Introduction
Growing human population demands intensive increment in agricultural activity to produce foods from plant origin. The impact of environmental stress and the appearance of diseases caused by fungal or bacterial species are the major constraints to production (Boyd et al., 2013). Maize (Zea mays L.) is one of the crops most used worldwide: an average harvested area of 157 million hectares and production of 781 mega tons from 2000 to 2014. It is a vital source of food security in many countries in North and Latin America and Sub-Saharan Africa (Ramírez-Cabral et al., 2017). México, with 8 million hectares of maize, contributes a production of 18 mega tons, of which 2.3 million tons are grown in the State of Mexico, the third largest producer of maize nationally, mostly by producers who farm ejidos or communal lands (Paulino-Flores et al., 2017). These producers are identified as being particularly sensitive to negative impacts of global economic integration and market liberalization (Eakin, 2005).
Among the biotic factors, bacteria are an important group of phytopathogens that affect crop health and the phytosanitary quality of seed. The first bacterial attacks in maize were reported in 1949. Now, reports of diseases include several symptoms, which are shown in Table 1.
Table 1: Reported diseases caused by bacterial attack in maize.
| Disease | Causal agent | Reference |
|---|---|---|
| Striped disease | Xanthomonas campestris pv. zeae | Coutingo and Wallis, 1991 |
| Goss's bacterial wilt | Clavibacter michiganensis subsp. nebraskensis (=Corynebacterium michiganense subsp. nebraskense) | Smidt and Vidaver, 1986 |
| Bacterial wilt or Stewart’s wilt | Pantoea stewartii (=Erwinia stewartii) | Forgey et al., 1982 |
| Holcus spot | Pseudomonas syringae pv. syringae | Kendrick, 1926 |
| Bacterial foliar wilt | Acidovorax avenae subsp. avenae (=Pseudomonas avenae | Johnson et al., 1949 |
| Bacterial stalk rot | Dickeya chrysanthemi f. sp. zeae (=Erwinia chrysanthemi) | Sah and Arny, 1990 |
| Bacterial stripe and leaf spot | Burkholderia andropogonis (=Pseudomonas andropogonis) | Vidaver and Carlson, 1978 |
| Chocolate spot | Pseudomonas syringae pv. coronafaciens | Ribeiro et al., 1977; White, 2004 |
| Maize brown stalk rot | Pantoea ananatis | Goszczynska et al., 2007; Krawczyk et al., 2010 |
| Bacterial stripe and stem rot | B. andropogonis | Gijón-Hernández et al., 2008 |
| Bacterial wilt | P. stewartii | Valencia-Torres et al., 2004 |
| Foliar bacterial wilt | A. avenae subsp. avenae | CIMMYT, 2004 |
| Brown stalk rot caused | P. ananwithatis | Gijón-Hernández et al., 2008 |
The causal agent of the diseases and chlorotic streak symptoms described above are Gram negative microorganisms from the Enterobacteriaceae family that have a Type III secretion system with the ability to export proteins involved in the process of colonizing the intercellular spaces of plant tissues and cause death (Collmer et al., 2000). This secretion system has been reported in plant pathogens including Pantoea stewartii subp stewartii, Pseudomonas, Erwinia, Xanthomonas, Ralstonia (Collmer et al., 2000) and Agrobacterium vitis and has been associated with the production of exopolysaccharide (EPS), which causes vascular occlusion (Chalupowicz et al., 2008). In P. agglomerans (Barash and Manulis-Sasson, 2007), because of the occlusions caused by EPS produced by this bacterium, symptoms such as chlorotic streaks, water-soaked necrotic spots and putrescence have been reported in microenvironments significantly different in terms of temperature, humidity and species of plants. Small or large climate variations and its correlation with bacterial diseases is a current global concern about the extent and importance of causes and of its effects that affect the incidence and prevalence of symptoms and loss of crops in a worldwide. Bacterial wilt, chlorotic streaks, necrosis are an important bacterial maize disease throughout the world from North to South America (Forgey et al., 1982; Smidt and Vidaver, 1986; Valencia-Torres et al., 2004; Albarracin-Orio et al., 2012), Africa (Coutingo and Wallis, 1991), Asia (Hui-Ying et al., 2011) and Europe (Krawczyk et al., 2010) and with different cultivar as pearl millet (Frederickson et al., 1997), rice (Lee et al., 2010) and cotton (Baird et al., 1997).
