28.54% and 19.29%, respectively, and compared to the results obtained for the same height and fresh biomass variables in plants with synthetic nutrition in dosage at 75% inoculated with the strain FA-56 and MIX, obtaining an increase of 25.03 and 18.80%, respectively; compared with the fertilized plants at 100%, such values were outstanding considering that 25% less of the synthetic fertilizer was used in bio-fertilized plant nutrition. In the dry biomass and stem diameter variables, the plants with the FA-56 strain and synthetic fertilization at 75% showed an increase of 27.25 and 37.85%, respectively, compared with the control plants with synthetic nutrition at 100%.
Table 2: Effect of Pseudomonas putida inoculation and synthetic fertilization on plant growth and fruit yield of bell pepper variety ‘California Wonder’ in greenhouse.
|
Treatment |
Height |
Stem diameter |
Radicle length |
Radicle volume |
Fresh biomass |
Dry biomass |
Yield |
SST °Brix |
|
cm |
mm |
cm |
cm3 |
-----------g------------ |
% |
|||
|
Synthetic fertilization 100 %† |
||||||||
|
Cepa FA-8 |
106.00 ab |
12.89 a |
56.97 abc |
79.00 ab |
265.11 abc |
82.30 ab |
335.20 ab |
8.5 a |
|
Cepa FA-56 |
108.30 ab |
13.47 a |
57.29 abc |
91.70 a |
298.60 ab |
87.80 ab |
330.55 ab |
8.8 a |
|
Cepa FA-60 |
100.50 abc |
12.62 a |
57.04 abc |
85.75 a |
280.85 abc |
79.50 abc |
370.70 ab |
8.5 a |
|
MIX ‡ |
118.75 a |
12.97 a |
57.60 ab |
89.50 a |
311.85 a |
94.40 a |
400.30 a |
9.0 a |
|
Control |
92.38 bc |
10.04 b |
50.08 bcd |
70.15 b |
261.43 bc |
75.05 bc |
325.00 ab |
8.5 a |
|
Synthetic fertilization 75 % |
||||||||
|
Cepa FA-8 |
96.88 abc |
12.59 a |
50.50 cd |
77.00 ab |
267.94 abc |
76.63 abc |
316.63 b |
8.5 a |
|
Cepa FA-56 |
115.50 a |
13.84 a |
56.60 abc |
86.60 a |
307.65 ab |
95.50 a |
353.75 ab |
9.0 a |
|
Cepa FA-60 |
110.70 ab |
12.07 a |
52.54 bcd |
86.40 a |
282.95 abc |
84.90 ab |
347.80 ab |
8.8 a |
|
MIX |
114.00 ab |
12.65 a |
59.55 a |
92.50 a |
310.60 a |
93.70 a |
408.80 a |
9.0 a |
|
Control |
86.38 c |
9.74 b |
48.60 d |
69.75 b |
240.54 c |
62.74 c |
300.55 b |
8.5 a |
SST = Total soluble solids. Means in the same column with different letters indicate significant differences, according to Tukey’s (P ≤ 0.05) test. † Nutritive solution made with: KNO3, KH2PO4, Ca(NO3)2∙4H2O, Mg(NO3)2 and micronutrients (Zn, Fe, B, Mn, Mo and Cu). ‡ MIX = Simultaneous inoculation with the three Pseudomonas putida strains in bell pepper plants.
For root length, radicle volume, and fruit yield the best result was obtained in bio-fertilized plants with the MIX treatment (combination of three rhizobacterial strains) with synthetic fertilization at 75%, obtaining increases of 18.91, 31.86, and 25.78%, respectively, compared with the control plants fertilized at 100%. In the percentage of total sugar content (Brix (total soluble solids) variable found in mature bell pepper, the results did not indicate the presence of significant (P ≤ 0.05) differences between the treatment of bio-fertilized plants with P. putida strains and synthetic nutrition in dosage of 75 and 100% (Table 2).
