Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Terra Latinoamericana
versão On-line ISSN 2395-8030versão impressa ISSN 0187-5779
Terra Latinoam vol.38 no.3 Chapingo Jul./Set. 2020 Epub 12-Jan-2021
https://doi.org/10.28940/terra.v38i3.646
Special number
Native mycorrhizal fungi as growth promoters in guava plants (Psidium guajava L.)
1 Laboratorio de Fitopatología, Biotecnología Vegetal, Centro de Investigación y Asistencia en Tecnología y Diseño del Estado de Jalisco, A.C. Camino Arenero 1227, El Bajío del Arenal. 45019 Zapopan, Jalisco, México.
2 Instituto de Investigaciones Agropecuarias y Forestales, Universidad Michoacana de San Nicolás de Hidalgo. Carretera Morelia-Zinapécuaro km 9.5. 58880 Tarímbaro, Michoacán, México.
This research study assessed the effect of five native consortia of arbuscular mycorrhiza on guava (Psidium guajava L.) plant growth. A completely randomized experimental design was established with seven arbuscular mycorrhizal fungus (AMF) treatments named: Las Campesinas (LC), Carlos Rojas (CR), Paso Ancho (PA), El Limón (EL), Cerro del Metate (CM) (native consortia), a commercial strain from the Instituto Nacional de Investigaciones Forestales, Agrícolas y Pecuarias (INIFAP®), and a control group without AMF (w/AMF), in a sterilized sand-soil mixture and under greenhouse conditions. Guava seeds germinated in sterilized sand; the seedlings were subsequently transplanted to a nursery bag with a sterilized sand-soil mixture where they were inoculated with the different AMF treatments. At 125 days after transplant, a destructive sampling was performed, recording plant height, stem diameter, leaf area, and fresh and dry biomass of each part. As variables of plant quality, dry biomass ratio of the aerial part/dry biomass of the root, index of robustness, and Dickson index were determined. The mycorrhizal colonization and spore production in the substrate were determined as microbiological variables. The results showed a differential effect on growth promotion in guava plants when they were inoculated with a native AMF consortium. Among the different consortia evaluated, EL promoted the best guava plant development and quality and where the highest colonization and spore production were reached in the substrate. The plants w/AMF or with the INIFAP inoculum had the highest mortality rate. The colonization percentages were higher than 60%, except for the CM consortium. Therefore, using AMF could be an advisable practice for the sustainable production of guava trees.
Index words: biofertilizer; plant quality; consortia; spores; mycorrhization
En esta investigación se evaluaron diferentes consorcios micorrízicos arbusculares nativos, en el crecimiento de plantas de guayaba (Psidium guajava L.). Se estableció un experimento totalmente al azar con siete inóculos de hongos micorrízicos arbusculares (HMA) denominados: Las Campesinas (LC), Carlos Rojas (CR), Paso Ancho (PA), El limón (EL), Cerro del Metate (CM) (consorcios nativos), una cepa comercial (INIFAP®), y un control sin HMA, en una mezcla de arena-suelo esterilizada y bajo condiciones de invernadero. Semillas de guayaba fueron germinadas en arena esterilizada y posteriormente, las plántulas fueron trasplantadas a bolsa de vivero con una mezcla de arena-suelo esterilizada, donde fueron inoculadas con los diferentes tratamientos de HMA. Después de 125 días, se realizó un muestreo destructivo donde se registró, la altura de planta, el diámetro del tallo, el área foliar, la materia fresca y seca de cada órgano. Así como, la relación materia seca de la parte aérea/materia seca de la raíz, el índice de robustez y el índice de Dickson, como variables de calidad de planta. Cómo variables microbiológicas se determinó, la micorrización radicular y la cantidad de esporas en el sustrato. Los resultados mostraron un efecto diferencial en el crecimiento en las plantas de guayaba, cuando se inocularon con algún consorcio nativo de HMA. Entre los diferentes consorcios evaluados, EL promovió el mejor desarrollo y calidad de planta, y fue donde se alcanzó la mayor colonización y producción de esporas en el sustrato. La mayor tasa de mortalidad se registró en las plantas sin HMA, o inoculadas con el inóculo INIFAP. Los porcentajes de colonización fueron superiores al 60%, excepto en el consorcio CM. Por lo anterior se puede mencionar que, el uso de HMA podría ser una práctica recomendable para el cultivo sustentable de guayabas en invernadero.
