Introduction
In Mexico, citrus species develop iron chlorosis when grown in alkaline and calcimorphic soils. In these soils, the availability of iron depends on the kind of iron minerals, particle size, crystalline structure, potential redox, presence of chelating agents and Fe antagonist elements (Cu, Ni and Mn) (Lindsay, 1984; Loeppert, 1986; Srivastava, 2012). Other factors are pH (pH 7.4-8.5), presence of bicarbonates (>200 mg kg-1, high carbonate content (>20%), active CaCO3, clay textures, and absence of oxygen in the radical zone (Chen and Barak, 1982). In conditions of low amounts of available Fe, it has been observed that some plants can develop adaptation strategies to increase their ability to translocate and absorb Fe in their leaves, up to nine times more than others in the same conditions or even in non-deficient conditions (Young and Terry, 1982). Adaptation strategies of plants to Fe deficient conditions may be transitory phenomena, activated before undergoing severe chlorosis when Fe is insufficient (Landsberg, 1984; Brown and Jolley, 1986). Such strategies occur in the subapical root zones, which promote excretion of protons, formation of organic acids (citrate and malate), release of flavine and phenol, increase in reductive capacity and production of ethylene (Römheld and Marschner, 1986; Romera and Alcántara, 1994). Moreover, El-Jendoubi et al. (2012) pointed out that under Fe deficiency, some fruit plants make Fe soluble in the rhizosphere through the excretion of great quantities of malate. Solubilized Fe3+ in the rhizosphere is conducted to the plasma membrane of rhizodermal cells, where it is reduced 10 to 20 times as compared with the situation of non-chlorotic plant (Moog and Brüggemann, 1994; Sueyoshi et al., 1997). Iron (Fe3+) reduction is attributed to a standard reductase constituent of all of the membranes of higher plants and to induced reductase (Bienfait, 1988).
According to Holden et al. (1991), Fe absorption implies that Fe is first reduced in the cell membrane by Fe3+ reductase; after that it goes to the cytoplasm as Fe2+ and is oxidized again forming the complex Fe3+-dicitrate, which travels through the xylem to the aerial part (Chaney, 1989).
After traveling through the xylem and having been previously reduced from Fe3+ to Fe3+ in the membrane, the Fe3+-dicitrate passes to the phloem, and in the cytoplasm of this tissue, it is complexed by nicotinamide for its cytoplasmatic distribution to the required sites (Stephan and Scholz, 1993). Lack of Fe in the foliage decreases the thylakoid stability, chlorophyll synthesis, quantity of carotenes and xantophylls, and photosystem activity, creating a significant reduction in photosynthesis (Römheld and Marschner, 1991) and the presence of visual symptoms of iron chlorosis, which maintains the venation (xylem) and the chlorotic mesophyll green (Abadía et al., 1991). This difference may alter concentrations and nutritive ratios in the foliage; for example, in peach trees (Prunus persica) it was found that the concentration of N, K, Ca, Mg, Mn and K/Ca ratio were higher in Fe-deficient chlorotic leaves than in non-deficient green leaves (Heras et al., 1976). Abadía et al. (1985) observed that the active Fe level of Fe2+, K, and K/Ca and P/Fe ratios were higher in chlorotic leaves of the same species, while in a similar experiment, Köseoglu (1995) registered increments in N, P, K, Mg, increments in P/Fe ratio, and a decrement in total Ca, Zn, Mn, and Fe contents.
For the reasons mentioned above, the objective of this research was to evaluate the adaptive strategies of Mexican lemon rootstocks through observation of changes in nutrients (anions and cations), chlorophyll concentration, active Fe in foliage, OH- or H+ excretion, reductive capacity of the root, and increment of fresh weight rootstocks. This is accomplished through chemical analysis of the plant to evaluate the strategies of response to Fe deficiency. This evaluation is the basis for selecting rootstocks adapted to alkaline soils.
Methods and materials
Fifty seeds of each rootstock species (Citrus macrophylla, Citrus volkameriana and Citrus aurantium) were germinated on Canadian peat in 200 cone polyurethane trays. The seedlings grew with natural light for a month until they reached a height of 10 cm, when they were transplanted to a closed hydroponic system with three liters of nutritive solution containing: 1.3 mM Ca(NO3)2, 1 mM KNO3, 0.8 mM MgSO4, 0.1 mM K2 HPO4, 0.56 μM ZnSO4, 6.7 μM MnSO4, 0.24 μM CuSO4, 33 μM H3BO4 (Manthey et. al.,1994). The treatments were the nutritive solution mentioned above at two levels of Fe, 0 and 35 μM as Fe-EDDHA and two levels of pH, 6.5 and 8. Two plants were set in each recipient and each treatment was replicated five times. Plants were irrigated twice a day, once in the morning and again in the afternoon. The nutritive solution was changed once a week and pH was revised three times per week. In these conditions the plants grew for two months.