With respect to the increase in fruit growth and yield determined on bell pepper plants bio-fertilized with P. putida rhizobacteria and reduced synthetic fertilization at 75%, the response observed was related with its metabolic capacity to produce regulating growth hormones, particularly of the auxin group, such as indole acetic acid (IAA) metabolic activity capable of inducing plant growth through cell division and differentiation of expressed tissues in yield and biomass increase (Nadeem et al., 2016; Ghosh et al., 2018).
On the other hand, rhizobacteria have been described as having the ability to improve the mineral nutrition process in plants, facilitating its availability, and increasing nutriment absorption, such as N, P and chelating ions, as Fe (Kumar-Solanki et al., 2014; Singh et al., 2018). In that respect, several authors have pointed out the importance of using rhizobacterial strains of the genus Pseudomonas as biofertilizer agents because of their capacity to stimulate growth and/or productivity in plants of economic interest, such as tomato (Hernández-Montiel et al., 2017), habanero pepper (Chiquito-Contreras et al., 2017), bell pepper (Bacilio et al., 2016), potato (Arseneault et al., 2015), soybean (Rubina et al., 2018), wheat (Imperiali et al., 2017), maize (Di Salvo et al., 2018), among others.
Díaz et al. (2015) indicated that the application in field of rhizobacterial agents of the species Azospirillum brasilense in sorghum plants with synthetic fertilization at 50%, increased the number of grains per panicle, protein content, and total grain yield compared with the sorghum plants without biofertilizer and with a fertilization dose at 100%.
In a similar study, Carlier et al. (2008) reported that biofertilization of wheat plants in field with Pseudomonas sp. strains and synthetic fertilization dose reduced at 50% showed a relevant effect on seed weight and ears of wheat number with a significant increase in both. Dubey et al. (2014) reported that inoculation in chickpea plants with rhizobacterial strains of Bacillus subtilis and synthetic fertilization in reduced dosage at 50%, improved seed yield compared with fertilized plants at 100% without inoculant. Chiquito-Contreras et al. (2017), while assessing the effect of different rhizobacterial P. putida strains and synthetic fertilization in a dose at 75% on habanero pepper plants, obtained a significant increase in fruit yield compared with plants with a dose at 100% of synthetic fertilization and without inoculation. These authors concluded that the application of a reduced dosage of synthetic fertilizers allowed a decrease in crop production costs, generating at the same time, a lower harmful impact toward the environment because of the continuous use of inorganic supplies. In this study, when bio-fertilized bell pepper plants were assessed with the three rhizobacterial P. putida strains (MIX treatment) and synthetic fertilization at 75%, fruit yield showed a relevant increase of 25% compared with fertilized plants at 100%.
With respect to nutritional fruit composition, particularly in total soluble solids content (°Brix), some studies have pointed out that this organoleptic quality in fruits of bio-fertilized plants could be influenced by the rhizobacterial metabolic activity and potential to favor absorption and assimilation of essential nutrimental elements; additionally, the stimulation regulated by ethylene (Gamalero and Glick, 2015) a volatile organic compound that intervenes in enzyme production with a reduction function of sugars located on the fruit cell wall gives rise to simple monosaccharides that increase progressively during the fruit maturity stage (Ordookhani and Zare, 2011; Pérez-Velasco et al., 2019).
As to the CFU of bacterial P. putida cells quantified in the bell pepper plant root samples, the rhizobacterial strains with the synthetic fertilization at 75 and 100%, showed differences between treatments (Figure 1). The plants of the MIX treatment, bio-fertilized simultaneously with the three P. putida strains (FA-8, FA-56, and FA-60) and synthetic fertilization at 75%, showed the greatest cell population rate with 545 CFU (107 g-1 of fresh root tissue) whereas the bio-fertilized plants with the individual and combined strains and synthetic fertilization at 100% showed the lowest CFU count.
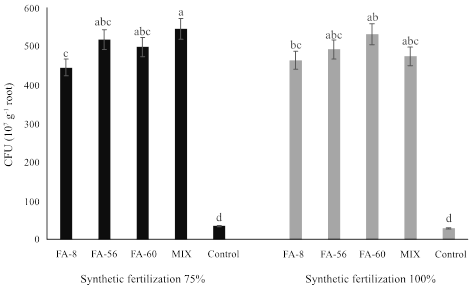
Different letters in bars indicate significant differences according to Tukey’s (P ≤ 0.05) multiple range test.