Palabras clave: biofertilizante; calidad de planta; consorcios; esporas; micorrización
Introduction
The guava (Psidium guajava L.) tree is one of the perennial fruit trees widely cultivated in the world. Its fruit is economical and consumed preferably fresh; it contains a great amount of vitamin C, B2, pectin, minerals, such as phosphorus, calcium, and iron besides having antioxidant properties (Mohandas et al., 2013). Mexico is the fifth world producer of this species; the State of Michoacan reported a cultivated surface of more than 11 thousand hectares in 2017, making this state one of the main producers of this fruit (SIAP, 2017). The increase in fruit demand has made the producers search for ecological and sustainable alternatives to increase production with the rise in organic product demand and face the environmental deterioration caused by chemical products. From 2008 to 2013, the organic production area in the world increased 109, 42, and 53% for temperate, citric and tropical, and subtropical fruit trees, respectively (Granatstein et al., 2016). One of the alternatives to increase production with a sustainable organic approach is, for example, using rhizosphere microorganisms that promote plant growth and help plants facing different production scenarios. In that respect, arbuscular mycorrhizal fungi (AMF) are obligate symbiotic microorganisms that colonize the root of the majority of plant species (Turrini et al., 2018). This symbiotic relationship promotes the host plant growth, and their roots may coinhabit with more than one AMF species. Mycorrhization also increases plant quality in greenhouse conditions and improves growth after transplant from the greenhouse to the field (Ortas and Ustuner, 2014; Machineski et al., 2018). On the other hand, no plant-fungus specificity exists, but the effect provided by the fungus to its host may be different, which could be promoting growth, increasing nutrient and water capture capacity, inducing biotic or abiotic stress resistance, among others (Azcón and Barea, 2015; Turrini et al., 2018); thus, we could mention that a differential effectiveness exists (Robles-Martínez et al., 2013). With this respect, Gavito and Varela (1995) and Bashan et al. (2000) mentioned that the inoculant constituted by more than one AMF species showed a greater benefit to the hosts. Additionally, Brussaard et al. (2007) mentioned that mycorrhizal diversity contributed positively to nutrition and water use. Native mycorrhizal consortia are also known to be more effective than those constituted by one or exotic species (Bashan et al., 2000; Ortas and Ustuner, 2014); this result is due to the fungus adaptation to specific natural conditions, and its inclusion into different environments may cause the inability to adapt to the environment (Rillig and Mummey, 2006). Similarly, studies have shown the efficiency of native consortia on plant growth in different species (Trejo et al., 2011; Carreón-Abud et al., 2015; Reyes-Tena et al., 2015; Machineski et al., 2018). In the case of guava, some studies have demonstrated the effectiveness of growth promotion by the AMF. Estrada-Luna et al. (2000) reported that the guava plants inoculated with a mixture of Glomus diaphanum, G. albidum and G. claroides showed greater stem growth, leaf production, leaf area, dry biomass, leaf concentration of P, Mg, Cu, and Mo, as well as an increase in gas exchange, more than in non-mycorrhized guava plants. Similar growth results were reported by Schiavo and Martins (2002) with Glomus clarum, Panneerselvam et al. (2012); Glomus mosseae, da Silva-Campo et al. (2013); Acaulospora longula, Gigaspora albida and Glomus etunicatum. On the other hand, Das et al. (2017) reported an increase in guava plant production mycorrhized with Glomus mosseae.
Likewise, guava has been reported as mycotrophic species (Estrada-Luna et al., 2000); some AMF species that have been isolated in their rhizosphere are Glomus mosseae (Panneerselvam et al., 2012; Mohandas et al., 2013), Sclerocystis coremioides, Acaulospora spp., and Scutellospora calospora (Kumuran and Azizah, 1995), which is why this species has been colonized by more than one AMF species and still have a certain differential effectiveness. However, no studies have been available where the effect of growth promoter on guava plant and transplant survival is reported with AMF consortia. Therefore, this study assessed five native AMF consortia and a monosporic commercial inoculant (INIFAP®) in growth and quality of guava (Psidium guajava L.) plants in greenhouse conditions.
Materials and Methods
Growth conditions
The assay was developed in a zenithal plastic type greenhouse of the Instituto de Investigaciones Agropecuarias y Forestales of the Universidad Michoacana de San Nicolás de Hidalgo (19° 45’ 95’’ N, 101° 09’ 16’’ W, 1900 m) under light, temperature, and environmental humidity conditions.
Arbuscular mycorrhizal fungus inoculant
Five native mycorrhizal consortia and a commercial inoculant made by INIFAP® (Instituto Nacional de Investigaciones Forestales, Agrícolas y Pecuarias, MX) were assessed. The consortia came from soils with Agave cupreata plantations of the State of Michoacán, Mexico and were named according to their collection site: Las Campesinas (LC), Carlos Rojas (CR), Paso Ancho (PA), El Limón (EL), Cerro del Metate (CM). The inoculants were previously propagated in tramp pots in the greenhouse for 12 months; each consortia contained from four to six species, of which the most abundant of each mycorrhizal consortia were the following: CM: Glomus glomerulatum, PA: Acaulospora delicata, CR and EL: Glomus deserticola, LC: Acaulospora scrobiculata (Trinidad-Cruz et al., 2017a). The commercial INIFAP inoculant was previously used in other experiments where its viability, colonization, and growth promotion was assessed in other plant species.