At the end of this study, the SPAD units and the concentration of chlorophylls a, b and a+b were determined in young leaves affected by ferric chlorosis. The chlorophylls were determined in 0.5 g of fresh leaves per treatment. Every sample was treated with 40 mL of pure acetone to prepare a homogenized sample that was filtered through fiberglass and the remnant was washed with 40 mL acetone before gauging 100 mL with distilled water. The final extract was composed of an 80% acetone solution, which was refrigerated and protected from the light. Measuring the absorption at 645, 652, and 663 nm, concentration of chlorophylls a and b was determined in the indicated extracts. Chlorophyll absorption was transformed using specific equations proposed by Bruinsma (1963).
Based on the principle that chelate preferably Fe2+ forms a very stable compound, which was determined by a wavelength of 510 nm (Katyal and Sharma, 1980), the active Fe (Fe2+) was extracted with 1-10 ortho-phenanthroline from young leaf samples (Zohlen, 2000).
The reducing capacity was determined in 200 mg of fresh weight of segments of root apex in active growth, through their immersion in 62 mL of a solution of 50 mM of MES buffer (pH 6.3), 1 mM of Ca (NO3)2, 0.8 mM of KNO3, 0.6 mM of MgSO4, 0.3 mM of FeHEDTA and 0.2 mM of bathophenanthroline disulfonic acid (BPDS).The reaction was carried out in darkness for an hour at 33 ºC and in agitation. Reduced Fe was determined through the formation of chelate Fe (BPDS)3 and colorimetrically measured at 536 nm (Manthey et al., 1994).
Nutrient concentration was measured in clean young undamaged leaves. The leaves were placed in plastic bags and transferred to the laboratory in a portable icebox. Each sample was washed according to the procedure proposed by Chapman (1966) and dried at 70 ºC for 48 hours in an oven with forced air circulation before being ground (mesh 40) in a stainless steel mill (Etchevers-Barra, 1988). The material was digested with a bi-acid mixture (HNO3/HClO4 = 4/2 mL) at 203 ºC. In the digested matter, K, Ca, Mg, Fe, Zn, Mn, Cu, and B were determined by spectrophotometry of AES-ICP emission in Varian Liberty II equipment. Nitrogen was digested in acid (H2SO4) and assessed in steam drive distillation (Bremner, 1965) adapted by plant analysis and modified with salicylic acid to include nitrates.
Chlorophyll concentration, active leaf Fe, root reducing capacity, leaf composition, fresh weight (ΔDFW), Σcations and Σanions were plotted and analyzed with ANOVA (Analysis of Variance), and means were compared with the Tukey test (α = 0.05) using the Statistical Analysis System version 6.12 (SAS Institute, 1988) and Microsoft Office ExcelTM.
Results and discussion
Organic and inorganic composition and nutrient balance of cations and anions
The concentration of N-organic was the same in all the rootstocks, while the concentrations of S-organic, NO3-, SO42-, Ca, Mg, Na, ΣC, ΣA, ΣC-ΣA were much higher in Volkameriana than in Macrophylla and Sour Orange. On the other hand, the concentrations of PO43- and K were higher in Macrophylla than in Volkameriana and Sour Orange. Hydroxide ion (OH-) excretion was higher in Sour Orange than in Macrophylla, but Volkameriana excreted H+ instead of OH- (Table 1). These results can be explained by the low levels of Fe in the plants, which promote reactions that modify the plant’s leaf and root structure, morphology, and physiology (CITE). All these strategies are aimed at developing conditions that increase mobilization of Fe in the rhizosphere, increasing absorption and translocation of this element (Brown, 1978) and modifying the concentration of other nutrients in the leaves. The reactions that increased may be H+ excretion, Fe reducing capacity by the roots, formation of radical hair, formation of epidermal “transfer” cells, synthesis and accumulation of organic anions (malate and citrate), flavine excretion and phenol compounds (Bienfait, 1988, 1996).
Table 1 Organic and inorganic composition and nutrimental balance of leaves, developed with or without Fe supplies in solutions with different pH.
