Introduction
Mediterranean region conditions are known for their low pluvial precipitation and high salts content in their water resources for irrigation (Azeñas et al., 2019). Furthermore, as the competition for high quality water increases, the use of saline waters has become an option for irrigating ornamental plants in urban gardening (Rodríguez et al., 2005; Acosta-Motos et al., 2014; Acosta-Motos et al., 2016). Many species of plants in this zone intended for ornamental purposes present morphologic and physiologic characteristics that allow some tolerance to these adverse environmental conditions, and to be used in gardening and reforestation programs (Álvarez et al., 2012). In this context, there is a great variety of native plants that have been studied to verify their potential for ornamental use, because they respond well to Mediterranean conditions, as is the case of some species such as Asteriscus maritimus, Myrthus communis, Phillyrea angustifolia, Pistacia lentiscus, among others (Martinez-Sánchez et al., 2008). According to Armas et al. (2010), salinity treatments applied to lentisco do not seem to have constrained its performance, since it is known to be a relatively salt-tolerant species: However, its adaptation strategies to high salinity levels in a reduced volume of substrate have not been studied enough.
Exposure to salt may affect plant metabolism through an osmotic effect, causing water deficit, or through a specific ion effect, causing excessive ion accumulations (Mazher et al., 2007). Some of these effects are attributed to changes in water relations. It is easily appreciable in plants by a reduction of the leaf transpiration surface, increase in depth of roots and increase of stomata number (Munns and Tester, 2008). Another effect is the daily stem fluctuations, which informs about the growth and the water status of the plants (Miralles et al., 2010). Also, many studies in plants in water-limited ecosystems have regarded the role of plant hydraulic conductivity and their relationship with other traits as key adaptations for plant growth and survival (Vilagrosa et al., 2010; Lens et al., 2013). Therefore, the main objective of this work was to study the physiological and hydric adaptability of lentisco to saline irrigation conditions to identify possible tolerance mechanisms to deal with saline stress related to plant-available water.
Materials and methods
Lentisco plants with five months of age were acquired in a commercial nursery and transferred to pots of 3.5 L, in a mixed substrate (1:1) of peat moss and coconut fiber, to which 3 g L-1 of slow release fertilizer were added only one time at the lentisco planting (Osmocote, The Scotts Co., Marysville, OH, USA). This experiment was conducted in a growth chamber in the Centro de Edafología y Biología Aplicada del Segura (CEBAS) in Murcia, Spain. The environmental conditions were scheduled to simulate changes in temperature and light [18 ºC with 9 h of darkness and 28 ºC with 15 h day (with lamps)], the relative humidity was 70%. During 90 days three saline treatments were applied (50, 100 y 150 mM of NaCl) and the control 0 mM, in the irrigation water, using 25 plants by treatment. The control was irrigated with distilled water. The irrigation time, was made by weighting the pots, when they reached 95% of its initial weight, (pot, substrate and water on the substrate, until it started to drip) taking as a reference this initial weight, representing this, the maximum retention capacity of water in plants.
The water content in the substratum (in percentage, %) was recorded by humidity sensors coupled to a data logger (ECH2O EC-5, Decagon Devices, Pullman, WA, USA) installed in one pot of each treatment. The percentage values of water in the substrate were calculated according to the transformation of the transmittance data from the linear equation recommended by Decagon Devices, specific for potting tests (θ = 1.04 * 10-3 * Raw - 0.50).
The following growth parameters were analyzed at the end of the experiment; weight, dry matter (Draying stove, TR 240 Naberdhrem, Germany), plant height, number of leaves, and total leaf area (portable scanner, ADC BioScientific Ttd, Herts, England).