Plant material
Commercial guava seeds were disinfected with a commercial chlorine solution at 3% (active chlorine at 6%) for 10 min and then washed with distilled water thrice for 5 min. Subsequently, they were placed in plastic germinator trays with sterilized sand under light and environmental conditions. The seeds and substrate were kept hydrated all the time. From the 28 days after sowing, the seeds were considered germinated when they showed a homogenous size (in average, 2 cm high, four leaves, and 9 mm of stem diameter), and transplanted to greenhouse bags.
Inoculation
The guava plants were transplanted in black polyethylene bags with 2.5 kg of sand and soil (1:1 v/v) sterilized with growth medium. The seedlings were placed in a cavity made in the center of the bag; at the moment of transplanting, inoculation of the different mycorrhizal consortia was performed directly in the radicle system. The number of spores of each AMF consortium was quantified per gram, and the necessary grams were taken to inoculate 80 spores, approximately; for the control group without AMF (w/AMF) treatment, 10 g of sterile sand per treatment were inoculated. Once inoculation was performed, the seedlings remained for 125 days in the greenhouse and were irrigated with demineralized water at field capacity as required. Seven treatments (five native consortia, the commercial INIFAP inoculant and one w/AMF) were generated; each treatment was replicated seven times, and the experimental unit was one guava plant. The factorial arrangement was a completely randomized block design.
Plant growth, quality, and mycorrhization variables
A destructive sampling was performed at 125 days after transplant; plant height was measured manually with a flexometer; the stem diameter with a Digital Vernier Caliper; leaf area with digital planimetry (LI‑COR, LI-3100, Lincoln, NE, U.S.A.); and the fresh biomass of each organ was weighed with an analytical balance (Mettler Toledo AT200, Columbus, OH, U.S.A.); subsequently, the samples were stove-dried at 60 °C for four days to determine dry weight. The relationship DAPB / DRB (dry aerial part biomass and dry root biomass) was determined as plant quality variable to know the balance between the plant transpiration and absorption surfaces (Prieto et al., 2009), robust index related to the plant photosynthetic capacity (Rueda-Sánchez et al., 2013), and Dickinson’s index, which expresses the equilibrium of biomass and robustness distribution, avoiding the selection of disproportionate plants and discarding those of lower height but with greater vigor (Dickson et al., 1960). With respect to the microbiological variables, the mycorrhizal colonization percentage was determined by Phillips and Hayman’s (1970) technique, subsequently determining the mycorrhizal colonization percentage with the methods described by Mcgonigle et al. (1990). For spore quantification in the substrate, the technique proposed by Gerdemann and Nicolson (1963) and Brundrett et al. (1996) was used.
Statistical analysis
A one-way analysis of variance (ANOVA) and Tukey’s multiple range test were performed to the data, both with a value of P ≤ 0.05. The percentage values were transformed (ArcSen n%/100) for homogenization of variance (Gomez and Gomez, 1984). The statistical software Statgraphics ver. XV (Statgraphics, 2005) was used for data processing.
Results and Discussion
Guava plant growth
The statistical analysis showed significant statistical differences (P ≤ 0.05) among the different treatments in all the growth variables assessed (Table 1). At 125 days after inoculation, the guava plants with the INIFAP inoculant and those w/AMF obtained the lowest growth values in all the cases and resulted statistically equal between them.
Table 1 Effect of arbuscular mycorrhizal fungus application on guava plant growth assessed 125 days after establishment.
|
Treatment |
|||||||
|
cm |
mm |
- - - - - - - - - - - - g - - - - - - - - - - - |
cm2 |
||||
|
2.17 c |
0.93 c |
0.01 d |
0.0005 c |
0.011 c |
0.011 c |
0.00 c |
|
|
INIFAP |
2.26 c |
0.82 c |
0.01 d |
0.0004 c |
0.006 c |
0.006 c |
0.00 c |
|
10.35 abc |
2.18 bc |
3.00 bcd |
0.41 bc |
0.73 abc |
1.14 bc |
19.78 c |
|
|
17.21 ab |
3.33 ab |
7.05 abc |
0.95 abc |
1.65 ab |
2.61 ab |
116.08 ab |
|
|
9.26 bc |
2.10 bc |
2.41 cd |
0.34 bc |
0.46 c |
0.80 bc |
43.45 bc |
|
|
18.12 ab |
3.81 a |
9.01 ab |
1.22 ab |
2.02 a |
3.25 ab |
152.72 a |
|
|
18.81 a |
4.07 a |
10.91 a |
1.72 a |
1.94 ab |
3.66 a |
145.19 a |
|
w/AMF = without arbuscular mycorrhizal fungi; PA = Paso Ancho; LC = Las Campesinas; CR = Carlos Rojas; EL = El Limón; CM = Cerro del Metate; H = plant height; D = stem diameter; TFB = total fresh biomass; DRB = dry root biomass; DAPB = dry aerial part biomass; TDB = total dry biomass; LA = leaf area. Measurements with different letters per column mean significant differences (P ≤ 0.05).