| Evaluated variables | Rootstocks | pH | Fe | ||||
|---|---|---|---|---|---|---|---|
| Volkameriana | Macrophyla | Sour orange | 6.5 | 8 | With | Without | |
| --------------------------------------------------------me 100 g-1-------------------------------------------------- | |||||||
| N-organic | 189.6 a1 | 177.1 a | 185.4 a | 185.7 a2 | 182.4 a | 188.0 a3 | 181.6 a |
| S-organic | 10.3 a | 10.0 ab | 9.5 b | 10.0 a | 9.9 a | 10.1 a | 9.8 a |
| NO3- | 0.7 a | 0.4 b | 0.3 b | 0.4 a | 0.5 a | 0.4 a | 0.5 a |
| PO43- | 3.4 b | 4.4 a | 3.7 ab | 4.2 a | 3.5 b | 3.9 a | 3.8 a |
| SO42- | 7.1 a | 4.5 b | 4.9 b | 5.4 a | 5.5 a | 5.3 a | 5.7 a |
| K | 68.3 b | 77.1 a | 42.3 c | 50.5 b | 74.7 a | 60.6 b | 64.0 a |
| Ca | 104.8 a | 83.5 b | 65.0 c | 71.9 b | 97.9 a | 87.3 a | 81.2 a |
| Mg | 49.5 a | 28.5 c | 37.6 b | 35.2 b | 41.9 a | 37.9 a | 40.3 a |
| Na | 1.7 a | 0.4 c | 0.4 b | 0.4 b | 1.2 a | 0.7 a | 0.9 a |
| Σ cations(ΣC) | 224.4 a | 189.6 b | 146.5 c | 158.2 b | 214.8 a | 108.1 b | 186.6 a |
| Σ anions (ΣA) | 11.4 a | 9.4 b | 8.9 b | 10.2 a | 9.6 b | 9.7 a | 10.1 a |
| ΣC - ΣA | 203.0 a | 180.2 b | 136.5 c | 148.0 b | 205.2 a | 171.2 b | 176.4 a |
| OH - Excretion | -13.0 c | 19.6 b | 50.0 a | 42.1 a | -9.9 b | 25.3 a | 12.5 b |
1Horizontal reading, varieties of rootstocks with the sae letter are not greatly different; 2 Horizontal reading, pH levels with different greatly different; 3 Horizontal reading, Fe levels with different letter are greatly different.
The high absorption of cations (K, Ca, Mg, Na), ΣC, ΣC-ΣA (organic anions), observed in Volkameriana and Macrophylla rootstocks, relative to that in Sour Orange, could be determined due to the processes involved during the development of strategies (acidification of the rhizosphere and reductase activity) for adaptation of these rootstocks when there is a scarcity of Fe.
Dakora y Phillips (2002) established that, when Fe is deficient, in Fe-efficient plants radical H+ excretion decreases the absorption of anions and increases both the accumulation of organic anions (ΣC-ΣA) and the absorption of cations. It has also been observed that these plants, when nourished with NO3-, decrease or change radical OH- excretion for H+ excretion but either alcalinization or acidification is present in all the radical system (Marschner and Römheld, 1994).
Most of the K concentration determined in the Volkameriana and Macrophylla rootstocks, relative to Sour Orange, could be attributed to greater excretion of H+ by the root apex, which helps to acidify the rhizosphere, increase the Fe activity around it and exchange it for K+ in order to maintain the electric potential of the membrane (Jolley and Brown, 1985; Römheld and Marschner, 1986). In the leaves affected by ferric chlorosis the synthesis of carbohydrates decreases and as a result the biomass also decreases, causing an increase on potassium level (Hamzé et al., 1986).
The Ca levels determined in Volkameriana were higher with regard to Macrophylla and Sour Orange.
Calcium is an element considered by Cohen et al. (1997) as a regulator of the ferric reductase activities that can favor adaptation of a plant to Fe stress, although there have been results that do not agree, such as those of Köseoglu (1995); the latter author found increments of N, P, K, and Mg, while Ca, Zn, Mn and Fe decreased in Prunus persica leaves affected by ferric chlorosis.
The Mexican lemon rootstocks grew in the solution with pH 6.5 increased the concentration of N-organic, S-organic, PO43-, ΣA and OH excretion; relative to thosegrew in the pH 8.0 solution.
However, K, Ca, Mg, Na concentrations, ΣC and ΣC-ΣA were higher in the rootstocks growing in the solution pH 8. These results coincide with the results reported by Larbi et al. (2010), who pointed out that more cations are absorbed in the interval close to neutral, while more anions are absorbed in the acid interval. The increment in the accumulation of organic anions (ΣC-ΣA) at alkaline pH coincides with the information reported by Smith and Raven (1979), who pointed out that this accumulation is a response of the plant to maintain the cytoplasm pH in the interval 7 to 7.5. Nevertheless, the OH- xcretion was greater at acid pH and there was little H+ production under the alkaline condition, a mechanism of the plant to maintain electro- neutrality to favor Fe activity (Marschner and Römheld, 1994). N-organic, S-organic, NO3-, SO42-, Ca, Mg, Na and ΣA concentrations were not affected by the treatments with or without Fe. However, the K level and the ΣC and the ΣC-ΣA were higher in the treatments without Fe, while the OH- excretion was higher when Fe was applied to the Mexican lemon rootstocks.
