Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Terra Latinoamericana
versão On-line ISSN 2395-8030versão impressa ISSN 0187-5779
Terra Latinoam vol.38 no.3 Chapingo Jul./Set. 2020 Epub 12-Jan-2021
https://doi.org/10.28940/terra.v38i3.647
Special number
Agave potatorum Zucc. growth promotion by free-living nitrogen-fixing bacteria
1 Instituto Politécnico Nacional, CIIDIR-Oaxaca. Hornos 1003. 71230 Santa Cruz Xoxocotlán, Oaxaca, México.
The free-living nitrogen-fixing bacteria (FLNFB) can be an important alternative to replace mineral fertilizers in agriculture. Agave potatorum Zucc., commonly known as “maguey tobalá”, is a wild species from which a highly demanded “mezcal” (liquor) is obtained and distinguished by its high quality. As it is a wild species, not much information is available referring to its agricultural management. This study assessed the effect of FLNFB inoculation on plant growth and solid soluble content (SSC) of the stem of A. potatorum plants under semi-controlled conditions and a randomized complete block design; three FLNFB (1) Burkholderia cepacia, (2) Flavobacterium sp., (3) Paenibacillus amylolyticus and a control (without FLNFB) were assessed in four blocks with 15 agave plants per block; each plant in the same block was randomly assigned to a different FLNFB. The plant growth variables assessed after 48 weeks were: plant height (PH), plant rosette diameter (ROD), plant stem diameter (PSD), unfolded leaves number (ULN), root volume (RV), root density (RD), stem dry biomass (SDB), total dry biomass (TDB), leaf area (LA) and SSC (°Bx). An analysis of variance and Tukey’s multiple range test for means separation (P ≤ 0.05) revealed that with respect to the control, B. cepacia increased RV 322.2%; ULN 42.6%; and SSC 72.9%. P. amylolyticus increased SDB 317.1%. B. cepacia, Flavobacterium sp., and P. amylolyticus increased the PSD approximately 50.3%; ROD 48.6%; LA 127.2%; and PH 51.8%. Flavobacterium sp. increased TB 164.8%. These results suggest that the FLNFB promoted growth of A. potatorum plants, making this environmentally friendly and inexpensive technology a good alternative for agave production.
Index words: diazotrophic bacteria; plant growth; maguey tobalá
Las bacterias fijadoras de nitrógeno de vida libre (BFNVL) pueden ser una importante alternativa para reemplazar los fertilizantes nitrogenados en la agricultura. Agave potatorum Zucc., coloquialmente conocido como maguey tobalá, es una especie silvestre de la cual se obtiene un mezcal muy demandado debido a su alta calidad. Al ser una especie silvestre, no existe mucha información referente a su manejo agronómico. En este estudio se evaluó el efecto de BFNVL en el crecimiento y contenido de sólidos solubles totales (SST) en plantas de A. potatorum bajo condiciones semi-controladas. Bajo un diseño en bloques completamente al azar se evaluaron tres BFNVL (1) Burkholderia cepacia, (2) Flavobacterium sp., (3) Paenibacillus amylolyticus y un control (sin BFNVL). En total se tuvieron 4 bloques con 15 plantas de agave por bloque, dentro de cada bloque a cada planta de agave se le aplicó al azar una BFNVL diferente. Las variables de crecimiento vegetal evaluadas después de 48 semanas fueron: altura de la planta (AP), diámetro de roseta (DRO), diámetro del tallo (DT), número de hojas desplegadas (NHD), volumen radicular (VR), densidad de raíz (DR), biomasa seca del tallo (BST), biomasa total (BT), área foliar (AF) y, SST en el tallo ((Bx). El análisis de varianza y la prueba de comparación múltiple de medias (Tukey, P ≤ 0.05) revelaron que con respecto al control, B. cepacia incrementó 322.2% el VR, 42.6% el NHD y 72.9% los SST. P. amylolyticus incrementó 317.1% la BST. B. cepacia, Flavobacterium sp. y P. amylolyticus incrementaron aproximadamente 50.3% el DT, 48.6% el DRO, 127.2% el AF y 51.8% la AP. Flavobacterium sp. incrementó 164.8% la BT. Estos resultados sugieren que las BFNVL promueven el crecimiento de A. potatorum, por lo que pueden ser una tecnología ambientalmente amigable y económica para la producción de este agave.