On the other hand, the greatest mortality after transplant and inoculation was recorded in the plants with the treatments w/AMF and those with the INIFAP inoculant with 43 and 58%, respectively. In that respect, Estrada-Luna et al. (2000) and Machineski et al. (2018) mentioned that the AMF increased stress resistance in fruit trees during transplant and acclimation because of the advantages that the symbiotic association promoted by improving hydric relationships, nutrient acquisition, and plant growth, among others.
Likewise, the plants that survived in the treatments w/AMF and those with the INIFAP inoculant did not produce leaves during the time the experiment lasted (Table 1). The fact of finding the greatest mortality and the least plant growth in plants w/AMF or with the INIFAP inoculant might have been due to their disadvantage with respect to the mycorrhized plants; in the same manner, the effect that the colonization may have on guava plant survival was notorious in those inoculated with the CM consortium, which had a survival of 85% with only 3% of colonization, and their growth was greater than that reached by the plants w/AMF and those with the INIFAP inoculant.
On the other hand, the greatest guava plant growth obtained from seeds and inoculated with different species and the arbuscular mycorrhizal fungus combination has been reported previously (Estrada-Luna et al., 2000; Schiavo and Martins, 2002; da Silva-Campos et al., 2013; Mohandas et al., 2013; Das et al., 2017). In general, this study found an increase in guava plants propagated by seeds when a mycorrhizal consortium was applied. The effectiveness of some of this native consortia has been proven in maize, chili, and bean (Reyes-Tena et al., 2015, 2016), Agave inaequidens (Quiñones-Aguilar et al., 2016), and Agave cupreata (Trinidad-Cruz et al., 2017b). This promotion effectiveness may have been caused by the diversity of the AMF species that contain inoculants; with this respect, Gavito and Varela (1995) and Bashan et al. (2000) mentioned that the inoculants constituted by more than one AMF species showed a greater benefit in their hosts. Furthermore, native mycorrhizal consortia are also known to be more effective than those constituted by exotic species or only one species (Bashan et al., 2000; Ortas and Ustuner, 2014). Another possible cause of greater growth promotion in mycorrhizal consortia could have been an effect of the propagation method (tramp pot) when rhizosphere soil propagates; besides the AMF, this soil had microorganism diversity, mainly bacteria associated to free-life spores that have the capacity of promoting plant growth. Some studies have reported a greater AMF effectiveness when they were applied jointly with other microorganisms (Panneerselvam et al., 2012; Mohandas et al., 2013). Similarly, other studies have reported the presence of actinomycetes, such as Streptmyces fradiae, S. avermitilis, S. cinnamonensis, S. canus and Leifsonia poae (Mohandas et al., 2013) and some pseudomonas species, such as Pseudomonas putida, P. aeruginosa, Brevibacillus sp. and Bacillus subtilis (Panneerselvam et al., 2012), colonizing AMF spores of the guava plant rhizosphere. Guava plant growth promotion was differentiated and only significant in the plants inoculated with the LC, PA, and EL consortia, with respect to those w/AMF or with the INIFAP inoculant. The difference found among the consortia was likely due to the species diversity of each one of them, which could indicate differential effectiveness (Costa et al., 2001; Robles-Martínez et al., 2013). The inoculated plants with the LC and EL consortia reached the greatest values in the variables assessed. The increments reached by these plants with respect to those w/AMF were 9, 4.4, 332 in average and more than 145 times for plant height, stem diameter, total dry biomass, and leaf area, respectively. Similar increments in growth variables have been reported in guava by the inoculation effect with AMF (Estrada-Luna et al., 2000; da Silva-Campos et al., 2013; Das et al., 2017). On the other hand, despite recording an increase in size in guava plants inoculated with the CR, PA, and CM consortia, the result was not significant with respect to those w/AMF or with the INIFAP inoculant (Table 1). Mycorrhization of guava plants with some AMF species has not promoted growth; da Silva-Campos et al. (2013) did not find differences in leaf number and dry biomass of the aerial part of guava plants when they were inoculated with Acaulospora lonbgula and Gigaspora albida.
Guava plant quality parameters
With respect to the quality attributes for broad-leaf trees (Rueda-Sánchez et al., 2013), which are considered growth variables, an increase was found when guava plants were inoculated with any AMF consortia (Table 2).