The results on Fe partially coincide with the findings of Abadía et al. (1985, 1989) who determined that in peach and pear leaves, affected by a lack of Fe, the levels of P, Ca, Mg, Na, Fe, Mn, Cu and Zn levels did not change significantly with regard to non-chlorotic plants. However, in another study carried out in Prunus persica, by Heras et al. (1976) N, K, Ca, Mg and Mn were concentrated in Fe- deficient chlorotic leaves rather than in non-deficient green leaves. In chlorotic leaves of the same species, the active Fe and K levels increased (Abadía et al., 1985), while in a similar experiment the N, P, K and Mg concentrations increased, and the Ca, Zn, Mn, and Fe concentrations decreased (Köseoglu, 1995). These results clearly show that the nutrient concentrations and sensitivity of leaves to ferric chlorosis may vary among plant species (Figure 1 and 2). The tree rootstocks had an increment in K, Ca, Mg, and Na absorption, when pH was modified from 6.5 to 8. But in the treatments without Fe, Volkameriana consistently showed an increase in the quantity of absorbed cations, except for Ca. Of the four cations, except K, Volkameriana absorbed more Ca, Mg and Na than Macrophylla and Sour Orange (Figure 1 and 2). According to Cohen et al. (1997), the greatest absorption of cations (K, Ca, Mg, Mn and Zn) was associated with the greatest activity of ferric reductases, which, regulated by these, decreased ferric chlorosis.
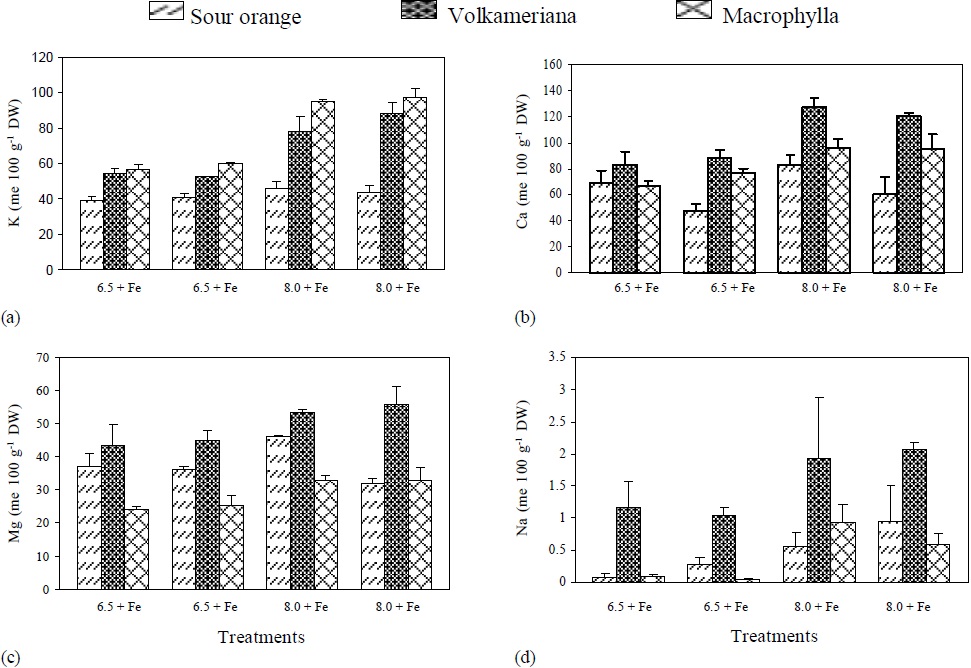
Figure 1 pH effect, with (+) or without (-) Fe application on the concentration of: (a) K, (b) Ca, (c) Mg and (d) Na, in different rootstocks of Mexican lemon. DW = dry weight.

Figure 2 pH effect, with (+) or without (-) Fe application on the concentration of: (a) Σ cations, (b) Σ anions, (c) Σ cations- Σ anions and (d) OH- excretion in different rootstocks of Mexican lemon.
The Σ Cations and the ΣCations-ΣAnions (organic anions) were higher in Volkameriana, followed by Macrophylla and Sour Orange. In Volkameriana and Macrophylla, the increments were even higher when pH changed from 6.5 to 8, and such increments were even higher in the treatments where Fe was suppressed (Figure 2).
Volkameriana presented a similar anion absorption in the three treatments where Fe was shortly available (pH 6.5-Fe, pH 8+Fe, pH 8-Fe). In contrast, Macrophylla showed a decrease in anion absorption when pH changed from 6.5 to 8, while Orange Sour did not show a clear trend. In general, the results were similar to those reported by Egmond and Aktas (1977), who obtained a decrease in anion absorption in Fe-deficient tomato and bean plants.
It was observed that at pH 6.5, Volkameriana and Macrophylla decreased OH- excretion when Fe was suspended, while at pH 8, they excreted H+. On the other hand, Sour Orange only excreted OH- . In all the treatments no trend appeared (Figure 2).