Chlorophyll content was determined with a portable device (Chlorophyll meter SPAD-502, Konica Minolta) and the fluorescence of PSII by fluorometry with a portable equipment (Fluorimeter mod. OS-30, OptiScience, Hudson, NH, USA). The stomatal conductance of the leaves was evaluated by using a steady state porter, LI-1600, LI-COR Inc., Lincoln, NE, USA) at maximum illumination time (Sanchez-Blanco et al., 2002). The leaf water potentials were performed by simulating the predawn (before lighting the lamps) and the midday (performed 7 h after lighting the lamps inside the growth chamber), this variable was measured with a pressure chamber (Soil Moisture Equipment Co., Santa Barbara, CA, USA), according to Scholander et al. (1965). The roots hydraulic conductivity was measured with a high-pressure flow meter (HPFM) (Dynamax, Houston, TX, USA) according to Tyree et al. (1995). The radial stems growth movement was monitored by Ecomatik DD dendrometers (Munich, Germany), installed four cm above from the substrate level on the main stem of each plant. The measurements were recorded every 30 min by a data logger (Ulogger Ecomatik, Munich, Germany) according to Miralles et al. (2010). The concentration of Cl− was analyzed by chloride analyzer (Chloride Analyser Model 926, Sherwood Scientific Ltd.) in the aqueous extracts obtained when mixing 100 mg of dry vegetable powder with 40 mL of water and shaking for 30 min before filtering. The concentrations of Na+ were determined in a digestion extract with HNO3:HClO4 (2:1, v/v) by Inductively Coupled Plasma optical emission spectrometer (ICP-OES IRIS INTREPID II XDL Thermo, England).
The experimental design consisted in a completely randomized design with five replicates (pots) per treatment. The effects of salinity and measures of the parameters were subjected to the comparison of means using Tukey's test, calculating it with significance P < 0.05. Statistical procedures were performed using the SPSS statistical package (IBM Company, Madrid, Spain).
Results and discussion
The substrate moisture data recorded (Figure 1) indicated that the salinity treatments retain higher water content in the substrate and this effect was directly proportional to the saline concentration, resulting in a smaller amount of water used for irrigation in saline treatments. Conversely, control plants had to be watered throughout the essay more constantly (Figure 1). This response shows the difficulty of plants in absorbing water by the effect of salts in the irrigation water, due to the alteration of the ionic balance in the solution (Yamaguchi and Blumwald, 2005). In response to this, the plants developed salt exclusion mechanisms to regulate the salt accumulation, favoring the absorption of other ions in lower concentration that are in the lentisco plants, such as K+ and NO3 - (Jha et al., 2010; Munns and Tester, 2008). Potassium also is crucial for crop growth and is strongly related to stress tolerance and water-use efficiency (WUE). A major physiological effect of K deficiency is the inhibition of net CO2 assimilation during photosynthesis (Du et al., 2019). Whether this reduction originates from limitations either to photochemical energy conversion or biochemical CO2 fixation or from a limitation of CO2 diffusion through stomata and the leaf mesophyll is discussed (Jákli et al., 2017).
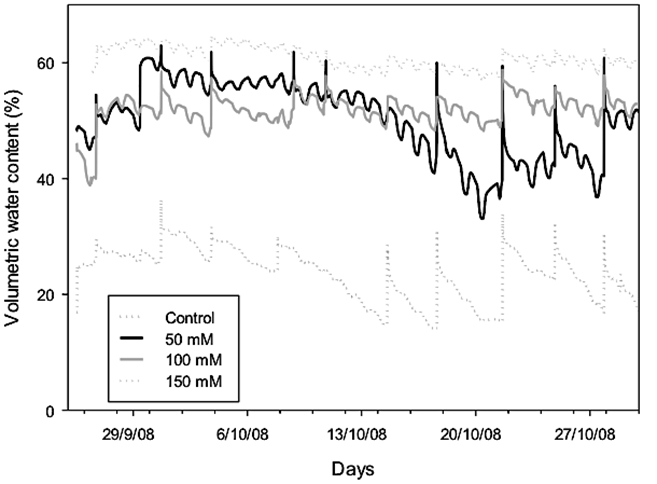
Figure 1 Volumetric water content, determined by transmittance sensors in the substrate, irrigated with different concentrations of NaCl. Measurements obtained every 10 min and stored in a data logger.
On the other hand, Cassaniti et al. (2009) mentioned that exclusion mechanisms are more effective at low concentrations of NaCl, suggesting that under conditions of high salinity, inhibition of important metabolic processes may occur, leading to a sharp decline in growth rates. Plant height was reduced in all saline treatments compared to control plants, and this effect was more evident in the treatment of 150 mM NaCl (Figure 2B). Bañon et al. (2006) demonstrated similar effects in Cistus albidus and Nerium oleander plants under salt or water deficit conditions.