Table 2: Effect of arbuscular mycorrhizal fungus inoculation on quality attributes of the plants assessed at 125 days after establishment.
|
Treatment |
Relationship DAPB/DRB |
Quality |
Robustness index |
Quality |
Dickson index |
Quality |
|
23.08 |
L |
2.46 |
H |
0.0004 |
L |
|
|
INIFAP |
17.05 |
L |
3.00 |
H |
0.0003 |
L |
|
1.55 |
H |
4.65 |
H |
0.34 |
M |
|
|
1.81 |
H |
5.06 |
H |
0.45 |
M |
|
|
1.29 |
H |
4.46 |
H |
0.14 |
L |
|
|
1.62 |
H |
4.66 |
H |
0.50 |
H |
|
|
1.15 |
H |
4.63 |
H |
0.64 |
H |
w / AMF = without arbuscular mycorrhizal fungus; PA = Paso Ancho; LC = Las Campesinas; CR = Carlos Rojas; EL = El Limón; CM = Cerro del Metate; DAPB = dry aerial part biomass; DRB = dry root biomass; H = high; M = medium; L = low.
The plants w/AMF or with the INIFAP inoculant showed the lowest quality, according to the DAPB/DRB relationship and Dickinson index. According to the DAPB/DRB relationship, two types of plant quality were obtained, low (DAPB/DRB > 2.0) for plants without AMF or with the INIFAP inoculant and high (DAPB/DRB < 2.0), when they were inoculated with any AMF consortium, which had a bearing in greater plant survival at the moment of transplanting to the field or in drought conditions (Prieto et al., 2009). For the robustness index, no difference was found in plant quality between the plants inoculated with AMF and those w/AMF, which according to this index, the plants showed high quality (< 6.0). With respect to Dickinson’s Index, two types of plant quality were obtained: low (< 0.2), for guava plants w/AMF and with the INIFAP inoculant and CM; average (0.2 - 0.4), for the plants inoculated with CR and PA; and high (≥ 0.5), for plants inoculated with EL and LC (Table 2). According to plant quality parameters, those inoculated with AMF were the ones with the best size and vigor, which would be benefitted at the moment of transplanting to the field (Ortas and Ustuner, 2014). This greater vigor in plants may have been caused by an increase in the photosynthetic activity that plants show when they are mycorrhized. In this particular case, Estrada-Luna et al. (2000) reported that guava plants propagated by seeds and inoculated with a mixture of Glomus diaphanum. Glomus albidum and Lomus claroides increased their net stomatal conductance and photosynthesis compared to those without mycorrhizal.
Mycorrhizal colonization percentage
Guava has been mentioned to be a highly mycotrophic plant with a mycorrhizal dependence of more than 100% (Estrada-Luna et al., 2000). These authors reported colonization values of 94% when a mixture of Glomus diaphanum, G. albidum and G. claroides was used. On the other hand, da Silva-Campos et al. (2013) reported percentages of 32, 48, and 62% when guavas were inoculated with G. etunicatum, Gigasora albida, and Acaulospora longula, respectively. Panneerselvam et al. (2012) reported 65% of colonization in guavas inoculated with Glomus mosseae. This study found that guava plants with the INIFAP inoculant did not show colonization (Figure 1), which could have generated the high mortality and stunted growth found in these plants. Several causes might have taken place if the INIFAP inoculant did not function, for example, spore viability and growth medium, among others. The EL consortium reached the greatest colonization (90%) although it resulted significantly similar to that obtained with the consortia PA, LC, CR. In average, a colonization of 70% was recorded among the four consortia, which was 23 times more than that obtained with the CM consortium that only colonized 3%. The low colonization for this consortium could have been due to species diversity that this inoculant contained, indicating a possible plant selectivity by the AMF symbiont (Costa et al., 2001; Barea and Azcón-Aguilar, 2013).
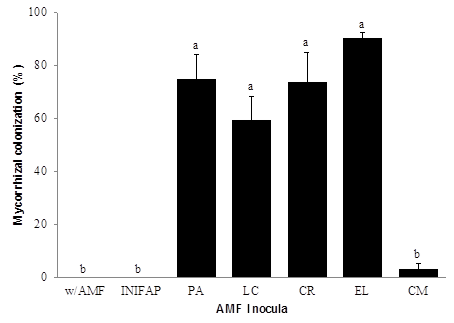
w/AMF = without arbuscular mycorrhizal fungus; PA = Paso Ancho; LC = Las Campesinas; CR = Carlos Rojas; EL = El Limón; CM = Cerro del Metate. Lines on bars indicated standard error. Different letters on bars indicate significant differences (P ≤ 0.05).
Figure 1: Mycorrhizal colonization in guava plant roots with different AMF, at 125 days of establishment.