It is important to mention that Volkameriana always showed the lowest levels in OH- production and excretion, with regard to Macrophylla and Sour Orange, and greater H+ production than Macrophylla, as it is shown in Figure 2 d. This phenomenon is important because, according to Römheld et al. (1984) and Römheld and Marschner (1986), H+ excretion allows acidification of the root’s interphase and promotes Fe solubilization, reductase activity, and phenol release. These processes help the plant to better utilize Fe. Egmond and Aktas (1977) have studied OH- and H+ excretion in plants subjected to Fe deficiency and determined that plants that excreted low levels of OH- showed less ferric chlorosis, and they even showed a change in the ionic balance in favor of H+ excretion. Abadía et al. (2002) also says that plants subjected to Fe stress and acidify the rhizosphere show an increased level of organic acids (citrate and malate), which contribute to greater acidification of the rhizosphere when exuded and generate reductive power, controlling the cation/anion balance in order to regulate cell pH and increase Fe transport from the root to the aerial part (Egmond and Aktas, 1977; Landsberg, 1986). Acidification alone may influence the pH of the first 2 mm of the root surface, increasing the reductase activity of the cell membrane, weakening the Fe-O bonds and releasing the metal, even in calcimorphic soils (Schwertmann, 1991; Susín et al., 1996).
Minor elements, nutrient ratios, root reductive capacity and increase of fresh weight in rootstocks with Fe stress
Volkameriana showed a significantly higher level of active Fe than Macrophylla and Sour Orange (Table 2). In Volkameriana, concentrations similar to those reported by Mohammad et al. (1998) in lemon leaves were determined. The authors mentioned above determined concentrations of 11 mg kg-1 of active Fe in chlorotic leaves and concentrations of 17 mg kg-1 in leaves with moderate chlorosis. The results of active Fe found in our research in Macrophylla and Sour Orange were similar in concentration to those reported by Mohammad et al. (1998) for chlorotic leaves. While Volkameriana showed an active Fe concentration similar to that reported by the author mentioned for moderately chlorotic leaves. The reason for determining active Fe, according to Zohlen (2000), is that it correlates better with chlorophyll concentration than with total Fe. Richier et. al. (2012) explains that this is due to the function of Fe2+ associated with chloroplast development, protein formation (cytochromes), ferredoxin, electron transport chain, and chloroplast mRNA and rRNA. The results showed a higher concentration of this element in the treatments with pH 6.5 and with Fe, than those with pH 8 and without Fe. The lower concentration of active Fe at pH 8 may be attributed to a lower quantity of available Fe, according to Lindsay and Schwab (1982), who pointed out that for every unit of increase in pH the dissolved Fe activity decreases thousand times because of the balance between solubility and precipitation. At pH lower than 7.4, ferrihydrite dominates, between 7.4 and 8.5 goethite dominates, while at a pH above 8.5 ferric hydroxides dominate; all of these compounds are of low solubility (Loeppert, 1986). According to Lindsay and Schwab (1982), the lowest concentration of Fe (10-10.4 M) is reached at a pH between 7.4 and 8.5, that is, 250 times lower than the critical level (10-8 M ) in which plants such as lemon may present Fe deficiency. At pH 8, the reductive capacity of the root is low and, consequently, the transfer of Fe to specific sites is also low (Romera et al., 1991; Fox et al., 1996).
Table 2 Microelements concentration, nutrient rations, chlorophyll concentration, Fe reduced by the roots and increment of fresh weight of rootstocks of Mexican lemon developed at different pH and with or without Fe.
| Evaluted variables | Rootstocks | pH | Fe | ||||
|---|---|---|---|---|---|---|---|
| Volkameriana | Macrophyla | Sour orange | 6.5 | 8 | With | Without | |
| Active Fe (mg kg-1) | 17.1 a1 | 10.0 b | 11.1 b | 14.4 a2 | 11.1 b | 15.7 a3 | 9.8 |
| Fe (mg kg-1) | 23.3 a | 26.0 a | 22.9 a | 25.5 a | 23.1 a | 24.8a | 23.4 |
| Mn (mg kg-1) | 33.7 a | 36.1 a | 14.1 b | 29.1 a | 27.2 a | 24.5 b | 31.4 |
| Zn (mg kg-1) | 25.8 a | 21.8 ab | 19.1 b | 22.6 a | 22.0 a | 22.2 a | 22.2 |
| Cu (mg kg-1) | 15.9 a | 15.0 a | 14.4 a | 15.5 a | 14.5 b | 15.2 a | 15.0 |
| B (mg kg-1) | 221.0 a | 116.3 b | 66.3 c | 132.4 a | 136.0 a | 149.4 a | 119.6 |
| K/Ca4 | 1.7 a | 1.2 b | 1.3 b | 1.4 a | 1.5 a | 1.5 a | 1.4 |
| K/Zn4 | 1043.6 b | 1415.4 a | 884.8 b | 940.1 b | 1288.9 a | 1072.9 a | 1156.2 |
| P/Fe4 | 49.7 a | 56.0 a | 52.0 a | 53.4 a | 51.9 a | 54.7 a | 50.7 |
| μmoles Fe2 + h-1 g FW | 35.5 ab | 36.2 a | 25.2 b | 36.3 a | 28.8 b | 28.8 b | 35.8 |
| Chlorophyll a (mg L-1) | 4.1 a | 3.9 a | 3.2 a | 4.8 a | 3.9 a | 4.8 a | 4.0 |
| Chorophyll b (mg L-1) | 7.7 a | 5.4 b | 4.6 b | 6.5 a | 5.5 a | 6.5a | 5.5 |
| Chorophyll a+b (mg L-1) | 13.6 a | 9.5 b | 8.1 b | 11.8 a | 10.0 a | 11.7 a | 10.1 |
| ∆ Fresh weight (g) | 31.0 b | 42.5 a | 13.0 c | 32.3 a | 25.8 b | 29.3a | 28.8 |
1 Horizontal reading. Rootstock varieties with the same letter are not significantly different; 2 Horizontal reading. pH levels with different letter are significantly different; 3 Horizontal reading. Fe levels with different setter are significantly different; 4 P, K and Ca concentrations in per cent and Fe and Zn in mg.kg-1 regarding dry weight.