Palabras clave: bacterias diazótrofas; crecimiento vegetal; maguey tobalá
Introduction
Plants require nitrogen in great quantities because this element is an essential component of proteins, nucleic acids, and other cellular constituents (Shridhar, 2012). The elevated nitrogen quantity in the Earth atmosphere (close to 80% in N2 gaseous form) cannot be used directly by the biological systems in the production of the necessary chemical compounds for their growth and reproduction (Shin et al., 2016). The reduction of N2, commonly known as “nitrogen fixation” is performed chemically or biologically (Shin et al., 2016). Biological nitrogen fixation (BNF) is one of the most important processes that provides the greatest external N source for the different ecosystems and agroecosystems and constitutes an important option for recovery and maintenance of soil fertility (Figueiredo et al., 2013). Some of the bacteria that live in soil are called free-living because they do not depend on plant root exudates for their survival (Sharma et al., 2014). The microorganisms that perform BNF are a limited group of free-living symbiotic bacteria called diazotroph, which have the capacity of reducing and transforming atmospheric nitrogen (N2) to ammonium (NH4 +), a form of readily assimilated nitrogen for plants (Bano and Iqbal, 2016). Among the free-living nitrogen-fixing bacteria (FLNFB), some are obligate or facultative anaerobic, such as Azospirillum sp., Clostridium pasteurianum, Klebsiella spp., Desulfovibrio sp.; other obligate aerobic, such as Azotobacter spp., Beijerinckia sp., and photosynthetic, such as purple sulfur, non-sulfur bacteria, and green sulfur bacteria (Allan and Graham, 2002). Some FLNFB may promote plant growth by means of synthesis and the release of phytohormones, such as auxins, gibberellins, cytokinins and abscisic acid (Hernández-Rodríguez et al., 2014). Some Azotobacter strains, for example, have the capacity of producing amino acids that promote plant growth; they are also capable of producing siderophores, which may provide iron to plants (Jnawali et al., 2015). The results of previous studies on incorporating FLNFB to soil have shown high yields in a great diversity of crops, such as rice, maize, bean, and tomato, minimizing the use of chemical fertilizers, especially nitrogenous (Hernández, 1998; Bonilla et al., 2000; Meunchang et al., 2005). Some reports have shown that inoculation with Azospirillum lipoferum in rice plants under greenhouse conditions increased yield above 6.7 g plant-1 (Mirza et al., 2000). Cotton plant height and dry biomass increased with Azospirillum brasiliense inoculation under greenhouse conditions (Bashan, 1998).
In Mexico, agave plants have had and still have a great economic and cultural importance for numerous indigenous and mixed-race populations that have used them for 7000 years as source of food, medicine, fuel, shelter, ornament, fiber, manure, housing construction, agricultural tool production, and mainly in the production of different types of alcoholic beverages, such as mezcal and tequila (García-Mendoza, 2007). Recent studies have indicated that agave plants also have a high potential for fixing carbon (García-Moya et al., 2010; Núñez et al., 2010). In the State of Oaxaca, around nine agave species are used, of which Agave angustifolia Haw. is the one with the greatest demand and the only one cultivated significantly in semi-arid soils, where approximately 8422.7 ha are cultivated with this agave (OEIDRUS-SAGARPA, 2011). The other eight agave species were collected from wild or semi-cultivated populations, mainly in living fences with little or null cultural management. Such is the case of Agave potatorum Zucc., colloquially known as maguey papalomé or maguey tobalá, a species whose mezcal is highly demanded (Sánchez-López, 2005) and distinguished for its top quality because of the proportion of the volatile aromatic compounds it contains (Vera et al., 2009). In 2018, 5.9 million L of mezcal were produced at national level. Oaxaca contributed with 92.3% of this production, of which 3.3% was made with A. potatorum (CRM, 2019). This agave develops in low fertility arid and semi-arid soils, estimating 42 ha of such species in the regions of Valles Centrales and Sierra Sur of Oaxaca, which represents 0.45% of the total plants of the genus Agave that exist in the state (OEIDRUS-SAGARPA, 2011). Because it is a wild species, not much information is available with respect to its agronomic management; thus it is necessary to carry out research in different aspects, including its nutrition within an agro-ecologic context. The analysis of sugars in the stems or agave “piñas” (stem without leaves) is important because the alcohol obtained in the fermentation depends on the quantity of reducing sugars. In the tequila and mezcal distilleries, two type of analyses are made, such as measuring °Brix (°Bx) degrees and determining reducing sugars by using Fehling reagent produced in the laboratory. °Brix represents an arbitrary scale to measure sugar densities in solutions and is equivalent to the percentage in weight of the soluble solids in one sample, which are mainly sugars. For example, the stems without leaves (piña) of A. tequilana should have at least 24% of total sugars to be considered of good quality (Bautista-Justo et al., 2001). In this study, the effect of FLNFB on plant growth and total solid soluble content of the stem (SSC) in plants of A. potatorum under semi-controlled conditions was evaluated.
