INTRODUCTION
In tropical forest, light is a limiting factor for plantlets survival, growth and establishment (Montgomery, 2004). Light conditions under which plants develop in the forest understory can change rapidly, and the adaptation presented by these plants, in response to selection pressure to use the light efficiently is what allows them to exist in such variable environmental conditions (Walters and Reich, 1999).
In tropical forests, early or pioneer successional species develop efficiently in a wide variety of environments. As these species grow in an unpredictable and variable habitat, they have greater plasticity than those that develop in a more homogenous habitat (late successional species).
In contrast with the reports published on species growing in temperate zones, little is known about the photosynthetic response of tropical species to different light conditions, (Koch et al., 1994). Plants growing in the understory probably experience the most variable light conditions and, as they are under selective pressure to use the light efficiently, a rapid response is required in the activation of photosynthesis and stomatal aperture (Ögren and Sundin, 1996).
Brosimum alicastrum, is a perennial evergreen tree of the tropical forest that has been classified as a late successional species (Oberbauer, 1985; Strauss-Debenedetti, 19891), tolerant to shade (Montgomery and Chazdon, 2002; Peters, 19892; Walters and Reich, 1999), and widely distributed in the tropical regions of Mesoamerica and South America. In north part of the Yucatan state this tree is commonly planted in streets and backyards of homes where despite of almost no soil present, they do develop as a very prominent evergreen tree. Under this soil conditions the early growth pattern of this tree is about 0.65 cm per year and star to flower at about seven years after plantation (Hernández et al., 2014).
Reports of these trees of the Veracruz area have estimated a photosynthetic rate of 5 µmol m-2 s-1 in plantlets developed under greenhouse conditions (Peters, 19892). Strauss-Debenedetti, (19891) and Montgomery (2004) reported it to be a species with low photosynthetic plasticity in a light gradient of 0 to 70%, with its highest photosynthetic value at 6 µmol m-2 s-1. Ramos and Grace (1990) found that plantlets developed in growth chambers with light (800 µmol m-2 s-1) and shade (80 µmol m-2 s-1) present differences in their growth rate without any effect on their photosynthetic rate.
The present study was proposed because no reports were found in the literature on photosynthesis, transpiration, stomatal conductance, chlorophyll fluorescence or chlorophyll content, of adult Brosimum alicastrum trees grown on these poor soils with a top limestone that are not irrigated during the dry season of northern Yucatan. Neither comparative studies of this tree from two distant localities of the country one collected in Mazatlan near the pacific coast and other from the northern part of the Yucatan peninsula.
MATERIALS AND METHODS
Plant Material and Growth Conditions
Three 3-year-old Brosimum alicastrum trees with a height of 3 m grown under the same field conditions with sun exposure at an average temperature of 28 °C were studied. The trees selected for the present study were grown in the botanical garden of the Center for Scientif ic Investigation of Yucatan, Merida, Yucatan, Mexico (20º 59’ N; 89º 38’ W). Soil characteristic have been defined as extremely rocky with hard limestone layer (‘‘laja’’) in the 25 cm near the surface. The soil was classified as Lithic Leptosol according to the FAO system (Querejeta et al., 2006) and define as Ek lu´um according with the maya classification. It is has a warm humid climate (Aw0) with summer rains; annual average temperature of 25 °C, maximum temperature in May (42 °C) and minimum temperature in December (15 °C); annual average rainfall of 946 mm and prevailing winds from the southeast and northwest (Ayala et al., 2008).
A parallel study carried out in a nursery of the same scientific center in 2-year-old B. alicastrum plantlets growth from seeds collected in two Mexican locations Sinaloa and Yucatan. Seeds were planted in polyethylene bags filled with a mixture of soil (leptosol) and sand (3:1, v:v) and watered twice a week. Plantlets from Sinaloa had an average of 109.5 cm in height and 1.1 cm of stem diameter, while the Yucatan plantlets had 98.64 cm and 0.92 stem diameter. These studies were conducted at the end of the dry season.