Total Fe concentration in the evaluated rootstocks did not show any significant difference. In different studies, it has been determined that this element does not match chlorophyll concentrations because it is being accumulated in inactive form in chlorotic leaves (Abadía et al., 1989).
Inactive iron accumulation originates total Fe concentrations equal to and higher than the concentrations that have been found in green leaves (Morales et al., 1998), and the plant may recover chlorophyll concentrations by activation with acid solutions sprayed on the foliage (Tagliavini et al., 1997). Neither pH nor Fe application caused significant difference in total Fe concentration. This may be explained by the existence of root factors and strategies developed by plants that determine its availability, absorption, and transfer (Kosegarten et al., 1999).
Volkameriana and Macrophylla had higher concentrations of Mn and Zn than Sour Orange. This may be attributed not only to the effect of acidification of the rhizosphere that both rootstocks promoted, but also to the simultaneous effect of the formation of “transfer” cells in the root apex that favor the absorption of these elements. According to Jolley and Brown (1985), Hydrogen ion excretion and the radical apical formation of “transfer” cells coincide in time and space; this phenomenon demands K, Mn, Zn and Fe absorption to maintain membrane electrical potential.
Moreover, concentration of Cu, P/Fe ratio and chlorophyll did not show significant differences among the rootstocks and Fe supply. However, concentration of Cu was affected by pH. The K/Ca ratio was significantly higher in Volkameriana than in Macrophylla and Sour Orange (Table 2). This ratio has been used in citrus as an indicator of resistance to Fe deficiency. It has been said that a ratio higher than 1.2 is high and is associated with high resistance to ferric chlorosis, while a lower ratio is considered low and indicates ferric chlorosis sensitivity (Hamzé et al., 1986).
Volkameriana showed a high ratio making it the species with the highest resistance to ferric chlorosis, while Macrophylla is borderline and Sour Orange is slightly above the level indicating resistance to ferric chlorosis. pH and Fe dose promoted values of K/Ca ratio above the limits for resistance to ferric chlorosis; however, there was no statistically significant difference from the effect of these factors.
The K/Zn ratio was higher in Macrophylla than in Volkameriana and Sour Orange (Table 2). This ratio has been used as an indicator of Fe-deficiency (Igartua et al., 2000) because the K concentration increases and the Zn levels decrease in chlorotic plants, resulting in higher and more constant ratios than individual values over the years (Belkhodja et al., 1998).
The root’s reductive capacity was significantly higher in Macrophylla and Volkameriana than in Sour Orange. Reductive capacity is attributed to specific reductase located in the membrane of root’s apical cells, which in plants subjected to Fe deficiency increases their activity 10 to 20 times than in non-deficient plants (Sueyoshi et al., 1997). The results obtained in this study coincide with those of Hamzé et al. (1986) and Manthey et al., (1994). Based on the root’s reductive capacity, Volkameriana and Macrophylla are considered resistant to Fe stress, while Sour Orange is classified as sensitive. When considering only the pH effect or the Fe dose, higher values of reductive capacity in treatments at pH 6.5 and without Fe were obtained. According to Susín et al. (1996), the highest root reductive power due to higher reductase activity occurs around pH 5.5, and it increases under Fe deficiency (Sueyoshi et al., 1997).