Spore production
Spore production in the environment varied significantly (P ≤ 0.05) depending on the AMF inoculant (Figure 2). The greatest spore production was recorded in the EL consortium, producing more than 40 spores/g of dry substrate, which was statistically different to the rest of the inoculants. In average the production in this consortium was five times more than that produced by the rest of the inoculants, followed by LC and CR inoculants where they produced 15 spores/g in average; the least spore production was recorded for the INIFAP, PA, and CM inoculants where they produced 3 spores/g of substrate in average. On the other hand, Panneerselvam et al. (2012) reported a production of 14.7 spores g-1 of dry substrate when the guava plants were inoculated only with Glomus mosseae. The spore production with the EL and CM consortia was related to the colonization percentage and plant growth, which indicated a certain selectivity by the species contained in the respective inoculants. In the case where a greater spore production was observed, it could have been due to a greater red mycelial production by these AMF that increased spore production (Azcón and Barea, 2015).

w/AMF = without arbuscular mycorrhizal fungus; PA = Paso Ancho; LC = Las Campesinas; CR = Carlos Rojas; EL = El Limón; CM = Cerro del Metate. Lines on bars indicate standard error. Different letters on bars indicate significant differences (P ≤ 0.05).
Figure 2: Spore production in the substrate of guava plants with different arbuscular mycorrhizal fungi (AMF) at 125 days of establishment.
Conclusions
Among the different consortia assessed, the one named El Limón (EL) promoted the best plant development and quality. The plants showed a height dependent on mycorrhization, which was likely colonized by more than one arbuscular mycorrhizal fungi species (AMF). The greatest mortality rate was recorded in plants w/AMF or with the INIFAP inoculant. The colonization percentages found were similar to those reported in other studies using monospecific inoculants or AMF mixtures. The use of AMF should be a practice for sustainable production of guava plants under greenhouse conditions.
Acknowldegments
This research was financed by the Fondo Mixto CONACYT-Gobierno del Estado de Michoacán under the project “Utilización de recursos microbianos para el control biológico de la pudrición del cogollo de agave tequilero en la DOT-Michoacán” (MICH‑2010‑03‑148208) and the 2014 research program of the Coordinación de la Investigación Científica at Universidad Michoacana de San Nicolás de Hidalgo. Likewise, the authors are grateful to Iván Eduardo Torres Valladares for his support in this research and Diana Fischer for editorial services in English.
REFERENCES
Azcón-Aguilar, C. and J. M. Barea. 2015. Nutrient cycling in the mycorrhizosphere. J. Soil Sci. Plant Nutr. 25: 372-396. doi: http://dx.doi.org/10.4067/S0718-95162015005000035. [ Links ]
Barea, J. M. and C. Azcón-Aguilar. 2013. Evolution, biology and ecological effects of arbuscular mycorrhiza. pp. 1-34. In: A. F. Camisao y C. C. Pedroso (eds.). Symbiosis: Evolution, biology and ecological effects. Nova Science. New York, NY, USA. ISBN: 978-1-62257-211-3. [ Links ]
Bashan, Y., E. A. Davis, A. Carrillo-Garcia, and R. G. Linderman. 2000. Assessment of VA mycorrhizal inoculum potential in relation to the establishment of cactus seedlings under mesquite nurse-trees in the Sonoran desert. Appl. Soil Ecol. 14: 165-176. doi: https://doi.org/10.1016/S0929-1393(00)00050-0. [ Links ]
Brundrett, M., N. Bougher, B. Dell, T. Grove, and N. Malajczuk. 1996. Working with mycorrhizas in forestry and agriculture. ACIAR Monograph 32. Canberra, Australia. ISBN: 186320 181 5. [ Links ]
Brussaard, L., P. C. de Ruiter, and G. G. Brown. 2007. Soil biodiversity for agricultural sustainability. Agric. Ecosyst. Environ. 121: 233-244. doi: https://doi.org/10.1016/j.agee.2006.12.013. [ Links ]