Materials and Methods
The experiment used three FLNFB strains, previously isolated from the rhizosphere of A. angustifolia cultivated in the District of Tlacolula, Oaxaca, Mexico in the communities of Magdalena Teitipac (16° 54´ N and 96° 33´ W), San Baltazar Guelavila (19° 80´ N and 96° 29´ W) and San Juan del Río (16° 53´ N and 96° 09´ W). Based on the Analytical Profile Index (API20 NE, bioMérieux, Inc., Durham, NC, USA) system, the identity of Burkholderia cepacia was confirmed with the phenotypical characteristics of gram negative bacteria, large white-colored colonies, entire borders, circular mucoid shape with yellow pigments in the center; Flavobacterium sp., gram negative bacteria, yellow-colored colonies with entire borders, circular shape with greenish excreted pigment; and Paenibacillus amylolyticus, gram positive bacteria, translucid colonies with entire borders and circular shape. These FLNFB were selected because of their high efficiency to fixate atmospheric N in vitro by quantifying the NH4 + ion. The diazotrophic capacity obtained was 1.10 µg N-NH4 + mL-1 for B. cepacia; 1.54 µg N-NH4 + mL‑1 for Flavobacterium sp.; and 1.68 µg N-NH4 + mL-1 for Paenibacillus amylolyticus.
Effect of free-living nitrogen-fixing bacteria in Agave potatorum Zucc. growth
The FLNFB selected were inoculated following the streak plate method in specific agar for nitrogen-fixing bacteria (NFb), and incubated in aerobic conditions at 30 °C for 48 h. Colonies from each strain developed in NFb agar were taken and transferred to a test tube with 10 mL of sterile saline solution at 0.85% and adjusted to a concentration of 1.5 × 108 cells mL-1, utilizing McFarland scale as reference. Subsequently, 1 mL of this bacterial suspension was inoculated in 9 mL of sterile nutritive medium that contained (g L-1): glucose 0.5; yeast extract 0.5; peptone 0.5; casein 0.5; starch 0.5; K2HPO4 0.30; MgSO4 0.05; pH 7.0 (Sánchez-López and Pérez-Pazos, 2018), and incubated at 30 °C for 24 h, thus obtaining one pre-inoculant per strain. For the inoculant production, 5 mL of the pre-inoculant were re-inoculated in 45 mL of the sterile nutritive medium and incubated at 30 °C at 150 rpm for 48 h. After incubation time, each bacterial strain was centrifuged at 10 000 rpm for 10 min. The cellular pellet obtained was diluted in sterile nutritive medium until a concentration of 15 × 108 cell mL-1 was obtained, according to Mc Farland scale as reference (Sánchez-López and Pérez-Pazos, 2018).
The agave seedlings used in this experiment were obtained from seeds of mature Agave potatorum Zucc. plants with an average from 5-7 years of age. Seed boxes of 200 wells were used with sterile soil of the area of San Baltazar Chichicapam, District of Ocotlán, Oaxaca, where A. potatorum grows wild. This soil showed the following characteristics: organic matter (1.7%), NO3 - (470 mg kg-1), pH (7.5) and available phosphorus (5.8 mg kg-1). One agave seed per cavity was sown, and irrigation was performed every other day until true leaf emergence was observed. After 60 days, seedlings were transferred to pots containing 2000 g of non-sterile soil, so the FLNFB were capable of colonizing agave plant roots, considering the possible synergic or antagonistic relationships that could be among them and the existing natural biota in soil. Under a randomized complete block design, three FLNFB were assessed: (1) Burkholderia cepacia, (2) Flavobacterium sp., (3) Paenibacillus amylolyticus and one control group with sterile nutritive medium without FLNFB to counteract the effect of the nutrients present in the culture medium on plant growth. In total, four blocks with 15 agave plants per block were prepared; within each block, a different FLNFB was applied randomly to each agave plant; 50 mL of the inoculant were applied directly to the agave plant root with a final colony forming unit (CFU g-1 of soil) of 3.75 × 107. Irrigation was performed twice per week. The experiment was established in semi-controlled conditions within a macro tunnel (day temperature from 26-32 °C, night temperature 18-20 °C; photoperiod, 14 h light: 10 h darkness). The growth variables of each plant assessed after 48 weeks after transplant were: plant height (PH) and rosette diameter (ROD) with a flex-o-meter graduated in centimeters; plant stem diameter (PSD) with a digital vernier; the unfolded leaves number (ULN) was counted visually; root volume (RV) was determined by the approximate test tube method; root density (RD) was obtained by the relationship mass-volume. Following Huang et al. (2017), only were the dry weight based variables considered; thus stem dry biomass (SDB) was determined after drying at 70 °C for 72 h; total dry biomass (TDB) was quantified by the total dry weight of each plant; leaf area (LA) was estimated with the ImageJ software; SSC (°Bx) was measured with a refractometer.