Figure 1: Colony forming unit count in bell pepper plant roots, variety ‘California Wonder’, biofertilized with Pseudomonas putida strains and control group of plants with synthetic fertilization at 135 days after transplant.
Evidence has pointed out the negative effect of synthetic fertilizers on population rate and metabolic activity of the rhizobacteria inoculated in plants, as it occurs in different rhizobacterial species capable of performing biological fixation of atmospheric nitrogen, an essential nutritional element for optimum plant growth and metabolism (Pankievicz et al., 2015); generally, this type of microorganisms in the presence of inorganic nitrogen show a reduction on the metabolic biologic N fixation because the action of this type of rhizobacteria is particularly significant when this nutrimental element is scarce in the plant rhizosphere (Nadeem et al., 2016; Moreau et al., 2019).
Some studies have described that fluctuations in rhizobacterial population rate is conditioned to the type of the rhizobacterial inoculation method used on plants, as well as the quantity and quality of the organic compounds produced in root exudation, which highlights the presence of different hormone groups (such as, auxins, gibberellins and cytokinins), sugars, vitamins, amino acids, enzymes, organic acids, and phenols (Aung et al., 2016; Wu et al., 2018). Finally, both the amount and quality of the root exudate compounds were capable of stimulating rhizobacterial activity through competence of the metabolites present in the exudates; furthermore, the area where roots emerge, spaces between the unions of epidermal cells and growth zones, are the ideal points for proliferation of the microbial populations with greater adhesion, activity, and microbial attraction (Vacheron et al., 2013; Sasse et al., 2018).
Conclusions
The determined response in bell pepper variety ‘California Wonder’ bio-fertilized with Pseudomonas putida strains, both individually and combined with synthetic fertilization dosage reduced at 75%, showed the greatest increase in the different morphologic growth variables (root length, root volume, stem diameter, and dry biomass), bacterial population, and fruit production. The use of rhizobacteria may contribute to a decrease in synthetic fertilizer quantity supplied to plants up to 25%, which allows a considerable reduction in production costs of bell pepper crop and contributes to soil fertility conservation besides minimizing environmental impact. Detailed studies in field conditions should be performed where the implementation of synthetic fertilization is contemplated in a reduced dosage to validate the capacity of the species P. putida rhizobacterial strains as bio-fertilizers capable of promoting plant growth and fruit yield in bell pepper plants with a sustainable agriculture production approach and ecologically viable.
Acknowldegments
The authors are grateful to the Facultad de Ciencias Agrícolas of Universidad Veracruzana Campus Xalapa, for using the facilities and technical support provided by the responsible staff of the Agricultural Chemistry Laboratory and greenhouse area; to D. Fischer for translation and editorial services.