In Mexico, there are no reports of this disease or its symptoms, and because maize is the most important food source for the Mexican population, it is important to know how the disease manifested as chlorotic streaks caused by P. agglomerans affects maize production. This knowledge will enable the development of management programs. The aim of this work was to study how the bacteria P. agglomerans affects maize yield when it is inoculated in different environmental conditions on three maize cultivars adapted to two locations in the Central High Valleys of Mexico.
Materials and Methods
Experimental zone
Two experimental locations were established in the state of Mexico (Figure 1). Temamatla (19° 12’ N and 98° 52’ W), altitude of 2260 m above sea level, humidity of 45 - 70% and temperature of 17- 20 °C and the second location, Ayapango (19° 8’ N and 98° 48’ W), altitude of 2440 m above sea level, humidity of 50 - 90% and temperature of 15 - 18 °C. (SMN, 2012; INEGI, 2013).
Genetic material
Three maize cultivars were used: the tri-linear HS2 hybrid (cross: CL12 × CL11 × CL7) from the Colegio de Postgraduados in Montecillo, Mexico; a simple-cross Triunfo hybrid (cross between lines L-10 and L-52) (Bravo-Quirino and Muñoz-González, 2003) from the National Institute of Research in Forestry, Agriculture and Fishing (INIFAP); and the native Cacahuacintle from CHVM. The two improved cultivars and native maize were developed at altitudes of 2200 to 2750 meters above sea level.
Experimental units
A split-plot design with randomized complete blocks was used (Figure 2). The largest unit was represented by the maize cultivars, with subunits for each of the isolates. Each block consisted of 12 treatments with three replicates, resulting in a total of 36 experimental units. Each unit consisted of four 10-m-long rows 0.8 m apart and 0.2 m between plants. A fertilization formula of 160N-60P-00K was applied. Fieldwork was performed manually or with machinery, depending on the conditions in CHVM.
Origin of the inoculum source
The P. agglomerans bacterial isolates A, B and C were obtained from maize plants with symptoms of chlorotic streaks in the experimental f ields of the Colegio de Postgraduados. These isolates were previously identified by sequencing the 16S rRNA gene. Molecular analysis of sequences showed that they were 99% similar to the sequences for the P. agglomerans cluster, while the control strain of P. agglomerans ATCC 27155 showed 98-99% similarity. Sequences were deposited in GenBank (Accession Nos. EF050806 to EF050810). The isolates were maintained at -80 °C in a solution of 25% glycerol (Morales-Valenzuela et al., 2007; Silva-Rojas et al., 2007).
Activation and inoculum preparation
To activate the bacteria, each isolate was seeded with the cross-streak method over CPG (Bioxon®, México) (10 g casamino acid, 10 g peptone, 10 g glucose and 18 g agar) and the cells were grown at 28 °C. After 48 h, pale yellow colonies characteristic of P. agglomerans were observed. The isolates of P. agglomerans were inoculated in chalk-agar culture medium (10 g yeast extract, 20 g glucose, 20 g calcium carbonate and 17 g agar). The isolates were incubated at 28 °C for 48 h. For each isolate, a bacterial suspension of 108 CFU mL-1 was prepared using sterile distilled water (Schaad et al., 2001).
Inoculation of maize seedlings with P. agglomerans
For each experimental unit, 10 maize plants from the central rows were selected. P. agglomerans inoculation was performed on maize plants with six true leaves (phenological stage V3) (CIMMYT, 2012) 30 days after sowing by the injection method on the stem 2 cm above the ground (Hui-Ying et al., 2011; Lamka et al., 1991). One mL of bacterial suspension with 108 CFU was injected. As the negative control, 1 mL of sterile distilled water was used. At the end of the experiment, leaves with symptoms were collected for each strain. Koch’s postulates were validated in maize plants inoculated with P. agglomerans.