Regarding chlorophyll concentration, Volkameriana presented significantly higher levels of chlorophylls b and a+b than Macrophylla and Sour Orange (Table 2). This result can be attributed to the high reductive capacity of the radical system in Volkameriana, which allowed Fe2+ absorption and traslocation, important steps in chlorophyll synthesis (Terry and Zayed, 1995). Some studies have found that active Fe regulates chloroplastic protein synthesis, important in the formation of the complex chlorophyll-proteins, which constitutes the thylakoid membrane. For this reason, photosynthetic pigment synthesis (chlorophyll and carotenoids) can be negatively affected by up to 95% because of a decrease in Fe2+ (Fodor et al., 1995; Zholem, 2000). In our study, it was found that Volkameriana had high reductive capacity in roots, higher levels of active Fe in foliage, and coincidently higher levels of chlorophyll a+b. These facts allow classifying this rootstock as that having the best response to Fe deficiency. Regarding the pH effect and the Fe dose, there were no significant differences.
Most of the increments in fresh matter registered in Macrophylla and Volkameriana rootstocks are the result of Fe-deficiency response strategies such as more reductive capacity of the root, reduction in OH- excretion, increase in H+ excretion, more K, Ca, Na, Mn, Zn, and B absorption, high K/Ca, K/Zn and active Fe ratios that originated higher concentrations of chlorophylls b and a+b.
The K/Ca ratio in Volkameriana was very similar in the four treatments; in Macrophylla this ratio increased when pH changed from 6.5 to pH 8, and in Sour Orange this ratio was lower in the treatments without Fe at pH 6.5 and pH 8. Volkameriana rootstock showed a more stable value of K/Ca ratio, regardless of the treatment, while in Macrophylla and Sour Orange there was a greater variation (Figure 3), which, according to Hamzé et al. (1986), is associated with lower resistance to ferric chlorosis.
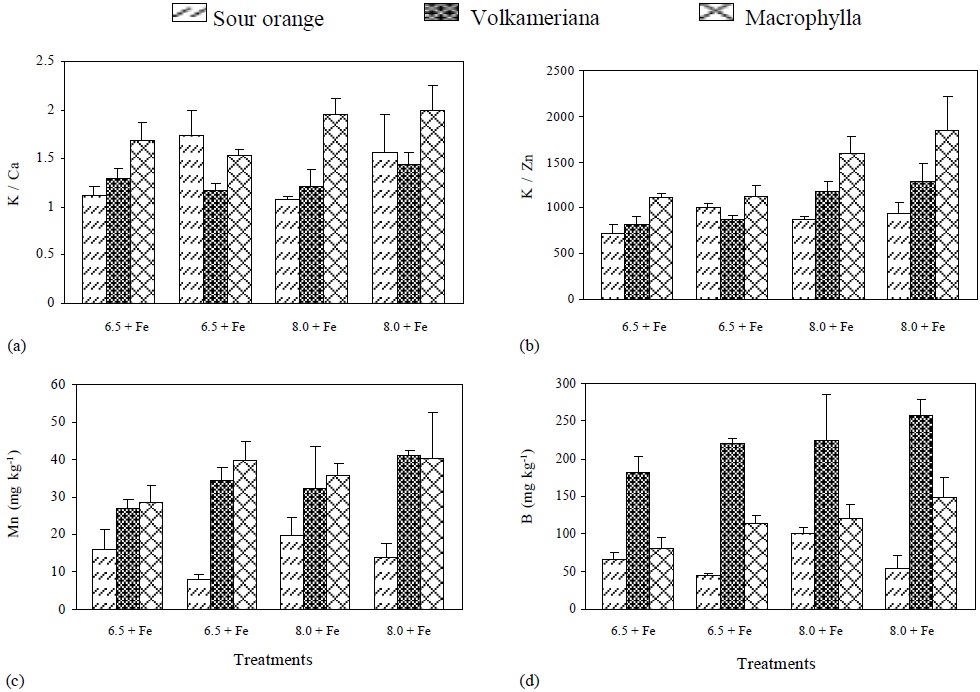
Figure 3 pH effect, with (+) or without (-) Fe application on the concentration of: (a) K/Ca, (b) K/Zn, (c) Mn and (d) B, in different rootstocks of Mexican lemon.
The K/Zn ratio tended to be higher when the pH of the nutritive solution was increased, and it was higher in Macrophylla followed by Volkameriana and Sour Orange. Mn absorption was higher when pH increased from 6.5 to 8. It was significantly higher in both Volkameriana and Macrophylla rootstocks (Figure 3) due to the activity of ferric reductases and H+ excretion (Sijmons and Bienfait, 1986; Cohen et al., 1997).
In the three rootstocks, B increased when pH changed from 6.5 to 8 (Figure 3); however, it was much higher in Volkameriana rootstock due to B participation in cell division (Larbi et al., 2010). In Fe-deficient plants, B must be highly required because side root formation (Moog et al., 1995), radical hairs, and apex enlargement are promoted (Landsberg, 1996).
The lowest levels of active Fe were found at pH 6.5 without Fe and at pH 8 with Fe. On the other hand, the highest levels of active Fe were found at pH 6.5 with Fe and at pH8 without Fe, being Volkameriana the rootstock with the highest concentration of active Fe (Figure 4).