REFERENCES
Arseneault, T., C. Goyer, and M. Filion. 2015. Pseudomonas fluorescens LBUM223 increases potato yield and reduces common scab symptoms in the field. Phytopathology 105: 1311-1317. doi: https://doi.org/10.1094/PHYTO-12-14-0358-R. [ Links ]
Aung, H. P., A. D. Mensah, Y. S. Aye, S. Djedidi, Y. Oikawa, T. Yokoyama, S. Suzuki, and S. D. Bellingrath-Kimura. 2016. Transfer of radiocesium from rhizosphere soil to four cruciferous vegetables in association with a Bacillus pumilus strain and root exudation. J. Environ. Radioact. 164: 209-219. doi: https://doi.org/10.1016/j.jenvrad.2016.07.006. [ Links ]
Bacilio, M., M. Moreno, and Y. Bashan. 2016. Mitigation of negative effects of progressive soil salinity gradients by application of humic acids and inoculation with Pseudomonas stutzeri in a salt-tolerant and a salt-susceptible pepper. Appl. Soil Ecol. 107: 394-404. doi: https://doi.org/10.1016/j.apsoil.2016.04.012. [ Links ]
Carlier, E., M. Rovera, A. Rossi Jaume, and S. B. Rosas. 2008. Improvement of growth, under field conditions, of wheat inoculated with Pseudomonas chlororaphis subsp. aurantiaca SR1. World J. Microbiol. Biotechnol. 24: 2653-2658. doi: https://doi.org/10.1007/s11274-008-9791-6. [ Links ]
Coy, R. M., D. W. Held, and J. W. Kloepper. 2019. Rhizobacterial colonization of bermudagrass by Bacillus spp. in a Marvyn loamy sand soil. Appl. Soil Ecol. 141: 10-17. doi: https://doi.org/10.1016/j.apsoil.2019.04.018. [ Links ]
Chiquito-Contreras, R. G., B. Murillo-Amador, C. J. Chiquito-Contreras, J. C. Márquez-Martínez, M. V. Córdoba-Matson, and L. G. Hernández-Montiel. 2017. Effect of Pseudomonas putida and inorganic fertilizer on growth and productivity of habanero pepper (Capsicum chinense Jacq.) in greenhouse. J. Plant Nutr. 40: 2595-2601. doi: https://doi.org/10.1080/01904167.2017.1381119. [ Links ]
Cordero, I., L. Balaguer, A. Rincón, and J. J. Pueyo. 2018. Inoculation of tomato plants with selected PGPR represents a feasible alternative to chemical fertilization under salt stress. J. Plant Nutr. Soil Sci. 181: 694-703. doi: https://doi.org/10.1002/jpln.201700480. [ Links ]
Dar, Z. M., A. Rouf, A. Masood, M. Asif, and M. A. Malik. 2018. Review on plant growth promoting rhizobacteria and its effect on plant growth. J. Pharmacogn. Phytochem. 7: 2802-2804. [ Links ]
Di salvo, L. P., G. C. Cellucci, M. E. Carlino, and I. E. García de Salamone. 2018. Plant growth-promoting rhizobacteria inoculation and nitrogen fertilization increase maize (Zea mays L.) grain yield and modified rhizosphere microbial communities. Appl. Soil Ecol. 126: 113-120. doi: https://doi.org/10.1016/j.apsoil.2018.02.010. [ Links ]
Díaz Franco, A., D. Gálvez López y F. E. Ortiz Cháirez. 2015. Bioinoculación y fertilización química reducida asociadas con el crecimiento de planta y productividad de sorgo. Rev. Int. Contam. Ambient. 31: 245-252. [ Links ]
Díaz Franco, A. , F. E. Ortiz Cháirez, O. A. Grageda Cabrera y E. Fernández Cruz. 2018. Nutrición mineral y rendimiento de sorgo inoculado con cepas microbianas en dos agroambientes. Terra Latinoamericana 36: 229-238. doi: https://dx.doi.org/10.28940/terra.v36i3.295. [ Links ]
Dinesh, R., M. Anandaraj, A. Kumar, V. Srinivasan, Y. K. Bini, K. P. Subila, R. Aravind, and S. Hamza. 2013. Effects of plant growth-promoting rhizobacteria and NPK fertilizers on biochemical and microbial properties of soils under ginger (Zingiber officinale Rosc.) cultivation. Agric. Res. 2: 346-353. doi: https://doi.org/10.1007/s40003-013-0080-8. [ Links ]