Data were subjected to an analysis of variance (ANOVA) and Tukey’s multiple comparison of means test (level of significance of 5%) with the statistical package SAS®, version 9.1 (SAS, 2004).
Results and Discussion
The ANOVA and multiple comparison of means test (Tukey, P ≤ 0.05) revealed that with respect to control, B. cepacia increased RV 322.2%, ULN 42.6%, and SSC 72.9%; P. amylolyticus increased SDB 317.1% (Figure 1). B. cepacia, Flavobacterium sp., and P. amylolyticus increased PSD approximately 50.3% (Figures 1 and 5), ROD 48.6%, LA 127.2%, and PH 51.8% (Figures 2 and 3). Flavobacterium sp. increased TB 164.8% (Figure 2).
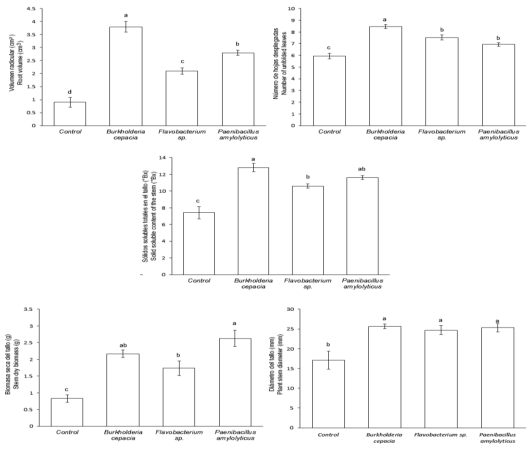
Different small letters on each bar indicate the inoculant effect (Tukey’s test, P ≤ 0.05).
Figure 1: Mean value ± standard error in some growth variables and total solid soluble content of the stem or “piña” (stem without leaves) of the Agave potatorum Zucc. plants inoculated with free-living nitrogen-fixing bacteria in semi-controlled environments after 48 weeks of assessment.
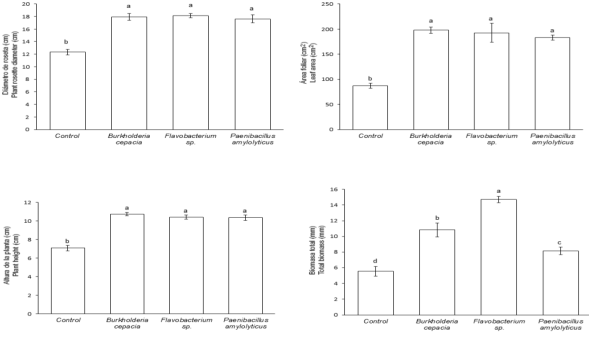
Different small letters on each bar indicate the inoculant effect (Tukey’s, test P ≤ 0.05).
Figure 2: Mean value ± standard error in some plant growth variables of Agave potatorum Zucc. inoculated with free-living nitrogen-fixing bacteria in semi-controlled environments after 48 weeks of assessment.

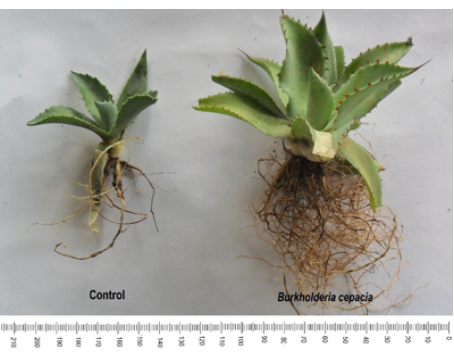
Divisions are in scale = 1 mm.
Figure 3: Effect of free-living nitrogen-fixing bacteria on plant height of Agave potatorum Zucc. in semi-controlled environments after 48 weeks of assessment.
