Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Terra Latinoamericana
versión On-line ISSN 2395-8030versión impresa ISSN 0187-5779
Terra Latinoam vol.27 no.4 Chapingo oct./dic. 2009
División II
In vitro System to Determine the Role of Aspergillus ustus on Daucus carota Roots
Un sistema in vitro para determinar el papel del Aspergillus ustus en raices de Daucus carota
Pedro Osuna-Avila1* and Jerry Barrow2
1 Universidad Autónoma de Ciudad Juárez. Henry Dunant 4016. 32310 Cd. Juárez, Chih., México. *Autor responsable (osunapedro@hotmail.com).
2 USDA-Agricultural Research Service. Jornada Experimental Range P.O. Box 30003MSC-3JER 88003 Las Cruces, NM, USA.
Recibido: abril de 2009.
Aceptado: septiembre de 2009.
ABSTRACT
Fourwing saltbush (Atriplex canescens) (Pursh) Nutt, is naturally colonized by a dark septate fungus indentified as Aspergillus ustus. An in vitro culture system was developed to demonstrate a mutualism between A. ustus and fungus free Ri T-DNA transformed Daucus carota roots. The individual response of the fungus and the roots and the combined response of dual cultured fungus and roots were evaluated without phosphorus (WP), with plant available P (PAP) (KH2PO4) and plant unavailable P as either rock phosphate (RP) or tricalcium phosphate (TCP). Preliminary experiments showed that D. carota roots only responded to P as KH2PO4 and were not able to utilize either RP or TCP. A. ustus, however responded best to TCP, which is greater than PAP, which was greater than RP and did not grow WP. A dual culture system was developed using divided Petri dishes to separate carbon in a root chamber and P in the fungal chamber and a chemical barrier to restrict the fungus to the fungal chamber. The dual culture system demonstrated that A. ustus solubilized and transported P from both RP and TCP, enhanced the number and length of branches in D. carota roots within the carbon supplemented root chamber. When PAP was added to the fungus chamber, branching occurred mainly in the chamber with the available P. In addition to enhanced root growth and branching, it was also demonstrated that the fungus accessed carbon from the root. The dual culture system effectively demonstrated a classical mutualistic association between A. ustus and D. carota roots.
Keywords: fourwing saltbush, mutualistic association, phosphorus, carrot.
RESUMEN
El chamizo (Atriplex canescens) (Pursh) Nutt, es colonizado naturalmente por el hongo septado de color oscuro identificado como Aspergillus ustus. Un sistema in vitro se desarrolló para demostrar un mutualismo entre A. ustus y las raíces de zanahoria (Daucus carota) transformadas Ri-TADN libre de hongos. La respuesta individual del hongo y raíces y la respuesta combinada de la asociación del hongo cultivado con las raíces fueron evaluadas sin fósforo, WP, con P disponible para las plantas (PAP) (KH2PO4) y P no disponible para las plantas tales como roca fosfórica (RP) o fosfato tricálcico (TCP). Experimentos preliminares mostraron que las raíces de D. carota solo respondían al P como KH2PO4 y no pudieron utilizar la RP o el TCP. Sin embargo, A. ustus respondió mejor con TCP el cual fue mejor que PAP, y este fue mejor que RP y no respondió sin P. Un sistema dual de cultivo fue desarrollado usando cajas petri divididas para separar el carbono en una cámara de de raíz y P en la cámara del hongo y una barrera química para restringir al hongo a la cámara del hongo. El sistema dual de cultivo demostró que A. ustus solubilizó, transfirió y transportó P de ambos RP y TCP, aumentó el número y longitud de ramificaciones en raíces de D. carota dentro de la cámara de raíz suplementado con carbono. Cuando el P disponible se adicionó a la cámara del hongo, las ramificaciones ocurrieron mayormente en la cámara con el P disponible. Además, de aumentar el crecimiento y ramificaciones en la raíz, también se demostró que el hongo accedió al carbono de la raíz. El sistema dual de cultivo efectivamente demostró una clásica asociación mutualística entre A. ustus y las raíces de D. carota.
Palabras clave: chamizo, asociación mutualística, fósforo, zanahoria.
