Introduction
Oils serve as a carbon and energy reserves and are precursors for membrane lipid and steroid biosynthesis in plants (Hanisch et al., 2006; Demirbas, 2011). Oil or lipid bodies called oleosomes are spherical storage organelles embedded within the cytoplasm of eukaryotes (Waltermann and Steinbuchel; 2005; Martin and Parton, 2006). Oleosomes occur in cells of most plant tissues and are prominent in seeds, pollen grains, embryos and endosperm. They are also in stems, fruits and leaves, where they are frequently associated with plastids (Lersten et al., 2006). The most common forms of lipids stored in plants are triacylglycols (TAG), triesters of fatty acids that are attached to glycerol (Moellering and Benning, 2010; Goncalves et al., 2011). Oil bodies consist of a TAG matrix covered by a stabilizing layer of phospholipids and unique proteins, which are thought to originate from the endoplasmic reticulum (Goldberg et al., 2009; Iwanaga et al., 2008; Lersten et al., 2006; Lin and Tzen, 2004). These universal components of eukaryotic cells provide a rapidly mobilized lipid source for many important biological processes (Martin and Parton, 2006; Rezanka and Sigler, 2009).
Lipid Bodies in Plant Cells
To function normally, embryos, seeds and pollen are physiologically programmed to cope with severe desiccation during maturation and storage (Liu et al., 2008). Regulation of fatty acid composition may be one mechanism by which plants regulate tolerance to temperature and moisture stress (Volk et al., 2007). TAGs in Cuphea sp. seeds is crystallized at low storage temperatures. After storage, if seeds were imbibed with water before TAGs liquefy, cells suffer irreversible membrane damage and fail to germinate. Depending upon fatty acid composition, TAGs in some species liquefy at 22 ºC while other species need to be warmed to 45 ºC before liquefaction and normal germination.
Oleosins, proteinaceous matrices associated with the surfaces of lipid bodies, are thought to maintain stability of lipid bodies in seeds of desiccation-tolerant plants to prevent them from coalescing during seed dehydration and germination (Murphy, 2001, Purkrtova et al., 2008; Jain et al., 2011). Oleosins that coat oil bodies in soybeans protect them from environmental stresses (Iwanaga et al., 2008). Pollen grains characteristically contain large numbers of oil bodies that serve as energy reserves for subsequent germination (Piffanelli et al., 1998; Updegraff et al., 2009). Surface proteins contribute to oil body stability in lily (Lillium longiflorum Thumb.) pollen (Jiang et al., 2007). This stability may enhance pollen viability as it is subjected to environmental extremes in transit by insects or wind. Adequate lipid storage compounds are essential for successful development during final stages of somatic embryogenesis in Picea abies (Grigova et al., 2007).
Lersten et al. (2006) examined freehand cross sections of fresh leaves of 302 plant species and reported oil bodies in mesophyll cells in 71 species, but did not observe them in epidermal cells, concluding that oil bodies are significant cellular components of many plant taxa. They found that oil bodies were common in eudicots and less frequent in monocots. They observed that in recent years oil bodies have been neglected because of a shift in plant tissue preparation. Early microscopic studies involved analysis of freehand sections that allowed vivid staining of lipids. Later methods involved killing fixatives and alcoholic dehydration that dissolved lipids and obscured their microscopic detection.
In addition to physiological roles, plant oils have wide economic importance. Two major plant oil classes are recognized: 1) volatile, low-molecular mass oils that are used commercially in perfumes and food extracts and 2) larger molecular mass, non-volatile oils that are commercially extracted from seeds of canola, castor, corn, soybean and other important crop plants (Lersten et al., 2006).