From the assessed growth variables, RD was the only one that did not show statistically significant changes among the inoculated plants and the control group. The increase in agave plant growth variables could be attributed to different mechanisms performed by the FLNFB previously reported as plant growth promoters (Requena et al., 1997); those found are (1) increase in elongation and cellular multiplication because of a better nutrient absorption as N; (2) phytohormone production, among those, auxins, cytokinins, gibberellins, and abscisic acid; (3) siderophore production, which may provide iron to plants; (4) enzyme synthesis, such as 1-aminocyclopropane-1-carboxylate deaminase, which decreases ethylene levels in plants (Lucy et al., 2004; Podile and Kishore, 2006; Appanna, 2007).
The RV (Figure 4), ULN, and LA in agave plants increased with B. cepacia inoculation. The results that agree with those established by Argüello-Navarro and Moreno-Rozo (2014). These authors inoculated cacao plants with FLNFB, such as Burkholderia sp., and after 120 days of nursery assessment, they found increments in leaf number, LA, TB, and root length in plants inoculated compared with the control group. Similarly, Roesch et al. (2005) evaluated the inoculation effect of diazotrophic bacteria from the genus Azospirillum in wheat and observed an increase in size and number of radicle hair compared with the control group. Numerous studies have shown that diazotrophic bacteria may promote plant growth, mainly by changing the root system morphology, improving development and productivity of several crops of economic interest (Bashan et al., 2004; Somers et al., 2004).
Divisions in scale = 1 mm.
Figure 4: Effect of free-living nitrogen-fixing bacteria (FLNFB) on root volume of Agave potatorum Zucc. in semi-controlled environments after 48 weeks of assessment.
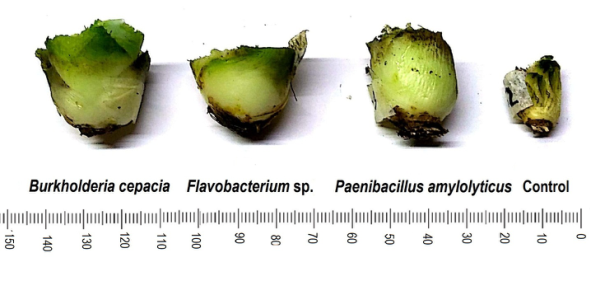
Figure 5: Effect of free-living nitrogen-fixing bacteria on stem or “piña” (stem without leaves) diameter of Agave potatorum Zucc. in semi-controlled environments after 48 weeks of assessment. Division in scale = 1 mm.
Flavobacterium sp. increased TB of agave plants; other studies have also reported that inoculation with FLNFB, such as Herbaspirillum seropedicae in rice plants, increased TB in 71.5% with respect to the control group. Under the conditions of pot cultivation, the bacteria of the genus Paenibacillus sp. RFNB4 increased the PH and TB of canola (Brassica campestris) plants significantly in 42.3 and 29.5%, respectively (Islam et al., 2009). Kifle and Laing (2016) found that inoculation with diazotrophic bacteria of the genus Bacillus, Pseudomonas, and Enterobacter in maize plants under greenhouse conditions, increased TB when compared with those non-inoculated. Govindarajan et al. (2006) assessed inoculation with Burkholderia vietnamiensis MG43 in the varieties Co 86032 and Co 86027 of micro-propagated sugar cane and found increments of 20 and 19% in the production of dry matter of Co 86032 and Co 86027, respectively.
Some authors have reported that when plants established these associations with endophytic fungi or FLNFB, their sugar content and development increased (Obledo et al., 2003; Ruiz et al., 2011). De la Torre-Ruiz et al. (2016) inoculated Agave americana plants with Rhizobium daejeonense, Acinetobacter calcoaceticus and Pseudomonas mosselii and found positive effects on TB, PSD, leaves number, root length and sugar content in stems without leaves (piñas) when compared with those non-inoculated agave plants. These results agree with those reported in this study where B. cepacia inoculation increased SSC (°Bx) in agave stems (piñas). The °Brix are equivalent to the percentage in solid soluble weight of one sample, which are mainly sugars (Bautista-Justo et al., 2001). The sugar content in agave plants may also vary with the plant physiological stage (Arrizon et al., 2010). The young A. tequilana plants, for example, possess higher levels of monosaccharides (e.g. glucose, fructose) than adult plants, which accumulate fructans starting from 8-10 years of age (Cedeño, 1995).
Conclusions
- Inoculation with free-living nitrogen-fixing bacteria in Agave potatorum plants had a positive effect on the evaluated plant growth variables of 90%. Burkholderia cepacia increased the root volume 322.2%; number of unfolded leaves 42.6%; and solid soluble content of the stem 72.9%. Paenibacillus amylolyticus increased stem dry biomass 317.1%. B. cepacia, Flavobacterium sp. and P. amylolyticus increased plant stem diameter approximately 50.3%; plant rosette diameter 48.6%; leaf area 127.2%; plant height 51.8%. Flavobacterium sp. increased total biomass 164.8%.