Measuring disease progress in maize
After harvesting and based on the presence of chlorotic streaks in inoculated maize leaves, the yield of each treatment was recorded by location, cultivar and isolates. For each treatment ear weight, width and length were evaluated, as well as grain number and weight.
Statistical analysis
The effect of the disease on yield was determined per treatment and analysed statistically with SAS V9.3 software (SAS, 2014). The program design was developed for a split-plot experimental design (Littell et al., 2006) with the following model:
where yijk is the response variable associated with level k of the factor in the small plot within the large plot in the jth block that received the ith level applied to the large plot, Bloj is the effect of the jth block, αi is the main effect of the ith level of the factor applied to the large plot (A), βk is the main effect of the kth level of the factor applied to the small plot (B), (αβ)ik is the interaction between factor A and factor B, ηij is a common random effect for all subplots in the large plot (i, j) and eijk is a random component particular to the subplot with the kth level of the small plot in the largest plot (i, j). Significant differences were analysed with ANOVA and the Tukey test with P < 0.05.
Results and Discussion
Chlorotic streaks in the inoculated plants were observed 8 days after inoculation. Figure 3 shows the development of chlorotic streaks parallel to the midrib of the leaf, located on the edge, the main ridge, the apex of the leaf blade and near the ligule. Dispersed aqueous stains, tissue contraction in the affected area, undulations in the area with chlorotic streaks, shortening of leaves, curl of the apex of the leaf blade and, in advanced stages of the disease, necrosis and loosening of the leaf blade were also observed.

Figure 3: Symptoms of chlorotic streaks on leaves of Triunfo hybrid maize in the experimental fields of Temamatla and Ayapango, México, during the 2010 spring-summer cycle: a) development of chlorotic streaks on the edges of the leaves parallel to the midrib; b) development of chlorotic streaks in the middle layer of the leaves; c) development of chlorotic streaks on the edges and shortening of the leaves in the middle lamella.
Chlorotic streak symptoms on maize plant
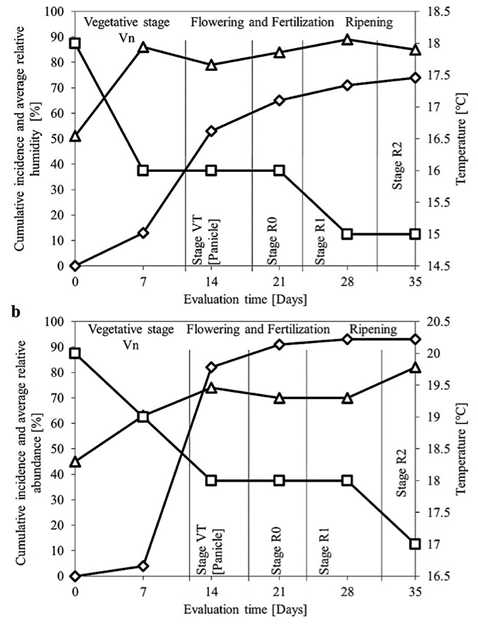
Figure 4 shows the cumulative incidence of plants with chlorotic streaks by location after 7 days of inoculation, the percentage of infected plants was less than 15%. For both microenvironments, the largest number of plants with chlorotic streaks was observed between 7 and 14 days after inoculation. The percentage of plants with chlorotic streaks was 92% in Temamatla and 70% in Ayapango. Both microenvironments showed significant differences among isolates (P < 0.0001) and over time (P < 0.0001). Differences in incidence were caused by different isolates: in Ayapango isolate A was highly virulent, and in Temamatla isolate B was more virulent.
Maize yield in plants with chlorotic streaks
Symptoms of chlorotic streaks were observed in 60% of inoculated plants (Figure 5). In Ayapango, the number of plants with fruit was lower in all the cultivars and for all the isolates (P < 0.0001). In Temamatla; all cultivars were highly susceptible, but to different isolates: HS2 to P. agglomerans isolate A; Triunfo to P. agglomerans isolate A and B and Cacahuacintle to P. agglomerans isolates B and C (Figure 5 and Table 2).