Lipid Bodies Associated With Fungi
Lipid bodies structurally similar to those found in vascular plants are also common in microbes. Oleogenic fungi are those that accumulate over 25% of their dry weight as lipids when carbon is available (Murphy, 2001). Microbial oils have potential commercial value as food supplements, pharmaceuticals, and biofuels (Peng and Chen, 2007). These may be produced more economically than those extracted from agricultural crops. Peng and Chen (2007) isolated fungal endophytes with large and copious quantities of lipid bodies within their hyphae from oleagenous plants. Those with enzymatic capability to decompose wheat straw are candidates for low cost microbial oil production.
Lipid Bodies Integrated with Fungal Endophytes
Plants host diverse populations of cryptic, symbiotic fungi (Arnold et al., 2000; Ganley et al., 2004; Van der Heijden et al., 2006; Vandenkoornhuyse et al., 2002). It is well documented that fungal symbionts contribute multiple benefits that enhance tolerance to abiotic and biotic stress, but the mechanisms of inducing stress tolerance are not well understood. In our research, we have heavily utilized a dual staining approach to reveal trypan blue staining fungal chitin and Sudan IV staining lipid bodies which are associated with fungal symbionts within plant tissues (Barrow et al., 2004; Barrow et al., 2007; Lucero et al., 2008; Barrow et al., 2008; Osuna and Barrow, 2009). We have also used molecular techniques to demonstrate the presence of complex fungal consortia that associate with plants even under aseptic conditions (Lucero et al., 2008, Lucero et al., 2011). Although Sudan IV stain would also reveal lipid bodies associated strictly with the plant, our collective experience suggests this is rare. Our objective in this study was to review dual stained images of plant tissues taken from studies examining endophyte distribution and other plant-fungal associations to specifically consider the association between plant lipid bodies and endophytic fungi.
Materials and methods
Micrographs of plant tissues retrieved from data archives of Jerry Barrow were prepared in association with several studies that occurred between 2000 and 2010. Plants used in these studies were harvested from native desert plant populations on the USDAAgricultural Research Service’s Jornada Experimental Range in southwestern New Mexico, USA. Root and leaf samples were collected from actively growing grasses and shrubs. Roots described as “aseptic” were disinfected with 1.8% sodium hypochlorite solution for 10 min, and then incubated on potato dextrose agar until mycelia appeared at the root tip. Staining methods are described in Barrow and Aaltonen (2001) and Barrow (2003). Briefly, hand sectioned tissues were cleared with potassium hydroxide followed by staining with a solution containing trypan blue, which stains fungal cell walls blue, and Sudan IV, which stains lipid bodies red. Slides containing small secondary roots and leaves were mounted and examined with a Zeiss Axiophot microscope with conventional and differential interference contrast (DIC) optics at 100X and 1000X magnification. Digital images were captured and processed using Auto-Montage 3D software by Syncroscopy.
Results and discussion
Analysis of root and leaf tissues frequently revealed amorphous trypan blue stained fungal structures associated with lipid bodies on leaf surfaces (Figure 1a), root meristems (Figure 1b), lateral roots (Figure 1c) and hydrated seeds (Figure 1d) of Bouteoula eriopoda (1a-c), and Sporobolus airoides (1d), respectively. Although hyphae were sometimes visible (Figures 1a-c), fruiting bodies and other clear details useful for identification were typically absent. Sometimes only blue stain, suggestive of fungal chitin, could be detected (Figure 1d). This amorphous fungal morphology observed among endophytes has been previously described (Barrow, 2003). Similar amorphous fungal structures, thought to be derived from yeasts, have been reported in surface sterilized seeds of Atriplex canescens and in micropropagated shoots of Bouteloua eriopoda (Osuna and Barrow, 2004) and of Atriplex canescens, both of which were demonstrated to contain DNA representing diverse fungal taxa (Lucero et al., 2011; Lucero et al., 2008).