INTRODUCTION
As in other ecosystems, plants in arid environments have evolved symbiotic associations with fungi which enable them to survive under extreme drought conditions (Read, 1992, Gianinazi-Pearson, 1996). Research has focused on vesicular-arbuscular (VA) mycorrhiza under dry conditions, primarily because they are involved in P uptake in other ecosystems. In these studies Atriplex canescens, Bouteloua gracilis, and Sporobolus flexuosus were more commonly colonized by dark septate fungi than by typical mycorrhizae (Barrow et ah, 1997a). However, there is evidence that VA expression is more common in plants of mesic environments than in many desert plants (Saif et al., 1977). Thus, desert plants can contain fungal hyphae and vesicles but arbuscules are rare. This indicates that dry ecosystems could have a strong influence on the exchange of P and carbon between the host and VA fungi (Saif et al., 1977). Williams et al. (1974) suggested that alternative methods of nutrient exchange with the host, such as those used by other mycorrhizal fungi with better adapted fungal associations, can be functioning under desert conditions. Other symptomless, systemic, biotrophic fungal endophytes associated with native desert plants are being studied. The term endophytes includes all the organisms that live within plant tissues at some time of their life cycle, but do not induce visible signs of disease within plant tissue (Bacon et al., 1986). Rigorous surface-sterilized plant organs and a verification of their internal colonization, by histological studies, are considered evidence that these fungi are endophytic (Bills, 1996; Pelaez et al., 1998; Pereira et al., 1999). Two groups of endophytic fungi have been found: those present in grasses (Neotyphodium sp) which provide protection against herbivores to the host (Mortimer et al., 1984; Clay, 1990; Schardl and Huei, 1992; Belesky and Milinowski, 2000), and those organisms that increase mineral uptake in shrubs such as Atriplex sp and cotton plants (Alternaria alternata and Cladorrinum foecundissimum), respectively (Barrow et al., 1997b; Gasoni and Stegman, 1997). Studies by Barrow et al. (1997a) show that grasses and shrubs in arid rangelands of the southwestern part of the United States are more extensively colonized by nonpathogenic fungi that are designated as melanized, dark septate endophytic (DSE) fungi than by VA mycorhiza. DSE fungal-root associations are common in all major ecosystems and are characterized by inter- and intracellular hyphae, and microsclerotia (Jumpponen and Trappe, 1998). Sometimes the hyphae formed a loose Hartig net-like structure similar to those found on ectendomycorrhizae (Ahlich and Sieber, 1996). These fungi produce protective polysaccharide sheaths on their hyphal surfaces that form a saturated matrix allowing them to remain active in dry soil (Barrow, 2003). Over 95% of native and crop plants are colonized by mycorrhizae fungi, except crucifers and some family Chenopodiaceae species. Atriplex spp. members of the family Chenopodiaceae are generally not considered to be mycorrhizal, because classical mycorrhizal fungi do not form typical mycorrhizal structures within the root (Hierrel et al., 1978). However, an analysis of Atriplex canescens, a palatable southwestern shrub, revealed atypical hyaline structures that non-destructively colonize the vascular cylinder, cortex and epidermis and also form melanized septate hyphae and microsclorotia, characteristic of DSE fungi (Barrow et al., 1997a). These fungi are similar to classical mycorrhizal fungi in several ways, but function is still unknown. They non-destructively colonize the root cortex of the host. The internal colonization is characterized by extensive inter and intracellular hyphae; coils and vesicles are also seen (Barrow and Aaltonen, 2001). As the roots age and decrease in physiological activity the incidence of microsclerotia increases. These DSE fungi are apparently from a wide assemblage of fungal species and can be either parasitic or symbiotic (Clay, 1990; Jumpponen and Trappe, 1998). Barrow et al. (1997b) found that the radicles of germinating fourwing saltbush seedlings, Atriplex canescens on sterile silica sand were immediately colonized by Alternaria alternata, which digested the seed capsule and transferred nutrients to the seedling enhancing vigor and growth of the seedling (Barrow and Osuna, 2002). In further unpublished studies by Barrow, other seed borne fungi, identified as Aspergillus ustus, also formed similar nondestructive interfaces with the roots. Extraradicle hyphae penetrated a root excluding screen, solubilized P from tricalcium and rock phosphate, and transported soluble P back to the roots subsequently enhancing both root and shoot biomass. The fungus also increased P use efficiency because plants treated with insoluble P had equivalent shoot biomass and greater root biomass as plants receiving sufficient soluble P for normal growth. In these experiments, extensive extra-radicle hyphae grew into the soil. This resulted in extensive aggregation of soil near the root zone (Barrow, 2003). Saprophytic fungi, like to those observed by Barrow et al. (1997b), have extensive symptomless, endophytic phases in plants that are overlooked by casual observation (Perry et al., 1989). Others studies, by Odell et al. (1993) and Gasoni and Stegman (1997), state that endophyte fungi might increase uptake of minerals by plants in the same manner as mycorrhizal fungi, and they proposed an ecological significance for the relationship. Although much work has been done in VA fungi because of its well-known role in P uptake in other ecosystems, more studies need to be conducted on other endophytic fungi to ascertain their function in relationship with Atriplex canescens. The ability to solubilize recalcitrant phosphates is widespread among fungi and other microorganisms. Among rhizosphere inhabiting fungi, Aspergillus spp. is the best P solubilizers in arid soils (Cunningham and Kuiack, 1992). Aspergillus and Penicilllium, similar to VA fungi, produce acidic metabolites which increase the dissolution ability of inorganic P from tri-basic calcium phosphate (TCP) and rock phosphate (RP) (Tarafdar and Rao, 1996; Ritz, 1995).