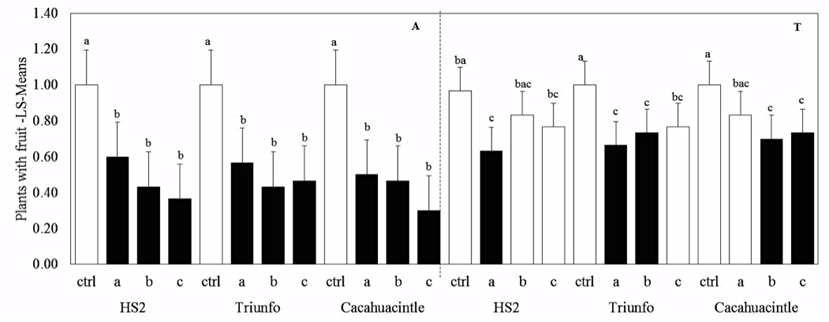
Figure 5: Percent of plants with chlorotic streaks and fruit development in two locations A (Ayapango) and T (Temamatla) and three isolate a, b, c.
Table 2: Effect of inoculating P. agglomerans on the yields of cob and grain by location and cultivars.
| Effect | Cob | Grain | |||||
|---|---|---|---|---|---|---|---|
| Plants with cob | Width | Length | Weight | Number | Weight | ||
| CHVM | |||||||
| Location | <0.0001 | <0.0001 | 0.8985 | <0.0001 | 0.116 | 0 | |
| Cultivar | 0.956 | <0.0001 | 0.002 | <0.0001 | <0.0001 | <0.0001 | |
| Isolate | 0.587 | 0.006 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | |
| Location*Cultivar | 0.739 | 0.335 | 0.748 | 0.141 | 0.378 | 0.294 | |
| Location*Isaloate | 0.22 | 0.604 | 0.537 | 0.019 | 0.99 | 0.165 | |
| Location*Cultivar*Isolate | 0.672 | 0.419 | 0.243 | 0.107 | 0.283 | 0.183 | |
| Cultivar*Isolate | 0.866 | 0.0382 | 0.015 | <.0001 | 0.002 | <.0001 | |
| Ayapango | |||||||
| Cultivar | 0.818 | <0.0001 | 0.025 | <0.0001 | <0.0001 | <0.0001 | |
| Isolate | 0.268 | 0.18 | 0.002 | 0.003 | 0.012 | 0.005 | |
| Cultivar*Isolate | 0.939 | 0.153 | 0.264 | 0.157 | 0.022 | 0.03 | |
| Temamatla | |||||||
| Cultivar | 0.894 | <0.0001 | 0.05 | <0.0001 | <0.0001 | 0 | |
| Isolate | 0.775 | 0.0308 | 0.007 | <0.0001 | 0.003 | <0.0001 | |
| Cultivar*Isolate | 0.418 | 0.1227 | 0.008 | <0.0001 | 0.022 | 0 | |
By location, in Ayapango, the cultivar and isolate effects on the variables cob length, width and weight were significant. Grain number and weight were influenced by the interaction cultivar * isolate. In Temamatla, the cultivar-isolate interaction affected all the yield variables (Table 2).
Average size of ears on plants with chlorotic streak was smaller, relative to the control: width 5.2 ± 0.57 cm, length 14.80 ± 3.07 cm and weight 175.73 ± 62.65 g. Plants without inoculation with P. agglomerans (controls) had ears 5.32 ± 0.46 cm wide, 15.77 ± 2.51 cm long, weighing 195.20 ± 55.29 g. The average values of grain number and weight, were 405.82 ± 116.38 and 136.38 ± 50.27g, significantly lower than the control: 443.12 ± 110.21 and 162.21 ± 49.26 g.
As shown in Table 3, the cultivar-isolate interaction was significant in all variables. In both locations, cultivar HS2 and Cacahuacintle had the smallest dimensions. Ear width was smallest in Ayapango with the interaction location-isolate C and in Temamatla with the cultivar Triunfo and isolate A, and in both locations the interaction Cacahuacintle-isolate C was significant.