Gas Exchange: CO2 Fixation and Transpiration Rate
Mature, fully exposed leaves were selected from any of the studies to carry out the measurements of gas exchange, transpiration, stomatal conductance and intercellular CO2 concentration. A portable photosynthesis analyzer (IRGA, LI-COR 6400, Nebraska, USA) was used. The CO2 concentration was maintained at 380 ppm throughout all the measurements.
Incident light, air temperature, leaf temperature and vapor pressure deficit (VPD) were measured during the determination of gas exchange, using the sensors of photon flux density (PFD) and temperature integrated in the leaf chamber of the IRGA.
Chlorophyll Fluorescence
In any of the experiments, chlorophyll fluorescence was measured in mature leaves exposed to full sunlight and in the shade. Quantum efficiency of the photosystem II (PSII) and the electron transport rate (ETR) were determined using a pulse-amplitude-modulated fluorimeter (Mini-PAM, Walz, Effeltrich, Germany). Temperature and photon flux density were measured with a thermocouple and a micro quantum sensor, respectively, integrated in the Mini-PAM leaf clip.
Light Response Curves
Light response curves were carried out on mature leaves trees exposed to sunlight at 15 h (when the maximum quantity of light, 2500 µmol m-2 s-1, was obtained). Prevailing average environmental conditions were 22 °C and 56% relative humidity. Photosynthesis curves were carried out as is commonly done starting at the lowest value of PFD up to 100% light. The readings were taken independently on four leaves per tree.
In addition, light curves were run using the Mini-PAM, in leaves selected under similar conditions to those described above. The Mini-PAM program used applies 8 pulses of actinic light every 2 s, increasing light intensity in each pulse until 200 µmol m-2 s-1 is reached. Five replicate samples were measured.
Water Use Efficiency
Water use efficiency (WUE) was calculated as CO2 absorption/transpiration rate.
Chlorophyll Content
During experiments, chlorophyll content was determined in the same leaves selected for the estimation of gas exchange. Chlorophyll determination was carried out with the Chlorophyll Meter SPAD-502 (Minolta, Co., Spectrum Technologies, Inc., Japan).
Statistical Analyses
In order to determine the differences between means of the variables analyzed at different times of the day, a repeated measures analysis of variance and Tukey tests were carried out; significance was determined when P < 0.01. The light curves were adjusted to a logarithmic model. All statistical analyses were performed with the statistical package, Statistical 7.0 (Statsoft Inc., 2004). To determine differences between means of variables from plants of Sinaloa and Yucatan, analysis of variance and post hoc Tukey test (P < 0.05) was carried out in SPSS 23 (IBM corp. NY, USA).
RESULTS AND DISCUSSION
Gas Exchange CO2 Fixation, Transpiration and Stomatal Conductance
From the results recorded in the present study, it is possible to establish the characteristics of the CO2 fixation pattern of B. alicastrum adult trees in their natural habitat, the tropical dry forest. It has been reported that B. alicastrum plantlets present a limited photosynthetic plasticity as well as a low acclimation potential to different light levels (Peters, 19892; Strauss-Debenedetti, 19891; Ramos and Grace, 1990; Strauss-Debenedetti and Bazzaz, 1991), however, these evaluations were performed under greenhouse conditions and in growth chambers, with environmental conditions, which were quite different from their natural habitat. From the results recorded in the present study, it is possible to establish the characteristics of the CO2 fixation pattern of B. alicastrum adult trees in their natural habitat of the northern part of Yucatan a tropical dry forest. Moreover, to compare them with data obtained from plantlets grown on pots of two populations already mentioned (Sinaloa and Yucatan).
In field, the ambient temperature presented a slight variation throughout the day, with an average of 28 °C and a maximum value of 30 °C. The CO2 fixation pattern of B. alicastrum is shown in Figure 1a where it can observe a very low photosynthetic rate during the early hours and an average maximum CO2 absorption peak of 4.2 µmol m-2 s-1 at 12 h.

Figure 1: Patterns of gas exchange from leaves of ramon grown in field conditions: (a) photosynthesis, (b) transpiration and (c) stomatal conductance. Each point is the mean of 30 measurements ± standard error. Values with the same letter do not differ significantly (Tukey; P < 0.001).