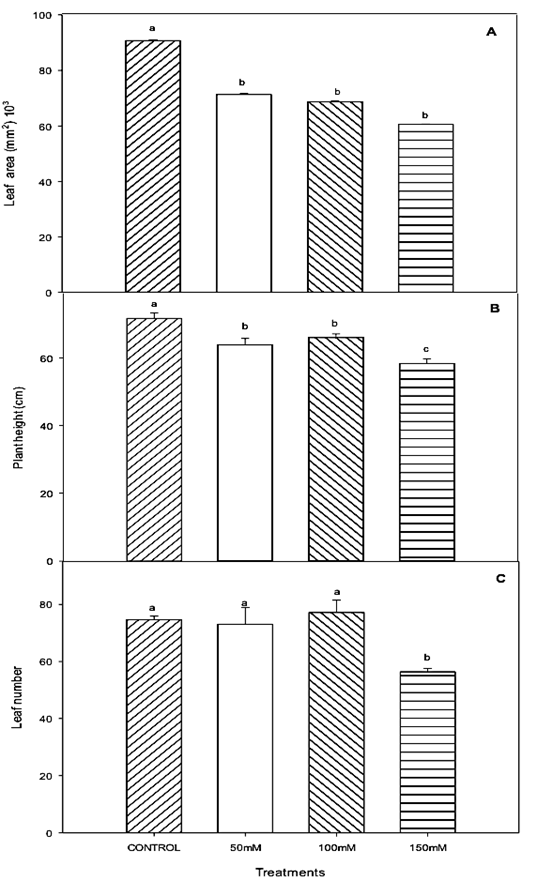
Figure 2 Growth of lentisco: (A) total leaf area (mm2), (B) plant height (cm), and (C) leaves number. Treatments with the same letter above the bars are equal at the end of the experiment to each other according to the Tukey test (P < 0.05) for each level of salt.
It is also important to mention that glycophytes plants with low tolerance to salinity generally present reduced growth rates as a strategy to redirect metabolic energy to withstand the effects of stress. These strategies are often related to the reduction of photosynthetic rates, stomatal closure mechanisms (Munns and Tester, 2008), membrane permeability, water channel activity and ionic balance (Cabañero et al., 2004), which are all limiting growth factors. The leaf area was reduced compared to the control in all the plants that received saline water, independently of the NaCl concentration in treatments (Figure 2A). Saline stress, as an initial symptom in plants seems to induce a restriction in leaf expansion and the reduction of leaf area. These responses are related to prevention mechanisms, which can minimize water losses, mainly due to stomata closure (Ruiz-Sánchez et al., 2000). In contrast, the number of plant leaves was affected only for the most severe salt treatment; the other treatments did not differ from the control (Figure 2C). This indicates a strategy adopted by plants that seek to increase the efficiency of water use. Since the results of this experiment, a smaller leaf area added to a lower leaves number in plants under salt stress contributed to the adaptation process of the plants to the lower availability of water, caused by the osmotic effect of the salt on the substrate, agreeing with the results of (Sheldon, et al., 2017). These strategies are apparently aimed to reduce water losses via leaf transpiration and contributing to improving WUE.
The hydraulic conductivity (Lp) measured in roots decreased in the most severe salt treatments, especially at 150 mM of NaCl (Figure 3). As we have mentioned, plants irrigated with salt had difficulty for uptaking water from the substrate, this results are in agreement with Acosta-Motos et al. (2017). A decrease in the hydraulic conductivity was probably an attempt by plants to adapt to the ionic alterations produced by the salt. Poscher (2005)1 mentioned that drought and salinity stresses might decrease hydraulic conductivity for water transport within the plants. Hydraulic conductivity can be influenced by external and internal factors such as water availability, salinity, soil conditions, embolisms in the xylem, and root traits. In this way, the efficiency of overcoming impaired hydraulic conductance and its related root characteristics differ considerably among species (Jákli et al., 2017).
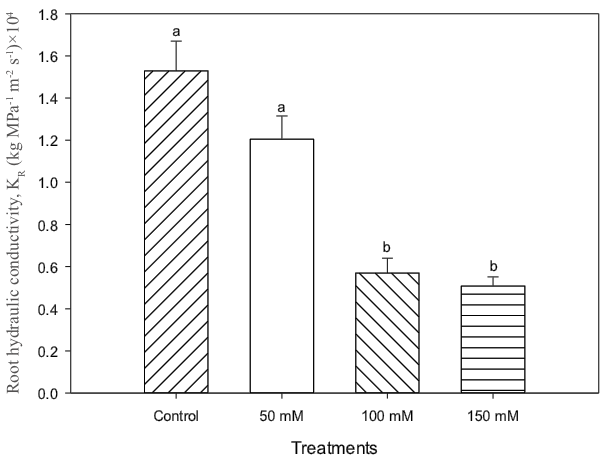
Figure 3 Hydraulic conductivity of lentisco. Treatments with the same letter above the bars are equal at the end of the experiment to each other according to Tukey’s test (P < 0.05) for each level of salt.