Table 3: Effect of inoculation of three P. agglomerans isolates on ear and grain yields by location and cultivar.
| Cultivar | Isolate | Cob | Grain | |||
|---|---|---|---|---|---|---|
| Width | Length | Weight | Number | Weight | ||
| - - - - - - - - cm - - - - - - - - | - - - - - g - - - - - | - - - - - g - - - - | ||||
| Ayapango | ||||||
| HS2 | ctrl | 4.96±0.34 dc† | 15.18 ± 1.67 ba | 176.49 ± 32.71 bc | 480.50 ± 87.79 bc | 155.94 ± 28.68 bac |
| a | 5.00±0.46 dc | 15.62 ± 1.79 ba | 191.81 ± 47.28 bac | 481.00 ± 102.97 bdc | 144.10 ± 41.20 ba | |
| b | 5.01±0.37 d | 14.82 ± 1.88 ba | 181.49 ± 49.85 bac | 454.47 ± 75.41 dc | 148.32 ± 35.27 bc | |
| c | 4.83±0.42 d | 14.16 ± 2.63 bc | 170.73 ± 47.30 bc | 451.69 ± 100.77 dc | 148.61 ± 45.54 bac | |
| Triunfo | ctrl | 5.48±0.54 ba | 16.69 ± 2.80 a | 225.14 ± 71.23 a | 557.70 ± 145.85 a | 185.03 ± 60.52 a |
| a | 5.45±0.75 bac | 15.08 ± 3.70 bc | 215.74 ± 113.84 ba | 446.71 ± 165.43 dc | 129.61 ± 72.07 bc | |
| b | 5.54±0.45 a | 15.92 ± 3.37 ba | 223.43 ± 57.64 a | 561.25 ± 137.12 ba | 170.16 ± 45.73 a | |
| c | 5.51±0.34 ba | 15.35 ± 3.30 ba | 194.97 ± 58 20 bac | 504.22 ± 169.37 bac | 137.09 ± 49.47 bac | |
| Cacahuacintle | ctrl | 5.52±0.49 a | 15.44 ± 3.07 ba | 183.96 ± 61.93 bac | 291.17 ± 96.99 e | 145.67 ± 58.60 bac |
| a | 5.31±0.78 bac | 14.73 ± 3.46 ba | 161.61 ± 76.82 c | 278.39 ± 94.99 e | 127.14 ± 66.62 bc | |
| b | 5.20±0.58 bdc | 15.00 ± 3.18 bc | 152.29 ± 64.62 c | 255.13 ± 101.10 e | 107.08 ± 59.60 dc | |
| c | 4.91±1.02 dc | 12.07 ± 4.37 b | 89.56 ± 48.34 d | 219.55 ± 100.33 e | 115.34 ± 36.90 d | |
| Temamatla | ||||||
| HS2 | ctrl | 4.63 ± 0.69 b | 14.84 ± 2.25 ba | 150.75 ± 57.59 dc | 480.50 ± 155.89 bc | 140.45 ±48.18 bcd |
| a | 4.56 ± 0.32 b | 14.32 ± 3.57 b | 144.93 ± 61.05 dce | 481.00 ± 141.58 c | 125.11 ± 56.88 fecd | |
| b | 4.69 ± 0.33 ba | 15.11 ± 2.73 ba | 164.35 ± 50.30 c | 454.47 ± 126.43 bc | 140.20 ± 45.58 bcd | |
| c | 5.24 ± 2.05 ba | 15.29 ± 3.07 b | 187.00 ± 61.71 bc | 451.69 ± 112.36 bc | 154.23 ± 61.11 bc | |
| Triunfo | ctrl | 11.18 ± 31.39 a | 32.25 ± 81.34 a | 224.31 ± 58.34 a | 557.70 ± 158.67 a | 182.52 ± 56.81 a |
| a | 4.90 ± 0.65 ba | 13.53 ± 2.70 b | 121.91 ± 64.92 dfe | 446.71 ± 160.22 c | 101.18 ± 59.78 gf | |
| b | 5.03 ± 0.73 ba | 16.65 ± 3.15 a | 142.60 ± 51.54 dce | 561.25 ± 123.64 ba | 151.98 ± 62.07 ba | |
| c | 4.87 ± 1.03 ba | 13.80 ± 3.51 b | 159.43 ± 54.62 c | 504.22 ± 141.00 c | 113.64 ± 51.85 gfecd | |
| Cacahuacintle | ctrl | 5.10 ± 0.42 ba | 15.07 ± 2.46 ba | 127.00 ± 49.62 dfe | 291.17 ± 75.12 d | 137.44 ±48.14 becd |
| a | 4.84 ± 0.62 ba | 14.57 ± 2.75 b | 193.90 ± 51.79 ba | 278.39 ± 77.18 d | 110.92 ± 45.34 gfe | |
| b | 4.70 ± 0.77 ba | 13.41 ± 2.56 b | 101.87 ± 52.56 f | 255.13 ± 89.59 d | 89.82 ± 44.24 g | |
| c | 4.68 ± 0.42 ba | 13.86 ± 3.75 b | 112.62 ± 57.24 fe | 219.55 ± 111.33 d | 111.35 ± 73.50 gfed | |
† Averages in rows marked with different letters indicate statistically significant differences. Tukey (P ≤ 0.05).