In potted trees experiment, ambient temperature average was 23.2 °C with a maximum of 30.3 °C. The CO2 absorption pattern was similar between plantlets of both localities. As in field trees, measurements, highest values of CO2 absorption were detected at 12 h, with values of 6.87 µmol m-2 s-1 for Sinaloa plantlets and 6.10 µmol m-2 s-1 for Yucatan plantlets (Figure 2a)

Figure 2: Patterns of gas exchange from leaves of ramon grown in pots in the open conditions: (a) photosynthesis, (b) transpiration and (c) stomatal conductance. Each point is the mean of 30 measurements ± standard error. Values with the same letter do not differ significantly (Tukey; P < 0.001). Each point is the mean of 15 measurements ± standard error. Values with the same letter do not differ significantly (ANOVA; P < 0.001).
Data recorded in the leaves of trees grown in field are lower than those reported in the study carried out on plantlets of the same species by other authors (Montgomery, 2004; Ramos and Grace, 1990). Nonetheless, the pattern data are like those recorded in the potted trees experiment.
The pattern determined in the present study shows that CO2 fixation at 8 h and 17 h in the leaves of trees grown in the field are at its lowest value. This pattern is different to those reported in other tree species such as Picea mariana (black spruce) and Larix laricina (tamarack larch) where maximum CO2 fixation occurred at 8 h in the morning and diminished around midday (Dang et al., 1991).
The highest CO2 fixation at midday is an indication that photoinhibition is not present in this plant species, as was mentioned by Thiele et al. (1998) who reported the value of 0.56 in potential photosynthetic efficiency (F v /F m ) at midday. In the present study, the pre-dawn Fv/F m , a value was of 0.71, which is similar to the values reported for Pinus halapensis and in a number of tree species growing in the Mediterranean regions (Navarro et al., 2004) with values below 0.83, which is considered optimal for many species (Björkman and Demming, 1987).
The flow of water through the plant is necessary in order to transport nutrients and carry out CO2 fixation. The transpiration rate (Figure 1b) and CO2 fixation in B. alicastrum in field conditions have a similar diurnal pattern; they lose the largest amount of water through transpiration (2.6 mmol H2O m-2 s-1) during the interval from 12 to 15 h when highest temperature and greatest incident light are present. In the nursery experiment, maximum values of transpiration were detected between 9 and 12 h, with values of 1.56 to 2.6 mmol H2O m-2 s-1 for plantlets from Sinaloa and 1.35 mmol H2O m-2 s-1 for plantlets from Yucatan (Figure 2b).
Oberbauer (1985) has reported that perennial evergreen species close stomata when conditions are most stressful; however, the diurnal courses of stomatal conductance in B. alicastrum in its natural habitat indicate that stomatal aperture was greatest at midday. It is important to note that stomatal conductance (or stomatal aperture) has a pattern similar to that of the photosynthetic rate and transpiration rate in the early hours of the day, with the highest value (132 mmol m‑2 s‑1) observed at midday; however, this diminishes by 20% at 15 h (Figure 1c). Stomatal conductance in plantlets of both localities in nursery condition had similar patterns. Highest values for plantlets of Sinaloa (0.088 mmol H2O m-2 s-1) and Yucatan (0.082 mmol H2O m-2 s-1) were measured at 9 h. These values decreased in hours with higher light incidence (Figure 2c).
The results indicate a gradual stomatal closure as the vapor pressure deficit (VPD) increases in natural (Table 1) and nursery (Table 2) conditions; nevertheless, although the stomata partially close, CO2 fixation remains high up to 15 h. Therefore, this could be a response indicating that B. alicastrum tolerates environmental water stress without its photosynthesis being affected. This may be due to its efficiency in extracting water from bedrock strata, as it is known to have deep roots (Querejeta et al., 2006). The internal CO2 concentration of the mesophyll remains high at 15 h, allowing the plant to continue carrying out photosynthesis, as has been reported for other perennial species (Niinemets et al., 2005).