Leaf water potential values at predawn and at midday indicated variations from -0.8 to -1.0 MPa and -2.1 to -2.5 MPa, respectively (Figure 4A and 4B). These results seem to express the effects produced by the application of different saline concentrations, which caused strong stomatal reduction. The saline irrigation level of 150 mM NaCl caused a decrease of approximately 40% in the stomatal conductance of the plants compared to the control (Figure 5), the other treatments did not show any differences.
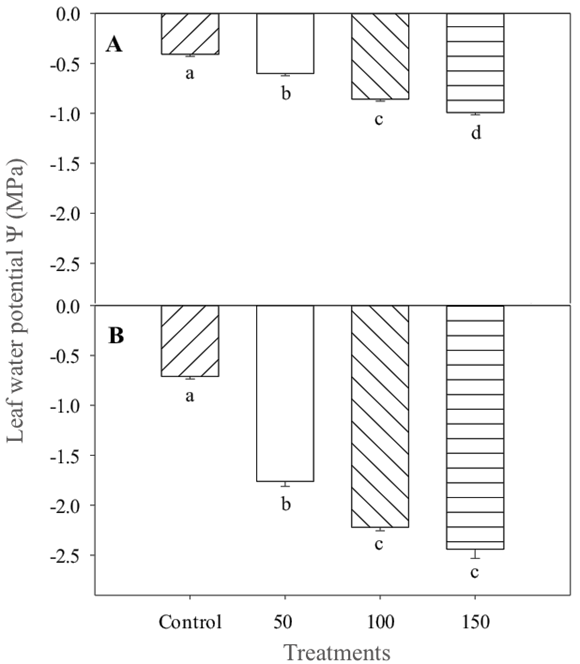
Figure 4 Water potential (ψ, MPa) at predawn (A) and at midday (B) of lentisco. Treatments with the same letter above the bars are equal at the end of the experiment to each other according to the Tukey test (P < 0.05) for each level of salt.
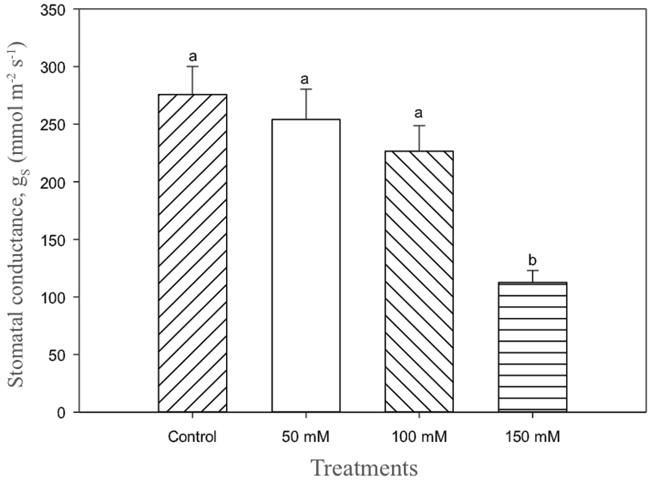
Figure 5 Stomatal conductance at midday (gs) of lentisco. Treatments with same letter above the bars are equal at the end of the experiment to each other according to the Tukey test (P < 0.05).
The plants showed a tendency to reduce stomatal opening in response to treatments under high NaCl levels. According to James et al. (2002) the physiological effects in plants induced by salt in the water can initially have correlation with the ionic effect. However, the stomatal changes observed by the lentisco plants seem to indicate that the more severe treatments and their osmotic effects are a direct cause of the root hydraulic conductivity reduction. Evaluations of chlorophyll content (Figure 6) and fluorescence (Figure 7) showed no effect on the application of salt to irrigation water for plants. According to Broetto et al. (2008), plants can produce chlorophyll fluorescence at PSII level in response to different stress factors, including saline, light intensity, osmotic, among others. In this particular case, and based on data obtained of the chlorophyll fluorescence, it is possible to affirm that there are no important correlations between the salt stress and the photochemistry of PSII, this is in agreement with the results obtained by Zhao et al. (2007), indicating slight variations of Fv/Fm due to PSII resistance to salt. According to Munné-Bosch et al. (2009), lentisco like other Mediterranean species, appear to be endowed with mechanisms of tolerance to adverse environments, independent of photosynthetic activity.