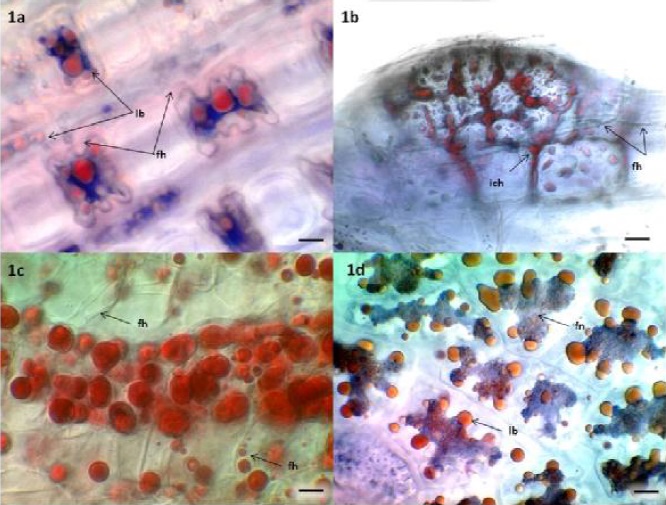
Figure 1a Leaf mesophyll cells of Bouteloua eriopoda. Trypan blue stain reveals a hyaline branched fungal network (fh), associated with red, sudan IV stained lipid bodies (lb). Bar = 10um. Figure 1b. A lateral root initial of Bouteloua eriopoda. Sudan IV stains intercellular lipid bearing fungal protoplasts (ich) and lipid bodies (lb) within meristematic cells. Finely branched fungal hyphae (fh) and amorphous fungal structures appear blue. Bar = 10um. Figure 1c. Sudan IV stained lipid bodies in vascular cells of developing lateral roots of Bouteloua eriopoda. Hyaline fungal hyphae remain unstained and clear (fh). These hyphae surround many of the large lipid bodies near the center of the page. Lipid body inclusions are clear in the hyaline hypha indicated with a black arrow on the lower right corner. Bar = 10um. Figure 1d. Endosperm cells of hydrated Sporobolus airoides seeds stained with trypan blue and sudan IV. A trypan blue stained fungal network (fn) with attached sudan IV stained lipid bodies (lb). Bar = 10 um.
In our studies of plants native to the Chihuahuan Desert we found extensive colonization by dark septate endophytes (DSE) (Figure 2e) (Barrow, 2003). DSE fungi are generally characterized by stained or melanized hyphae and microsclerotia (Jumpponen and Trappe, 1998), which were most prevalent in dormant plants (Figure 2a). Melanin, a natural dark pigment that makes DSE structures microscopically visible in plant tissues, was most prevalent in dormant plant tissues (mh). Lipid bodies associated with melanized hyphae in dormant plants were rare, but the abundance in physiologically active plants was remarkable (Figure 2b-d). Fungal endophytes were also detected as non-staining hyaline, clear, hyphae (Figure 1a, c, 2a, d) or with amorphous, trypan blue staining networks (Figure 1a, b, d). Lipid bodies were typically associated with all of these fungal structures.
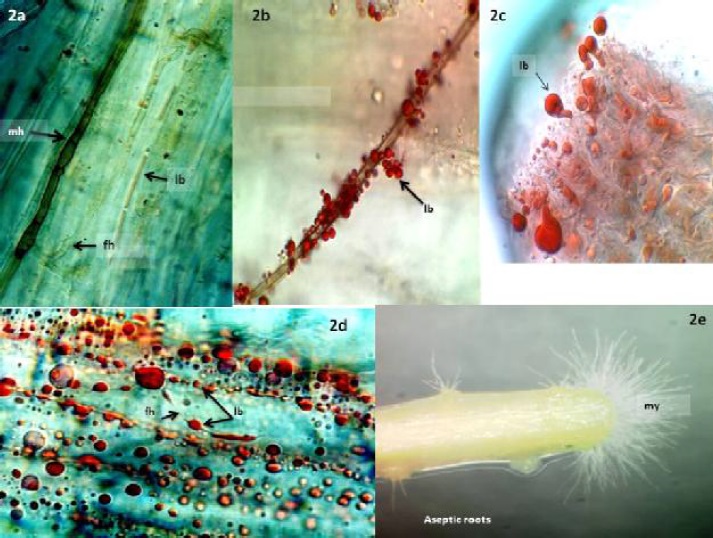
Figure 2a Trypan blue and sudan IV stain produce limited color on dormant B. eriopoda roots. Brown, melanized (mh) and fine hyaline hyphae (fh) are visible. A hyaline hypha near the center of the image contains weakly staining lipid bodies (lb) that are less developed than the lipid bodies seen during active growth stages. Figure 2b. A melanized hypha on the surface of B. eriopoda is covered with lipid bodies (lb). Figure 2c. B. eriopoda root tissue as shown in 2e, stained to reveal lipid body droplets (lb) emerging from the zone where mycelia are visible. Figure 2d. Dual stained, physiologically active roots of B. eriopoda exhibit abundant intercellular lipid bodies (lb) and fine hyaline hyphae. Figure 2e. Mycelia (my) emerging from aseptic roots of B. eriopoda aseptic roots are visible at the tip, below the root hair zone.