Dubey, R. C., S. Khare, P. Kumar, and D. K. Maheshwari. 2014. Combined effect of chemical fertilisers and rhizosphere-competent Bacillus subtilis BSK17 on yield of Cicer arietinum. Arch. Phytopathol. Plant Protect. 47: 2305-2318. doi: https://doi.org/10.1080/03235408.2013.876744. [ Links ]
Espinosa Palomeque, B., A. Moreno Reséndez, P. Cano Ríos, V. P. Álvarez Reyna, J. Sáenz Mata, H. Sánchez Galván y G. González Rodríguez. 2017. Inoculación de rizobacterias promotoras del crecimiento vegetal en tomate (Solanum lycopersicum L.) cv. afrodita en invernadero. Terra Latinoamericana 35: 169-178. doi: https://doi.org/10.28940/terra.v35i2.194. [ Links ]
Gamalero, E. and B. R. Glick. 2015. Bacterial modulation of plant ethylene levels. Plant Physiol. 169: 13-22. doi: https://doi.org/10.1104/pp.15.00284. [ Links ]
Ghosh, D., A. Gupta, and S. Mohapatra. 2018. Dynamics of endogenous hormone regulation in plants by phytohormone secreting rhizobacteria under water-stress. Symbiosis 77: 265-278. doi: https://doi.org/10.1007/s13199-018-00589-w. [ Links ]
Hernández-Montiel, L. G., C. J. Chiquito-Contreras , B. Murillo-Amador , L. Vidal-Hernández, E. E. Quiñones-Aguilar, and R. G. Chiquito-Contreras. 2017. Efficiency of two inoculation methods of Pseudomonas putida on growth and yield of tomato plants. J. Soil Sci. Plant Nutr. 17: 1003-1012. doi: http://dx.doi.org/10.4067/S0718-95162017000400012. [ Links ]
Holguin, G. and Y. Bashan. 1996. Nitrogen-fixation by Azospirillum brasilense Cd is promoted when co-cultured with a mangrove rhizosphere bacterium (Staphylococcus sp.). Soil Biol. Biochem. 28: 1651-1660. doi: https://doi.org/10.1016/S0038-0717(96)00251-9. [ Links ]
Imperiali, N., X. M. Chiriboga, K. Schlaeppi, M. Fesselet, D. Villacrés, G. Jaffuel, S. F. Bender, F. Dennert, R. Blanco-Pérez, M. G. A Van der Heijden, M. Maurhofer, F. Mascher, T. C. J. Turlings, C. Keel, and R. Campos-Herrera. 2017. Combined field inoculations of Pseudomonas bacteria, arbuscular mycorrhizal fungi, and entomopathogenic nematodes and their effects on wheat performance. Front. Plant Sci. 8: 1-17. doi: https://doi.org/10.3389/fpls.2017.01809. [ Links ]
Kamou, N. N., H. Karasali, G. Menexes, K. M. Kasiotis, M. C. Bon, E. N. Papadakis, G. D. Tzelepis, L. Lotos, and A. L. Lagopodi. 2015. Isolation screening and characterization of local beneficial rhizobacteria based upon their ability to suppress the growth of Fusarium oxysporum f. sp. radicis-lycopersici and tomato foot and root rot. Biocontrol Sci. Technol. 25: 928-949. doi: https://doi.org/10.1080/09583157.2015.1020762. [ Links ]
Kumar-Solanki, M., R. Kumar-Singh, S. Srivastava, S. Kumar, P. Lal-Kashyap, A. K. Srivastava, and D. K. Arora. 2014. Isolation and characterization of siderophore producing antagonistic rhizobacteria against Rhizoctonia solani. J. Basic Microbiol. 54: 585-597. doi: https://doi.org/10.1002/jobm.201200564. [ Links ]
Moreau, D., R. D. Bardgett, D. Finlay, D. L. Jones, and L. Philippot. 2019. A plant perspective on nitrogen cycling in the rhizosphere. Funct. Ecol. 33: 540-552. doi: https://doi.org/10.1111/1365-2435.13303. [ Links ]