Table 1: Water use efficiency (WUE), vapor pressure deficit (VPD) and chlorophyll content at different times of the day in Brosimum alicastrum plants grown in their natural habitat.
| Time | WUE† | VPD† | Chlorophyll |
|---|---|---|---|
| h | µmol mmol-1 | kPa | SPAD unit |
| 8 | 0.68 ± 0.026 a | 2.036 ± 0.007 a | 51.6 |
| 12 | 1.49 ± 0.037 bc | 2.160 ± 0.025 b | 50.8 |
| 13 | 1.40 ± 0.078 b | 2.151 ± 0.023 b | 52 |
| 15 | 1.57 ± 0.076 c | 2.419 ± 0.040 c | 52.8 |
| 17 | 0.55 ± 0.031 d | 2.310 ± 0.008 d | 52.8 |
† Each value is the mean of 30 replicates ± standard error. Values with the same letter do not differ significantly (Tukey; P < 0.001).
From the results obtained in the present study, this species can be considered shade tolerant and capable of maintaining an efficient photosynthetic rate despite receiving a light intensity of at least 1200 µmol m-2 s-1. CO2 fixation and high electron transport within the leaf, even with partial stomata closure, results in high water use efficiency (WUE). The presence of abaxial papillae in B. alicastrum leaves has been reported, which could explain, to some extent, the values obtained in stomatal conductance, transpiration and CO2 fixation. Strauss-Debenedetti (19891) has reported that the presence of papillae be an evolutionary adaptation to the environmental conditions of light and limited water availability in which this species grows.
Table 2: Water use efficiency (WUE) and vapor pressure deficit (VPD) of the day in Brosimum alicastrum plants grown in nursery.
| Time | WUE† | VPD† | |||
|---|---|---|---|---|---|
| Sinaloa | Yucatan | Sinaloa | Yucatan | ||
| h | - - - - - µmol mmol-1 - - - - - | - - - - - - - kPa - - - - - - - | |||
| 9 | 4 ± 0.76 b | 3.18 ± 0.34 a | 2.03 ± 0.16 a | 2.16 ± 0.08 a | |
| 12 | 4.42 ± 0.21 a | 4.52 ± 0.35 a | 2.3 ± 0.1 a | 2.25 ± 0.18 a | |
| 15 | 5.64 ± 0.92 a | 5.4 ± 0.28 a | 2.30 ± 0.15 a | 2.21 ± 0.04 a | |
| 17 | 2.98 ± 0.4 b | 2.86 ± 0.22 a | 2 ± 0.02 a | 1.94 ± 0.09 a | |
† Each value is the mean of 15 replicates ± standard error. Values with the same letter do not differ significantly in each hour of measurement (ANOVA; P < 0.05).
Water Use Efficiency
Water use efficiency (WUE) of trees grown the field increases gradually throughout the day, with the highest value of 1.57 µmol mmol-1 registered at 15 h and the lowest value was found at 17 h (Table 1). WUE may be related to the capacity displayed by B. alicastrum to absorb water from bedrock strata, because this plant species has developed roots extending deep into the earth. (Calderón, 1975; Querejeta et al., 2006).
Trees in nursery showed the highest value of WUE of 5.63 μmol mmol-1 for plants from Sinaloa and 5.40 μmol mmol-1 for plants from Yucatan at 15 h (Table 2).
WUE indicates the water use strategy of a species in its different life stages or among species (Donovan and Ehleringer, 1991). In order to predict the changes associated with plant productivity, it is essential to understand how WUE changes with the climate. In B. alicastrum, a change is observed in its WUE when the environmental conditions are different. At a higher temperature (at 15 h), water use efficiency was greater (Table 1), in contrast with reports of other perennial evergreen species (Dang et al., 1991). WUE also reflects an exchange between water loss and carbon gain in the carbon assimilation process through photosynthesis. In the case of B. alicastrum, under more extreme conditions (higher temperature and greater light incidence), a higher carbon absorption with less water loss was observed, which may contribute to maintaining biomass increase even under conditions which could be stressful for other perennial evergreen species.