Analysis of leaf tissues revealed lipid bodies integrated with fungal structures in photosynthetic mesophyll and bundle sheath and cells of the stomatal complex of grasses and shrubs (Barrow, 2003; Barrow and Aaltonen, 2004). In both shrub and grass species, lipid bodies were most prevalent in the root meristems, cortex and phloem. In leaves, they were most conspicuously present in photosynthetic mesophyll, bundle sheath and cells of the stomatal complex. Color images in the above cited references vividly illustrate lipid bodies associated with hyphae and other structures of endophytic fungi in tissues of native plants.
Like Lersten et al. (2006) we found that lipid bodies were prominent features of photosynthetic bundle sheath and mesophyll cells in many plant species. However, while Lersten et al. (2006) found lipid bodies to be less frequent in monocots, we observed them to be abundant if sampled during periods of high physiological activity (Figure 1, 2b-c, d). Lipid bodies were rarely observed in dormant plants (Figure 2a). Unlike Lersten et al. (2006) we also observed that lipid bodies in our samples were tightly associated with endophytic fungi, which varied from finely branched networks (Figure 1a) to very dense amorphous looking structures (1c, 2b,c) (Barrow, 2003; Barrow and Aaltonen, 2004). We presume that the differences in observations are due to different research objectives (our group was primarily interested in distribution of endophytic fungi), differences in imaging (Lersten et al. utilized brightfield microscopy, while we utilized differential interference contrast optics to enhance clear structures), and due to our utilization of dual staining techniques. Nonetheless, we feel that highlighting this difference is important, particularly because recent interest in plant oils for biofuels, in addition to agricultural value, is climbing. Contributions of endophytic fungi are too often overlooked in plant physiology studies. Failure to acknowledge the presence of endophytes too often leads to risky assumptions that biosynthesis, regulation, and transport of lipids and other biomolecules found within the plant is exclusively carried out by plant cells.
Conclusions
- Plant lipid bodies serve as a carbon and energy reserve for important biological processes, and are valued economically for producing food oils and biofuels. Our observations indicate that plant lipid bodies are frequently, if not always associated with endophytic fungi. This is novel because it highlights a largely unexplored possibility that oils in plants are produced and managed, in full or in part, by endophytic fungi.
- We propose that the physical association between lipid bodies and endophytic fungi in diverse plant species reveals a possibility that endophytic fungi may be directly involved in biosynthesis, transport, or regulation of plant oils. Considering increased global interest in accelerating production of plant oils to meet 21st century food and fuel needs, researchers exploring oil production in plants would do well to expand assessments of endophytic fungi which can play a valuable role in managing this important carbon source. More studies are necessary to explore the interfaces where plant and endophyte exchange organic carbon. Furthermore using modern imaging, molecular and genomic analysis to assess plant interactions with associated endophyte communities could transform understanding of plant carbon metabolism.











 nueva página del texto (beta)
nueva página del texto (beta)