Carreón-Abud, Y., M. Vega-Fraga, and M. E. Gavito. 2015. Interaction of arbuscular mycorrhizal inoculants and chicken manure in avocado rootstock production. J. Soil Sci. Plant Nutr. 15: 867-881. doi: http://dx.doi.org/10.4067/S0718-95162015005000060. [ Links ]
Costa, C. M. C., L. C. Maia, U. M. T. Cavalcante, and R. J. M. C. Nogueira. 2001. Influência de fungos micorrízicos arbusculares sobre o crescimento de dois genótipos de aceroleira (Malpighia emarginata D. C.). Pesqui. Agropec. Bras. 36: 893-901. doi: https://doi.org/10.1590/S0100-204X2001000600007. [ Links ]
F. S da Silva-Campos, M. A., . Barbosa-da Silva, A. M. Yano-Melo, N. F. de Melo, E. M. R. Pedrosa, and L. Costa-Maia. 2013. Responses of guava plants to inoculation with arbuscular mycorrhizal fungi in soil infested with Meloidogyne enterolobii. Plant Pathol. J. 29: 242-248. doi: http://dx.doi.org/10.5423/PPJ.OA.10.2012.0156. [ Links ]
Das, K., S. Sau, P. Datta, and D. Sengupta. 2017. Influence of bio-fertilizer on guava (Psidium guajava L.) cultivation in gangetic alluvial plain of west bengal, India. J. Exp. Biol. Agric. Sci. 5: 476-482. doi: http://dx.doi.org/10.18006/2017.5(4).476.482. [ Links ]
Dickson, A., A. L. Leaf, and J. F. Hosnerm. 1960. Quality appraisal of white spruce and white pine seedling stock in nurseries. For. Chron. 36: 10-13. doi: https://doi.org/10.5558/tfc36010-1. [ Links ]
Estrada-Luna, A. A., F. T. Davies Jr., and J. N. Egilla. 2000. Mycorrhizal fungi enhancement of growth and gas exchange of micropropagated guava plantlets (Psidium guajava L.) during ex vitro acclimatization and plant establishment. Mycorrhiza 10: 1-8. doi: https://doi.org/10.1007/s005720050280. [ Links ]
Gavito, M. and L. Varela. 1995. Response of “criollo” maize to single and mixed species inocula of arbuscular mycorrhizal fungi. Plant Soil. 176: 101-105. doi: https://doi.org/10.1007/BF00017680. [ Links ]
Gerdemann, J. W. and T. H. Nicolson. 1963. Spores of mycorrhizal Endogone species extracted by wet sieving and decanting. Trans. Br. Mycol. Soc. 46: 235-244. doi: https://doi.org/10.1016/S0007-1536(63)80079-0. [ Links ]
Gomez, K. A. and A. A. Gomez. 1984. Statistical procedures for agricultural research. John Wiley and Sons. New York, NY, USA. [ Links ]
Granatstein, D., E. Kirby, H. Ostenson, and H. Willer. 2016. Global situation for organic tree fruits. Sci. Hortic. 208: 3-12. doi: https://doi.org/10.1016/j.scienta.2015.12.008. [ Links ]
Kumuran, S. and H. C. Azizah. 1995. Influence of biological soil conditioner on mycorrhizal versus non-mycorrhizal guava seedlings. Trop Agric. 72: 39-43. [ Links ]
Machineski, G. S., C. A. Gotardo-Victola, C. H. O. Machineski, M. F. Guimarães, and E. L. Balota. 2018. Effects of arbuscular mycorrhizal fungi on early development of persimmon seedlings. Folia Hortic. 30: 39-46. doi: https://doi.org/10.2478/fhort-2018-0004. [ Links ]
Mcgonigle, T., M. H. Miller, D. G. Evans, G. L. Fairchild, and J. A. Swan. 1990. A new method wich gives and objective measure of colonization of roots by vesicular-arbuscular mycorrhizal fungi. New Phytol. 115: 495-501. doi: https://doi.org/10.1111/j.1469-8137.1990.tb00476.x. [ Links ]
Mohandas, S., S. Poovarasana, P. Panneerselvam, B. Sarithaa, K. K. Upretia, R. Kamala, and T. Sita. 2013. Guava (Psidium guajava L.) rhizosphere Glomus mosseae spores harbor actinomycetes with growth promoting and antifungal attributes. Sci. Hortic. 150: 371-376. doi: https://doi.org/10.1016/j.scienta.2012.11.019. [ Links ]
Ortas, I. and O. Ustuner. 2014. The effects of single species, dual species and indigenous mycorrhiza inoculation on citrus growth and nutrient uptake. Eur. J. Soil Biol. 63: 64-69. doi: https://doi.org/10.1016/j.ejsobi.2014.05.007. [ Links ]
Panneerselvam, P., S. Mohandas, B. Saritha, K. K. Upreti, Poovarasan, A. Monnappa, and V. V. Sulladmath. 2012. Glomus mosseae associated bacteria and their influence on stimulation of mycorrhizal colonization, sporulation, and growth promotion in guava (Psidium guajava L.) seedlings. Biol. Agric. Hortic. 28: 267-279. doi: https://doi.org/10.1080/01448765.2012.741108. [ Links ]