Chlorophyll content in the leaves of field plants was from 50.8 to 52.8 SPAD units (Table 1), while in nursery plants leaves from Sinaloa had 42.76 SPAD units and 44.6 in leaves plants from Yucatan. The chlorophyll values are known to facilitate a high CO2 fixation during the day. These values are like other of perennial evergreen species and remains constant throughout the day. Contrasting with other species where the chlorophyll value diminishes as the leaves receive more light (Hoel and Solhaug, 1998; Montgomery, 2004).
Photosynthetic Efficiency and Electron Transport Rate
In field conditions, photosynthetic efficiency (∆F/Fm’) in B. alicastrum at maximum light intensity was 0.2 on average and at 0.55 in shade plants (Figure 3a), indicating that the variation in photochemical efficiency of the PSII under illuminated conditions is very effective. The electron transport rate (ETR) in plants exposed to 100% light was 80 µmol m-2 s-1, while shade plants registered 15 µmol m-2 s-1 (Figure 3b).

Figure 3: (a) Photosynthetic efficiency pattern of Brosimum alicastrum under conditions of light and shade. (b) Electron transport rate (ETR) of Brosimum alicastrum leaves under conditions of light and shade. Each point is the mean of 10 measurements ± standard error.
In the nursery experiment, plantlets of both localities shown similar photosynthetic rate with highest values when light intensity was low (7 and 19 h), when maximum light intensity is present (13 h). Plantlets of Sinaloa registered values of 0.17 Fv/Fm while plantlets of Yucatan had 0.18 Fv/Fm (Figure 4a). Pattern of ETR also were similar with highest value reported at 13 h in both Sinaloa (21.04 mmol m-2 s-1) and Yucatan (20.89 mmol m-2 s-1) plantlets (Figure 4b).
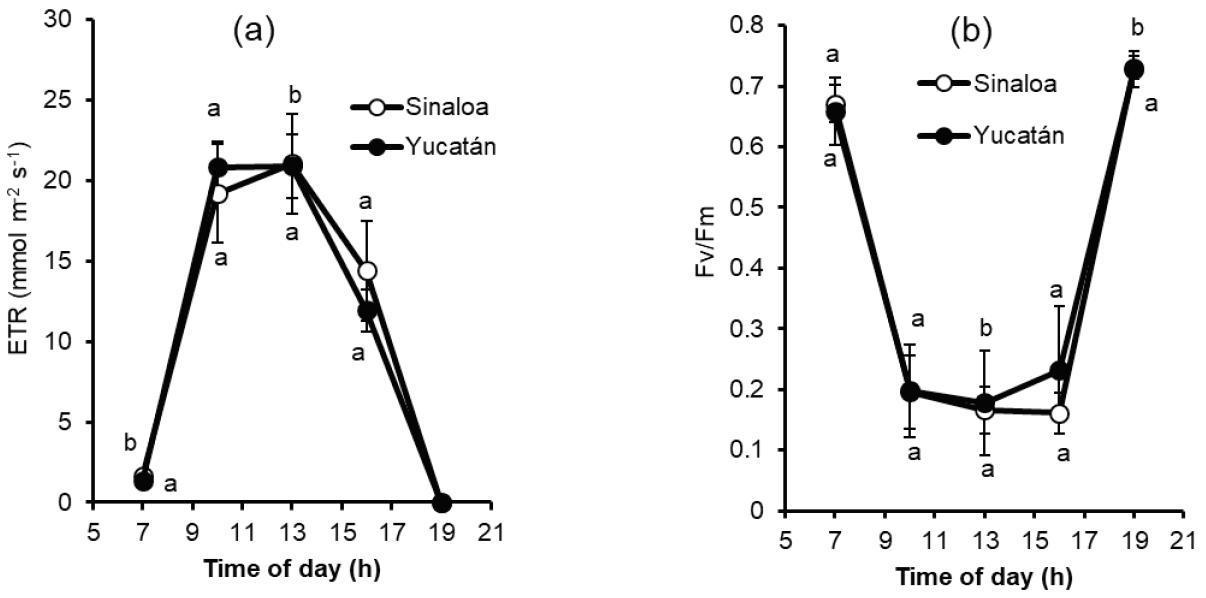
Figure 4: (a) Photosynthetic efficiency pattern of Brosimum alicastrum under nursery conditions. (b) Electron transport rate (ETR) of Brosimum alicastrum leaves under nursery conditions. Each point is the mean of 15 measurements ± standard error. Values with the same letter do not differ significantly in each hour of measurement (ANOVA; P < 0.05).