Ri T-DNA transformed root have a great growth potential, tolerate changes in medium composition, high growth rate of roots culture, and great consistency in the rate of apical dominance in roots (Becard and Fortin, 1988). The distinctive phenotype of transformed roots, are capable of autonomous hormone growth and appears to be controlled by the T-DNA linked genes of A. rhizogenesis (White et al., 1995). These transformed roots can be used for continuous axenic root growth system in tissue culture. In vitro methods using sterile D. carota roots were previously developed (Pfeffer et al., 1999, Bago et al., 2000) to study carbon transfer and metabolism between roots and vesicular-arbuscular mycorrhizae. An endophytic fungus was isolated from A. canescens roots in a P uptake study and was identified as Aspergillus ustus by Dr Marin Klich, USDA-REE-ARS-MA-SSRC-F&FAR. The general objective of this study was to develop in vitro experimental protocol to separate and identify individual contributions of symbiotic partner's A. ustus, a DSE fungus isolated from Atriplex canescens roots and sterile Ri T-DNA D. carota roots.
MATERIALS AND METHODS
Daucus carota roots were maintained on Vm culture medium (modified white medium). This medium was prepared with basic salts (except KH2P04 and NaH2P04H20) and organic substances 30 g L-1 sucrose and the pH was adjusted to 5.5. medium was solidified with 8 g L-1 Phytagel and autoclaved 15 min at 121 °C. The medium was poured into 150 x 15 mm the petri dishes. Segments 10 mm long were used for single petri plate experiments and segments 50 mm long were dissected for experiments using divided petri dishes. A dark septate fungal endophyte (Aspergillus ustus) which apparently enhanced P uptake from plant unavailable RP and TCP was isolated from aseptic Atriplex canescens root seedlings at the Jornada Experimental Range in Las Cruces, New Mexico. Fungal stock cultures were also maintained on Vm medium so both roots and fungi had the same nutritional environment. Mycelium taken from the growing edges of the cultures was used for inoculum. In order to administer P treatments in petri dishes with solid agar medium, it was necessary to construct disks to contain insoluble rock phosphate (RP) or tricalcium phosphate (TCP) to serve as a platform for growing the fungus in petri dishes with solid medium. The disks were constructed by cutting 4 mm rings of 20 mm PVC tubing. A piece of 10 urn polyester screens was glued to the bottom of the ring. The disk was then packed with either RP or TCP. The material was sealed within the disks by gluing a second 10 um polyester screen to the top of the ring. The 10 um pores diameter screen allowed passage of the fungus but restricted roots from entering the disk.
Development of dual culture
To establish and demonstrate a symbiotic association it was necessary to separate root and fungal responses in a divided petri dish (dual culture). For dual culture experiments, M medium pH adjusted to 5.5 and supplemented with carbon (30 g L-1 ultrapure sucrose) and all essential nutrients except P were added to the root compartment. The M medium lacking P and sucrose was added to the root compartment (this was the WP treatment). For the plant unavailable treatments, RP and TCP were added in disks as described above. The pH was adjusted to 7 in the fungal chamber to prevent solubilization of RP and TCP. The M medium in both compartments was gelled with 4 g L-1 agarose. Daucus carota root segments were placed with the basal portion in the root compartment and the apex in the fungus compartment adjacent to the disks.