Nadeem, S. M., M. Ahmad, M. Naveed, M. Imran, Z. A. Zahir, and D. E. Crowley. 2016. Relationship between in vitro characterization and comparative efficacy of plant growth-promoting rhizobacteria for improving cucumber salt tolerance. Arch. Microbiol. 198: 379-387. doi: https://doi.org/10.1007/s00203-016-1197-5. [ Links ]
Naili, F., M. Neifar, D. Elhidri, H. Cherif, B. Bejaoui, M. Aroua, Z. Bejaoui, M. Abassi, K. Mguiz, H. Chouchane, H. I. Ouzari, and A. Cherif. 2018. Optimization of the effect of PGPR-based biofertilizer on wheat growth and yield. Biom. Biostat. Int. J. 7: 226-232. doi: https://doi.org/10.15406/bbij.2018.07.00213. [ Links ]
Naseri, R. and A. Mirzaei. 2010. Response of yield and yield components of safflower (Carthamus tinctorius L.) to seed inoculation with Azotobacter and azospirillum and different nitrogen levels under dry land condition. American-Eurasian J. Agric. Environ. Sci. 9: 445-449. [ Links ]
Noh-Medina, J., C. Yam-Chimal, L. Borges-Gómez, J. J. Zúñiga-Aguilar y G. Godoy-Hernández. 2014. Aislados bacterianos con potencial biofertilizante para plántulas de tomate. Terra Latinoamericana 32: 273- 281. [ Links ]
Ordookhani, K. and M. Zare. 2011. Effect of Pseudomonas, Azotobacter and Arbuscular Mycorrhizal Fungi (AMF) on lycopene, antioxidant activity and total soluble solid in tomato (Solanum lycopersicum L.) F1 Hybrid, Delta. Adv. Environ. Biol. 5: 1290-1294. [ Links ]
Pankievicz, V. C., F. P. do Amaral, K. F. D. N. Santos, B. Agtuca, Y. M. J. Schueller, A. C. M. Arisi, M. B. R. Steffens, E. M. de Souza, F. O. Pedrosa, G. Stacey, and R. A. Ferrieri. 2015. Robust biological nitrogen fixation in a model grass-bacterial association. Plant J. 81: 907-919. doi: https://doi.org/10.1111/tpj.12777. [ Links ]
Pérez-Velasco, E. A., R. Mendoza-Villarreal, A. Sandoval-Rangel, M. Cabrera-de la Fuente, V. Robledo-Torres y L. A. Valdez-Aguilar. 2019. Evaluación del uso de endomicorrizas y Azospirillum sp. en la productividad y calidad nutracéutica de chile morrón (Capsicum annuum) en invernadero. ITEA-Inf. Tec. Econ. Agr. 115: 18-30. doi: https://doi.org/10.12706/itea.2018.029. [ Links ]
Puente, M. L., J. L. Gualpa, G. A. Lopez, R. M. Molina, S. M. Carletti, and F. D. Cassán. 2018. The benefits of foliar inoculation with Azospirillum brasilense in soybean are explained by an auxin signaling model. Symbiosis 76: 41-49. doi: https://doi.org/10.1007/s13199-017-0536-x. [ Links ]
Rubina Noreen, H., F. Urooj, H. Farhat, H. A. Shafique, A. Rahman, and S. Ehteshamul-Haque. 2018. Impact of endo-nodule fluorescent Pseudomonas and Rhizobia on root rotting fungi and growth of soybean (Glycine max L. Merr.). Int. J. Biol. Res. 6: 27-33. [ Links ]
Sasse, J., E. Martinoia, and T. Northern. 2018. Feed your friends: Do plant exudates shape the root microbiome? Trends Plant Sci. 23: 25-41. doi: https://doi.org/10.1016/j.tplants.2017.09.003. [ Links ]
SIAP (Servicio de Información Agroalimentaria y Pesquera de México). 2017. Producción agrícola, resumen nacional por cultivo. http://www.siap.gob.mx/cierre-de-la-produccionagricola-por-cultivo/ . (Consulta: junio 05, 2019). [ Links ]
Singh, A. V., B. Prasad, and R. Goel. 2018. Plant growth promoting efficiency of phosphate solubilizing Chryseobacterium sp. PSR 10 with different doses of N and P fertilizers on lentil (Lens culinaris var. PL-5) growth and yield. Int. J. Curr. Microbiol. App. Sci. 7: 2280-2289. doi: https://doi.org/10.20546/ijcmas.2018.705.265. [ Links ]