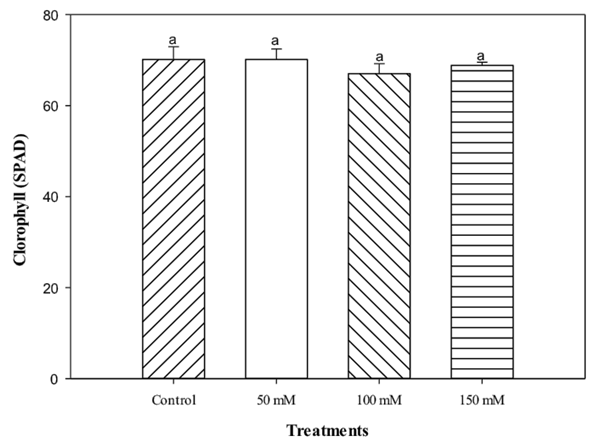
Figure 6 Chlorophyll concentration (SPAD) of lentisco. Treatments with the same letter above the bars are equal at the end of the experiment to each other according to the Tukey test (P < 0.05) for each level of salt.
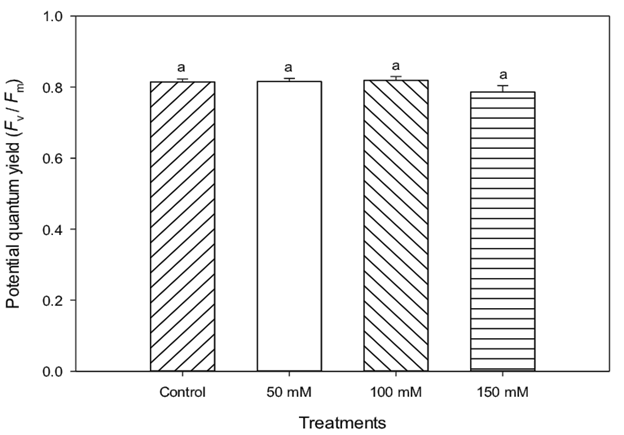
Figure 7 Fluorescence of chlorophyll of PS II (Fv/Fm) of lentisco. Treatments with the same letter above the bars are equal at the end of the experiment to each other according to the Tukey test (P < 0.05) for each level of salt.
Analysis of the mineral elements in roots and leaves showed a pattern of increase in Cl- and Na+ concentration proportional to increases in NaCl in irrigation water. According to Jha et al. (2010), Cassaniti et al. (2009), and Sheldon et al. (2017), it is possible that the increase of Na+ and Cl- concentration in leaves and roots will have a significant ionic effect, in addition to the initial osmotic effect. On the other hand, the ions Ca+, Fe+, Mg+ and P+ did not present any alterations in their compositions due to the increase in salt concentration in the two types of analyzed tissues (aerial part and roots) (Figure 8). The K+ concentration decreased in response to increased salinity in irrigation water.
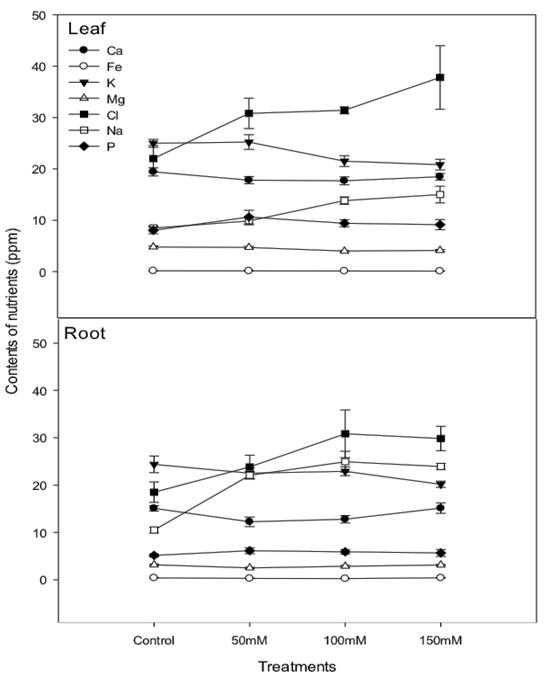
Figure 8 Macro and microelements of lentisco grown under different concentrations of salt into the substrate (NaCl).