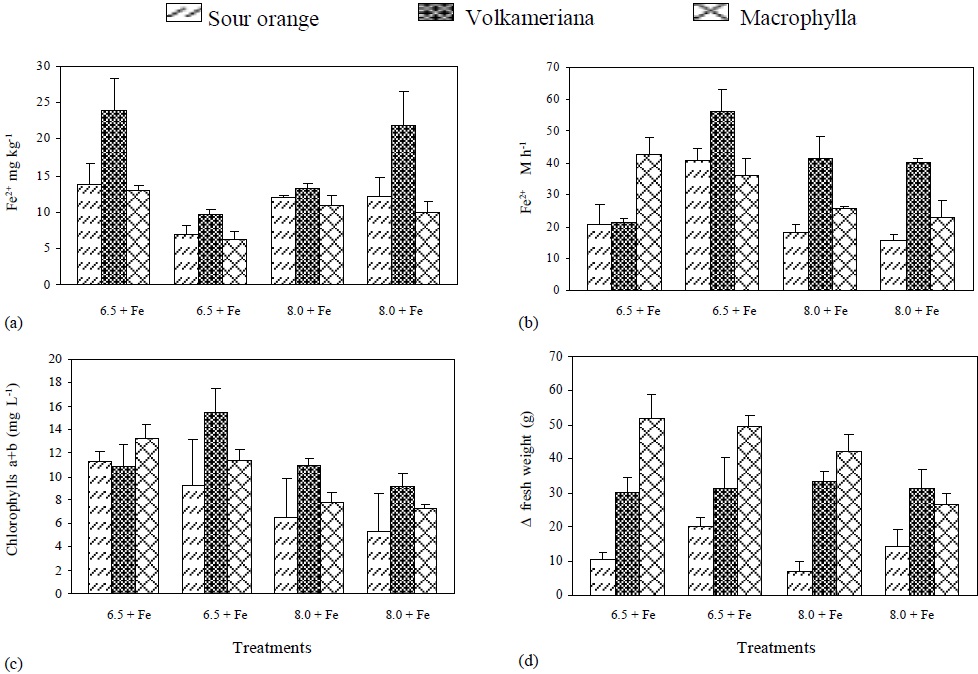
Figure 4 Active Fe-, root’s reductive capacity, chlorophyll concentration, and increase in plant’s fresh weight (DFW) to the different treatments of rootstocks of Mexican lemon.
When Fe supply was eliminated, the root’s reductive capacity at pH 6.5 increased in Volkameriana and Sour Orange rootstock, and an equal decrease at pH 8 was observed in treatments with or without Fe. At pH 6.5 and without Fe the same reductive capacity was observed (Figure 4), which coincides with the information provided by Sueyoshi et al. (1997) and González-Vallejo et al. (1999), who state that the best pH for promoting more activity of the membrane’s ferric reductases in beet leaves was between 6.5 and 7, increasing under Fe- deficient conditions. According to Bienfait (1996), H+ excretion is related to citrate accumulation. Citrate is isomerized in the form of a-oxoglutarate and reduced NADP+ (NADPH) which directly or indirectly transfers electrons to the membrane ferric reductases that are responsible for reducing Fe.
Furthermore, the concentration of chlorophyll a+b showed higher levels at pH 6.5 than pH at 8 with or without Fe; this concentration was higher in Volkameriana in all the treatments. Moreover, a direct ratio (or relationship) between the quantity of active Fe and the roots’ reductive capacity regarding the concentration of chlorophyll a+b was observed. Finally, the ΔFW of fresh weight was higher in Macrophylla and Volkameriana rootstocks than in Sour Orange. Macrophylla showed less growth when the pH increased and Fe was not applied, while Volkameriana had constant values in the four treatments. Sour Orange showed a very irregular increase in fresh weight in the different treatments, but it was always low (Figure 4).
Conclusions
The results obtained in this study showed higher leaf concentrations of N-organic, S-organic, NO3 -, SO42-, Ca, Mg, Na, ΣCations, ΣAnions, ΣCations-ΣAnions, active Fe, Zn, Cu, B, higher concentration of chlorophylls a, b and a+b, and K/Ca ratio in Volkameriana, than in Macrophylla and Sour Orange (was this difference a significative one). Macrophylla, however, showed higher levels of PO43+, K, total Fe, Mn, K/Zn ratio, root reductive capacity and increase of fresh weight than Volkameriana and Sour Orange.
Volkameriana and Macrophylla decreased OH- excretion in conditions of limited Fe, while extreme Fe- deficiency promoted H+ excretion. For this reason, nutrimental element absorption improved. These rootstocks developed better strategies to respond to Fe stress by promoting changes in leaf nutrient composition and reductive capacity in the root, originating better utilization of Fe to maintain higher levels of chlorophylls.











 nova página do texto(beta)
nova página do texto(beta)