- The results in this study suggest that these bacteria are capable of promoting A. potatorum growth. Therefore, they can be an eco-friendly and economic technology for the production of this agave species in the State of Oaxaca, Mexico and other places with similar management conditions.
Acknowledgments
This study formed part of the Project “Bacterias fijadoras asimbióticas de nitrógeno en Agave potatorum Zucc.” SIP 20180051, financed by the Instituto Politécnico Nacional (IPN). The authors appreciate the valuable comments and suggestions of two anonymous reviewers that contributed to improve this research study; to D. Fischer for translation and editorial services in English.
REFERENCES
Allan, D. and P. Graham. 2002. Soil 5611: Soil biology and fertility: Symbiotic nitrogen fixation, other N2-fixing symbiosis. Dep. of Soil, Water and Climate. University of Minnesota. http://www.soils.agri.umn.edu/academics/classes/soil3612/SymbioticNitrogen/Other.htm . (Consulta: mayo 28, 2019). [ Links ]
Appanna, V. 2007. Efficacy of phosphate solubilizing bacteria isolated from vertisols on growth and yield parameters of sorghum. Res. J. Microbiol. 2: 550-559. doi: 10.3923/jm.2007.550.559. [ Links ]
Argüello-Navarro, A. Z. y L. Y. Moreno-Rozo 2014. Evaluación del potencial biofertilizante de bacterias diazótrofas aisladas de suelos con cultivo de cacao (Theobroma cacao L.). Acta Agronómica 63: 238-245. doi: https://doi.org/10.15446/acag.v63n3.41033. [ Links ]
Arrizon, J., S. Morel, A. Gschaedler, and P. Monsan. 2010. Comparison of the water-soluble carbohydrate composition and fructan structures of Agave tequilana plants of different ages. Food Chem. 122: 123-130. doi: https://doi.org/10.1016/j.foodchem.2010.02.028. [ Links ]
Bano, S. A. and S. M. Iqbal. 2016. Biological nitrogen fixation to improve plant growth and productivity. Int. J. Agric. Innovat. Res. 4: 596-599. [ Links ]
Bashan, Y. 1998. Azospirillum plant growth-promoting strains are nonpathogenic on tomato, pepper, cotton, and wheat. Can. J. Microbiol. 44: 168-174. doi: https://doi.org/10.1139/w97-136. [ Links ]
Bashan, Y., G. Holguin, and L. E. Bashan. 2004. Azospirillum plant relationships: Physiological, molecular, agricultural and environmental advances (1997-2003). Can. J. Microbiol. 50: 521-577. doi: https://doi.org/10.1139/w04-035. [ Links ]
Bautista-Justo, M., L. García-Oropeza, R. Salcedo-Hernández y L. Parra-Negrete. 2001. Azúcares en agaves (Agave tequilana Weber) cultivados en el estado de Guanajuato. Acta Univ. 11: 33-38. doi: https://doi.org/10.15174/au.2001.325. [ Links ]
Bonilla Buitrago, R. R., R. Novo Sordo, N. Vanegas, A. M. Galvis Macias, M. M. Martínez S., D. Parra y O. Vanegas. 2000. Generación de tecnologías para la utilización de la fijación no simbiótica de nitrógeno como alternativa de fertilización. Corpoica. Regional tres. Boletín de Investigación núm. 5. Valledupar, Colombia. [ Links ]
Cedeño, M. C. 1995. Tequila production. Crit. Rev. Biotechnol. 15: 1-11. doi: https://doi.org/10.3109/07388559509150529. [ Links ]
CRM (Consejo Regulador del Mezcal). 2019. El crecimiento del mezcal 2018. Resumen del informe estadístico. pp. 10-19. In: El mezcal, la cultura líquida de México 2. http://www.crm.org.mx/publicaciones/revista/pdf/Revista_El_Mezcal2.pdf . (Consulta: mayo 30, 2019). [ Links ]