The K+: Na- and K+: Cl- ratio in leaves and roots of lentisco presented values predominantly favorable to the concentration of Na+ where the ionic effects of NaCl was concluded. Other nutrients such as P, Mg, Fe, and Ca did not suffer interference in their absorption due to salt. This result was surprising, considering that many cations are antagonistic to Na+ and Cl- (Francois et al., 1991). Other ions like P, Mg, Fe, and Ca apparently did not suffer interference in their absorption due to the salt. This surprisingly occurred, considering that many cations are antagonistic to Na+ and Cl- (Francois et al., 1991).
Finally, a trend was observed for the inhibition of the radial stem growth compared to the control plants, induced by the accumulated salts in the substrate (Figure 9). It should be mentioned that this effect was more evident after 30 days of the application of the treatments. It was also observed that the plants under salt treatments recorded high daily stem growth fluctuation, mainly in the concentration of 150 mM of NaCl. This measurement technique has been discussed for evaluations of plants subject to water deficiency (Jones, 2007), also as a tool for studies on irrigation programming (Fernández and Cuevas, 2010). Many authors have mentioned possible causes of daily variations in stem diameter, including changes in nocturnal temperature, daily water balance and water deficit (Drew et al., 2008). Particularly, in the present trial, where environmental conditions were controlled, daily variations in stem diameters could be directly related to the increase in salt concentration in the substrate. Wimmer et al. (2002) argue that daily increments in stem diameter are related to cell expansion rates, which should be maximal depending on the availability of water for tissues in the vascular cambium region. In this sense, it seems clear that the osmotic effect of the salt interfered negatively for the radial growth parameters.
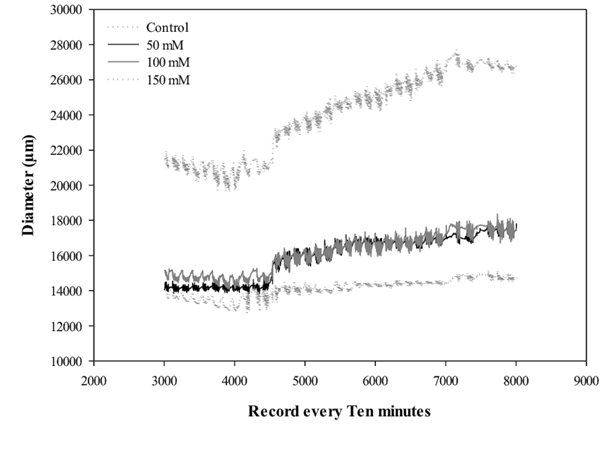
Figure 9 Daily variations of stem diameter of lentisco cultured under different concentrations of salt (NaCl) in the substrate.
The lentisco plants were subjected to an osmotic stress in the substrate, which caused low absorption of water by the plants. This effect led the plants to set adaptation strategies as variations in growth rates, height, leaf area and radial growth of the stem and in water relations, mainly. On the other hand, saline treatments accumulated Na+ and Cl- ions in the root and leaf tissues, which caused ionic effect in the cells. This effect may be related to the opening and closing of stomata, but apparently not related to PSII fluorescence and chlorophyll content. As a general conclusion, it is suggested that lentisco plants adopt important adaptive mechanisms in the face of salt stress, and may be recommended in revegetation or gardening programs in degraded areas, where the condition of the irrigation water is saline.
Conclusions
Pistacia lentiscus plants were subjected to osmotic stress resulting from the concentration of salt in the substrate, which reduced the absorption of water by the plants. This effect caused the plants to initiate adaptation strategies such as variations in growth rates, particularly in height, leaf area and radial growth of the stem, as well as changes in water relations. The salt treatments caused an accumulation of Na+ and Cl- ions in the root and leaf tissues, subjecting the cells to ionic stress. This stress may be related to the opening and closing of stomata, but is apparently not related to PSII fluorescence and chlorophyll content. As a general conclusion, P. lentiscus plants seem to have important adaptive characteristics that allow them to deal with salt stress, and may be used in revegetation or gardening programs in degraded areas where irrigation water contains salt.











 nova página do texto(beta)
nova página do texto(beta)