The Central Valleys of the State of Mexico are notable for their production of maize (500 000 ha), 80% of which is rainfed. Climate in this region is temperate and humid, with an average annual temperature of 16.5 °C, relative humidity of 85%, and annual rainfall of 800-900 mm, at an altitude of 2200 meters above sea level (SMN, 2012). These conditions are favourable for maize cultivation but allow the development of bacterial diseases, such as chlorotic streaks on maize leaves caused by P. agglomerans.
In this research, during the period of evaluation of chlorotic streaks, average humidity and temperature in Ayapango and Temamatla were 45% and 85% and 16-19 °C, respectively. Under these conditions, the incidence of maize plants with chlorotic streaks increased gradually in the three cultivars of maize (Figure 3). Differences between both locations (Temamatla and Ayapango) during the evaluation were found. In Temamatla the humidity was lower than Ayapango (70 and 90% respectively) and the temperature was 2 °C higher in Temamatla. These findings probably had influence in the incidence of plants with chlorotic streaks. Probably the bacteria requires a lower humidity to express its pathogenicity.
Morales-Valenzuela et al. (2007) found similar results when they inoculated maize and sorghum seedlings with P. agglomerans under greenhouse conditions. Frederickson et al. (1997) found that at 25 °C and 95% relative humidity, different isolates of P. agglomerans caused necrosis on the leaves and edges of pearl millet (Pennisetum glaucum (L.) R. Br.).
Similarly, Sabaratnam and Beattie (2001) reported that when maize plants are grown in relative humidity below 95%, colonisation of P. agglomerans in leaf tissue increases. However, when maize plants are exposed to 45% relative humidity, colonisation decreases drastically.
In the two locations, maize yield was affected by P. agglomerans when relative humidity was 85%. Differences of ± 3 °C in temperature caused lower yield compared with the controls. The affinity of P. agglomerans to the cultivar Cacahuacintle is probably due to the high concentration of carbohydrates, of which more than 73% is amylopectin (Carballo et al., 2010), in contrast to cultivar HS2 and Triunfo. Loss of grain weight of the cultivar Cacahuacintle reduces productivity by 1.9 Mg ha-1 meaning economic repercussions for the producers (Téllez-Silva et al., 2016).
Conclusions
Symptom development was mainly observed in maize leaves during vegetative growth in the three cultivars. The percentage of diseased plants exhibited behaviour characteristic of bacterial diseases: stage Vn was the most affected and in stage R2 the disease stabilised. This behaviour correlated with temperature and relative humidity in the CHVM during the spring-summer 2010 crop season. The three evaluated maize cultivars were susceptible to the three isolates of P. agglomerans. Disease symptoms correlated with loss of ear and grain yield, but posterior effects of these symptoms on yield is still unknown. Therefore, it is necessary to correlate these symptoms with maize growth and yield. This is the first report in Mexico that describes the infection of P. agglomerans and how the chlorotic streak symptoms can vary in two similar microenvironments.











 nueva página del texto (beta)
nueva página del texto (beta)