De la Torre-Ruiz, N., V. M. Ruiz-Valdiviezo, C. I. Rincón-Molina, M. Rodríguez-Mendiola, C. Arias-Castro, F. A. Gutiérrez-Miceli, H. Palomeque-Dominguez, and R. Rincón-Rosales. 2016. Effect of plant growth-promoting bacteria on the growth and fructan production of Agave americana L. Braz. J. Microbiol. 4: 587-596. doi: https://doi.org/10.1016/j.bjm.2016.04.010. [ Links ]
Figueiredo, M. V. B., A. C. E. S Mergulhão, J. Kuklinsky-Sobral, M. A. Lira Junior, and A. S. F. Araujo. 2013. Biological nitrogen fixation: Importance, associated diversity, and estimates. pp. 267-289. In: N.K. Arora (ed.). Plant microbe symbiosis: Fundamentals and advances. Springer. New Delhi. doi: https://doi.org/10.1007/978-81-322-1287-4_10. [ Links ]
García-Mendoza, A. J. 2007. Los agaves de México. Ciencias 87: 14-23. [ Links ]
García-Moya, E., A. Romero-Manzanares, and P. S. Nobel. 2010. Highlights for Agave productivity. Global Change Biol. Bioenerg. 3: 4-14. doi: https://doi.org/10.1111/j.1757-1707.2010.01078.x. [ Links ]
Govindarajan, M., J. Balandreau, R. Muthukumarasamy, G. Revathi, and C. Lakshminarasimhan. 2006. Improved yield of micropropagated sugarcane following inoculation by endophytic Burkholderia vietnamiensis. Plant Soil 280: 239-252. doi: https://doi.org/10.1007/s11104-005-3223-2. [ Links ]
Hernández, Y. 1998. La fijación biológica del nitrógeno. Rev. Cubana Cienc. Agríc. 32: 233-250. [ Links ]
Hernández-Rodríguez, A., N. Rives-Rodríguez, Y. Acebo-Guerrero, A. Diaz-de la Osa, M. Heydrich-Pérez y V. L. Divan Baldani. 2014. Potencialidades de las bacterias diazotróficas asociativas en la promoción del crecimiento vegetal y el control de Pyricularia oryzae (Sacc.) en el cultivo del arroz (Oryza sativa L.). Rev. Protec. Veg. 29: 1-10 [ Links ]
Huang, P., L. de-Bashan, T. Crocker, J. W. Kloepper, and Y. Bashan. 2017. Evidence that fresh weight measurement is imprecise for reporting the effect of plant growth-promoting (rhizo) bacteria on growth promotion of crop plants. Biol. Fert. Soils 53: 199-208. doi https://doi.org/10.1007/s00374-016-1160-2. [ Links ]
Islam, M. R., M. Madhaiyan, H. P. Deka Boruah, W. Yim, G. Lee, V. S. Saravanan, Q. Fu, H. Hu, and T. Sa. 2009. Characterization of plant growth-promoting traits of free-living diazotrophic bacteria and their inoculation effects on growth and nitrogen uptake of crop plants. J. Microbiol. Biotechnol. 19: 1213-1222. doi: 10.4014/jmb.0903.03028. [ Links ]
Jnawali, A. D., R. B. Ojha, and S. Marahatta. 2015. Role of Azotobacter in soil fertility and sustainability-a review. Adv. Plants Agric. Res. 2: 250-253. doi: 10.15406/apar.2015.02.00069. [ Links ]
Kifle, M. H. and M. D. Laing. 2016. Effects of selected diazotrophs on maize growth. Front. Plant Sci. doi: 10.3389/fpls.2016.01429. [ Links ]
Lucy, M., E. Reed, and B. R. Glick. 2004. Application of free kiving plant growth-promoting rhizobacteria. Antonie Van Leeuwenhoek 86: 1-25. doi: 10.1023/B:ANTO.0000024903.10757.6e. [ Links ]
Meunchang, S., S. Panichsakpatana, and R. W. Weaver. 2005. Inoculation of sugar mill by-products compost with N2-fixing bacteria. Plant Soil 271: 219-225. doi: https://doi.org/10.1007/s11104-004-2389-3. [ Links ]
Mirza, M. S., G. Rasul, S. Mehnaz, J. K. Ladha, R. B. So, S. Ali, and K. A. Malik. 2000. Beneficial effects of inoculated nitrogen-fixing bacteria on rice. pp. 191-204. In: J. K. Ladha and P. M. Reddy. (eds.). The quest for nitrogen fixation in rice. Int. Rice Res. Inst. Los Baños, Filipinas. ISBN: 9712201120, 9789712201127. [ Links ]
Núñez, H. M., L. F. Rodríguez, and M. Khanna. 2010. Agave for tequila and biofuels: an economic assessment and potential opportunities. Global Change Biol. Bioenerg. 3: 43-57. doi: https://doi.org/10.1111/j.1757-1707.2010.01084.x. [ Links ]