Phillips, J. M. and D. S. Hayman. 1970. Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Trans. Br. Mycol. Soc. 55: 158-161. doi: https://doi.org/10.1016/S0007-1536(70)80110-3. [ Links ]
Prieto R. , J. Á., J. L. García, J. M. Mejía, S. Huchin y J. L. Aguilar. 2009. Producción de planta del género Pinus en vivero en clima templado frío. Campo Experimental Valle del Guadiana. Centro de Investigación Regional Norte Centro. INIFAP. Publicación Especial Núm. 28. Durango, Dgo. México. ISBN: 978-607-425-133-3. [ Links ]
Quiñones-Aguilar, E. E., A. C. Montoya-Martínez , G. Rincón-Enríquez, P. Lobit, and L. López-Pérez. 2016. Effectiveness of native arbuscular mycorrhizal consortia on the growth of Agave inaequidens. J. Soil Sci. Plant Nutr. 16: 1052-1064. doi: http://dx.doi.org/10.4067/S0718-95162016005000077. [ Links ]
Reyes-Tena, A., L. López-Pérez, E. E. Quiñones-Aguilar yG. Rincón-Enríquez . 2015. Evaluación de consorcios micorrízicos arbusculares en el crecimiento vegetal de plantas de maíz, chile y frijol. Biológicas 17: 35-42. [ Links ]
Reyes-Tena, A. , E. E. Quiñones-Aguilar , G. Rincón-Enríquez , and L. López-Pérez . 2016. Mycorrhizae in Capsicum annuum L. to promote growth and biosecurity against Phytophthora capsici L. Rev. Mex. Cienc. Agríc. 7: 857-870. [ Links ]
Rillig, M. C. and D. L. Mummey. 2006. Mycorrhizas and soil structure. New Phytol. 171: 41-53. doi: https://doi.org/10.1111/j.1469-8137.2006.01750.x. [ Links ]
Robles-Martínez, M. L., C. Robles, F. Rivera-Becerril, M. P. Ortega-Larrocea y L. Pliego-Marín. 2013. Inoculación con consorcios nativos de hongos de micorriza arbuscular en Agave angustifolia Haw. Rev. Mex. Cienc. Agríc. 4: 1231‑1240. [ Links ]
Rueda-Sánchez, A., J. D. Benavides-Solorio, J. T. Saenz-Reyez, H. J. Muñoz Flores, J. Á. Prieto-Ruiz y G. Orozco-Gutiérrez. 2013. Calidad de planta producida en los viveros forestales de Nayarit. Rev. Mex. Cienc. For. 5: 58-73. [ Links ]
Schiavo, J. A. and M. A. Martins. 2002. Produção de mudas de goiabeira (Psidium guajava L.) inoculadas com o fungo micorrízico arbuscular Glomus clarum, em substrato agroindustrial. Rev. Bras. Frutic. 24: 519-523. doi: https://doi.org/10.1590/S0100-29452002000200048. [ Links ]
SIAP (Servicio de Información Agroalimentaria y Pesquera). 2017. Guayaba, reina de la Vitamina C [online ]. Disponible en: <Disponible en: https://www.gob.mx/siap/articulos/guayaba-reina-de-la-vitamina-c?idiom=es > (Consulta: marzo 20, 2019). [ Links ]
Statgraphics. 2005. StatGraphics centurion: ver. XV (User manual). StatPoint Technologies Inc. Warrenton, VA, USA. [ Links ]
Trejo, D., D. Ferrera-Cerrato, R. García, L. Varela, L. Lara, and A. Alarcón. 2011. Effectiveness of native arbuscular mycorrhizal fungi consortia on coffee plants under greenhouse and field conditions. Rev. Chil. Hist. Nat. 84: 23-31. doi: http://dx.doi.org/10.4067/S0716-078X2011000100002. [ Links ]
Trinidad-Cruz, J. R., E. E. Quiñones-Aguilar , L. V. Hernández-Cuevas, L. López-Pérez , and G. Rincón-Enríquez. 2017a. Arbuscular mycorrhizal fungi associated in the rhizosphere of Agave cupreata in mezcal regions from Michoacán, México. Sci. Fungorum. 45: 13-25. [ Links ]
Trinidad-Cruz, J. R. , E. E. Quiñones-Aguilar , G. Rincón-Enríquez , L. López-Pérez yL. V. Hernández-Cuevas . 2017b. Mycorrhization of Agave cupreata: Biocontrol of Fusarium oxysporum and plant growth promotion. Rev. Mex. Fitopatol. 35: 151-169. doi: http://dx.doi.org/10.18781/r.mex.fit.1607-5. [ Links ]
Turrini, A., L. Avio, M. Giovannetti, and M. Agnolucci. 2018. Functional complementarity of arbuscular mycorrhizal fungi and associated microbiota: The challenge of translational research. Front. Plant Sci. 9: 1407. doi: https://doi.org/10.3389/fpls.2018.01407. [ Links ]
Received: October 05, 2019; Accepted: December 12, 2019











 texto em
texto em 