Schulz, H. and B. Glaser. 2012. Effects of biochar compared to organic and inorganic fertilizers on soil quality and plant growth in a greenhouse experiment. J. Plant Nutr. Soil Sci. 175: 410-422. doi: https://doi.org/10.1002/jpln.201100143. [ Links ]
Sunar, K., P. Dey, U. Chkraborty, and B. Chakreborty. 2015. Biocontrol efficacy and plant growth promoting activity of Bacillus altitudinis isolated from Darjeeling hills, India. J. Basic Microbiol. 55: 91-104. doi: https://doi.org/10.1002/jobm.201300227. [ Links ]
Tahir, M., U. Khalid, M. Ijaz, G. M. Shah, M. A. Naeem, M. Shahid, K. Mahmood, N. Ahmad, and F. Kareem. 2018. Combined application of bio-organic phosphate and phosphorus solubilizing bacteria (Bacillus strain MWT 14) improve the performance of bread wheat with low fertilizer input under an arid climate. Braz. J. Microbiol. 49: 15-24. doi: http://dx.doi.org/10.1016/j.bjm.2017.11.005. [ Links ]
Tripti, A. Kumar , Z. Usmani, V. Kumar, and Anshumali. 2017. Biochar and flyash inoculated with plant growth promoting rhizobacteria act as potential biofertilizer for luxuriant growth and yield of tomato plant. J. Environ. Manage. 190: 20-27. doi: https://doi.org/10.1016/j.jenvman.2016.11.060. [ Links ]
Vacheron, J., G. Desbrosses, M. L. Bouffaud, B. Touraine, Y. Moenne-Loccoz, D. Muller, L. Legendre, F. Wisniewski-Dye, and C. Prigent-Combaret. 2013. Plant growth-promoting rhizobacteria and root system functioning. Front. Plant Sci. 4: 1-19. doi: https://doi.org/10.3389/fpls.2013.00356. [ Links ]
Watanabe, M., Y. Ohta, S. Licang, N. Motoyama, and J. Kikuchi. 2015. Profiling contents of water-soluble metabolites and mineral nutrients to evaluate the effects of pesticides and organic and chemical fertilizers on tomato fruit quality. Food Chem. 169: 387-395. doi: https://doi.org/10.1016/j.foodchem.2014.07.155. [ Links ]
Wu, L., Y. Kobayashi, J. Wasaki, and H. Koyama. 2018. Organic acid excretion from roots: a plant mechanism for enhancing phosphorus acquisition, enhancing aluminum tolerance, and recruiting beneficial rhizobacteria. Soil Sci. Plant Nutr. 64: 697-704. doi: https://doi.org/10.1080/00380768.2018.1537093. [ Links ]
Yang, J., J. W. Kloepper, and C. M. Ryu. 2009. Rhizosphere bacteria help plants tolerate abiotic stress. Trends Plant Sci. 14: 1-4. doi: https://doi.org/10.1016/j.tplants.2008.10.004. [ Links ]
Yousefi, A.A. and A. R. Barzegar. 2014. Effect of Azotobacter and Pseudomonas bacteria inoculation on wheat yield under field condition. Int. J. Agric. Crop Sci. 7: 616-619. [ Links ]
Zaidi, A., E. Ahmad, M. S. Khan, S. Saif, and A. Rizvi. 2015. Role of plant growth promoting rhizobacteria in sustainable production of vegetables: Current perspective. Sci. Hortic. 193: 231-239. doi: https://doi.org/10.1016/j.scienta.2015.07.020. [ Links ]
Recommended citation:
Hernández-Montiel, L. G., B. Murillo-Amador, C. J. Chiquito-Contreras, C. E. Zuñiga-Castañeda, J. Ruiz-Ramírez y R. Gregorio Chiquito-Contreras. 2020. Respuesta morfo-productiva de plantas de pimiento morrón biofertilizadas con Pseudomonas putida y dosis reducida de fertilizantes sintéticos en invernadero. Terra Latioamericana Número Especial 38-3: 583‑596. DOI: https://doi.org/10.28940/terra.v38i3.651
Received: October 09, 2019; Accepted: January 06, 2020











 texto en
texto en 