Obledo, E. N., L. B. Barragán-Barragán, P. Gutiérrez-González, B. C. Ramírez-Hernández, J. J. Ramírez, and B. Rodríguez-Garay. 2003. Increased photosynthetic efficiency generated by fungal symbiosis in Agave victoria-reginae. Plant Cell Tissue Organ Cult. 74: 237-241. doi: https://doi.org/10.1023/A:1024046925472. [ Links ]
OEIDRUS-SAGARPA (Oficina Estatal de Información para el Desarrollo Rural Sustentable de Oaxaca-Secretaria de Agrícultura Ganaderia, Desarrollo Rural Pesca y Alimentación). 2011. Maguey mezcal, regiones productoras de Oaxaca. 2011. http://www.oeidrus-oaxaca.gob.mx/biblioteca.html . (Consulta: octubre 15, 2009). [ Links ]
Podile, A. R. and G. K. Kishore. 2006. Plant growth-promoting rhizobacteria (PGPR). pp. 195-230. In: S. S. Gnanamanickam (ed.). Plant-associated bacteria. Springer. Dordrecht, The Netherlands. ISBN: 978-1-4020-4538-7. [ Links ]
Requena, N., T. M. Baca, and R. Azcón. 1997. Evolution of humic substances from unripe compost during incubation with lignolytic or cellulolytic microorganisms and effects on the lettuce growth promotion mediated by Azotobacter chroococcum. Biol. Fertil. Soils 24: 59-65. doi: https://doi.org/10.1007/BF01420221. [ Links ]
Roesch, L. F., F. O. Camargo, P. A. Selbach, e E. S Sá. 2005. Reinoculação de bactérias diazotróficas aumentando o crescimento de plantas de trigo. Ciênc. Rural 35: 1201-1204. doi: https://doi.org/10.1590/S0103-84782005000500035. [ Links ]
Ruiz, S., L. Adriano, I. Ovando, C. Navarro, and S. Salvador. 2011. Biofertilization of micropropagated Agave tequilana: Effect on plant growth and production of hydrolytic enzymes. Afr. J. Biotechnol. 10: 9623-9630. doi: https://doi.org/10.5897/AJB11.641. [ Links ]
Sánchez-López, A. 2005. Oaxaca, tierra de maguey y mezcal. Instituto Tecnológico de Oaxaca. Oaxaca de Juárez, México. ISBN: 9709916009, 9789709916003. [ Links ]
Sánchez-López, D. B. y J. V. Pérez-Pazos. 2018. Caracterización y evaluación de PGPRs sobre el crecimiento de plántulas de Dioscorea rotundata in vitro. Agron. Costarricense 42: 75-91. doi: http://dx.doi.org/10.15517/rac.v42i2.33780 [ Links ]
SAS Institute. 2004. SAS/STAT Guide to personal computers. Version 9.0l. SAS Institute Inc. Cary, NC, USA. [ Links ]
Sharma, J., T. Gurung, K. Nandy, and A. K. Mitra. 2014. Efficiency of different nitrogen fixing bacteria with respect to growth and development of legumes. Int. J. Curr. Microbiol. Appl. Sci. 3: 799-809. [ Links ]
Shin, W., R. Islam, A. Benson, M. M. Joe, K. Kim, S. Gopal, S. Samaddar, S. Banerjee, and T. Sa. 2016. Role of diazotrophic bacteria in biological nitrogen fixation and plant growth improvement. Korean J. Soil Sci. Fertil. 49: 17-29. doi: http://dx.doi.org/10.7745/KJSSF.2016.49.1.017. [ Links ]
Shridhar, S. B. 2012. Review: Nitrogen fixing microorganisms. Int. J. Microbiol. Res. 3: 46-52. doi: 10.5829/idosi.ijmr.2012.3.1.61103. [ Links ]
Somers, E., J. Vanderleyden, and M. Srinivasan. 2004. Rhizosphere bacterial signaling: A love parade beneath our feet. Critical Rev. Microbiol. 30: 205-240. doi: https://doi.org/10.1080/10408410490468786. [ Links ]
Vera-Guzmán, A., P. Santiago G. y M. G. López. 2009. Compuestos volátiles aromáticos generados durante la elaboración de mezcal de Agave angustifolia y Agave potatorum. Rev. Fitotec. Mex. 32: 273-279. [ Links ]
Received: July 07, 2019; Accepted: January 02, 2020











 texto em
texto em 