These results would suggest that electron transport in the PSII is very stable, even with high light intensity.
Light Response Curves
In the CO2 fixation curve of the leaves in response to different light intensities for B. alicastrum (Figure 4a), can be seen that it increases rapidly as there is an increment of incident light on the leaf. The light level required to saturate the photosystem II is approximately 500 µmol m-2 s-1 of photon flux with a maximum photosynthetic rate of 5 µmol m-2 s-1. The present results of CO2 assimilation rate with different amounts of light and its value of saturation to the light was similar to those reported for plantlets of this plant species by Peters (19892) and is similar to other perennial evergreen species growing in tropical forests (Langenheim et al., 1984), which, usually present low values in their photosynthetic rates in comparison with herbaceous plants and shrubs. Similarly, Mooney and Gulmon (1982) have reported that the low CO2 assimilation values of perennial evergreen species, such as B. alicastrum, may be related to their photosynthesis throughout the year and the longevity of their leaves.
B. alicastrum is very sensitive to incident light intensity such as 24 µmol m-2 s-1 (value corresponding to the light compensation point) that is enough for the metabolic system of this tree’s leaves to initiate CO2 fixation (Figure 4b). The light compensation point of this species was very low and is like those reported for other late successional species (Bazzaz and Carlson, 1982), thus allowing it to be included in this group of species. However, it is important to note that B. alicastrum has the capacity to maintain its photosynthetic rate of 5 µmol m-2 s-1, which is a value similar to that reported by Walters and Reich (1999). This value remains even though of light received is increased to 2500 µmol m-2 s-1 and no apparent photoinhibition is presented which is confirmed with the pre-dawn values of Fv/Fm and the values of ∆F/Fm’ recovery throughout the day. The CO2 fixation rate in adult plants of this tree is similar to those reported in plantlets during experiments carried out under greenhouse conditions (Peters, 19892; Strauss-Debenedetti, 19891) and in experiments performed in growth chambers (Ramos and Grace, 1990); thus we can appreciate the extensive photosynthetic plasticity of this plant species since it presents the same response under such different environmental conditions. Our report confirmed its classification as a shade tolerant species (Peters, 19892; Walters and Reich, 1999; Montgomery and Chazdon, 2002) it is also capable of tolerating high variations of light without affecting its diurnal pattern of CO2 fixation, and can therefore be considered a climax plant species, as indicated by Ramos and Grace (1990).
In its natural environment growth conditions, ramon trees leaves, data of the fluorescence emission of the photosystems PSI and PSII changes constantly as it continues to adapt to the environmental conditions in which the plants grow. In this study, the light curves of chlorophyll fluorescence and the changes presented by ∆F/Fm’ as the light received by the B. alicastrum leaves increases (Figure 5) indicate that, as the fluorescence diminishes with the continuous increase in light, photochemical efficiency also rises due to the fact that the centers of PSII reaction are receiving a greater proportion of electrons, therefore, photochemical efficiency and the resulting photosynthesis are very efficient in the summertime, when this research work was carried out.
CONCLUSIONS
Although Brosimum alicastrum has been classified as a late successional, shade tolerant species, it was found to have the capacity, as well as the physiological and photosynthetic plasticity, to adapt to highlight conditions, even in the dry season, while presenting high water use efficiency. This plant species, therefore, can thrive under a variety of natural conditions with limited water availability, conferring an adaptive advantage of great ecophysiological relevance.











 nueva página del texto (beta)
nueva página del texto (beta)