Effect of plant preservative mixture and benomilo to restrict A. ustus growth to the fungal compartment
The experiment was configured using divided petri-dishes, with M medium supplied to the root and fungus compartments as described above. A TCP filled disk (4 x 20 mm) was added to the fungus compartment. A 5 mm section of M medium agar was removed from the fungus compartment adjacent to the entire length of the barrier. An additional fungicide barrier was added to this strip. Two fungicides were tested, benomilo and Plant Preservative Mixture (PPM) a plant preservative compound developed by Plant Cell Technology to protect plant tissue cultures from fungal invasion. These materials were prepared by prepadding agarose agar (4 g L-1) with 50 mg L-1 benomilo or with 2 mL L-1 PPM. The center of the TCP disks were inoculated using mycelial fragments of A. ustus. After the fungus grew into the disks and extended 3 to 4 mm into the culture medium, fungicide medium was poured into the 5 mm strips. Three D. carota roots segments were then placed with the apex adjacent to the mycelia. Three treatments with benomilo or with PPM or without fungicide were each replicated 10 times. One way analysis of variance was performed and mean differences was tested using Tukey's test.
Potential of A. ustus to solubilize rock phosphate or tricalcium phosphate and enhance D. carota root growth
A 4 x 20 mm disk filled with RP was added to the fungus compartment. Two D. carota roots were arranged in each dish and the treatments consisted of inoculated (I) and non-inoculated (NI) roots. The center of the RP or TCP ring was inoculated with mycelium of A. ustus. An additional treatment was done for TCP as control: non-divided petri dishes were prepared with M medium, minus sucrose (-S), minus P (-P), solidified with 4 g L-1 agarose with pH adjusted to 5.5. Treatments were replicated 14 times. After 30 days, the number and length of root branches and the diameter of the fungal colony were measured in mm and the number of new root branches was recorded. Data were analyzed using a one way analysis of variance and Tukey's test.
Bi-directional transfer of carbon and P between D. carota root and A. ustus
Petri-dishes and medium were prepared with media and benomilo strip as described above for dual culture of D. carota roots and A. ustus. A set of control plates was prepared without sucrose in the root compartment. Four treatments were tested: 1, TCP inoculated (I) in the fungal compartment; 2, TCP not inoculated (NI) in the fungal compartment; 3, no P and no inoculation in the fungal compartment; 4, sucrose was omitted in the root compartment, P was omitted in the fungal compartment and the fungal compartment was not inoculated.
Three D. carota root segments were placed in each dish. In the Treatment 1, the center of the TCP ring was inoculated with mycelium of A. ustus. After 14 days of culture the number of branched roots, the length of branches and diameter of fungal colonies were measured in mm. Data were analyzed using a one way analysis of variance. Treatment means were separated using Tukey's test.
RESULTS AND DISCUSSION
Ability of plant preservative mixture or benomilo to restrict A. ustus growth to the fungal compartment
A. ustus responded differently to the fungicide barriers. During the first week of culture the fungus grew uniformly around the TCP rings. After 13 days the fungicide barriers were poured into the strips of removed M agar. After 26 days, there were no significant differences in the diameter of fungal colonies, PPM (x = 32 mm), benomilo (x = 33 mm) or without fungicides (x = 33.5mm). However, after 26 days the density of fungal colonies was drastically affected. In the PPM treatment, the colonies became very diffuse and were invisible to the naked eye. With the stereo microscope hyphae were seen growing along the root crossing both the physical and chemical barrier into the root compartment. Hyphae also crossed over both barriers without the root. Once the fungus invaded the root compartment having access to abundant carbon, it over grew and killed the root. Sporulation was also noted in the diffuse mycellia of the PPM treatment. The abundant growth of the fungus in the root compartment indicated the transport of P through the fungal hyphae. In contrast, the fungus was completely restricted to the fungus compartment in the benomilo treatments sufficiently long to allow demonstration of a mutualistic association. An interesting observation was made; after the TCP ring was inoculated the fungus initially radiated 360°. Once the roots were placed near the fungus, the mycelium grew more rapidly and densely toward the root suggesting a chemical attraction between the root and fungus. A remarkable observation was observed in a secondary root tip became swollen as it made contact with the fungus indicating colonization (Figure 2a, 2b).
The diffusion of PPM into the medium of the fungus chamber more severely affected fungal growth than the benomilo, but did not restrict the fungus from entering the root chamber. In the non-fungicide control, A. ustus crossed the divider and as in the PPM treatment it over grew the medium and root of the root chamber, killing the root. The benomilo strip sufficiently restricted the fungus for sufficient time to allow the mutualistic association to between A. ustus and D. carota roots. The effectiveness of benomilo over PPM could be explained because it is a systemic fungicide that is taken up and translocated by the plant cells (Pederson and Sylvia, 1997). It has also been used effectively and extensively to obtain soil free of arbuscular mycorrhizal fungal activity for greenhouse investigations (Habte, 1997). PPM is a strong biocide against microbial airborne, waterborne and endogenous contamination in plant tissue culture (Guri and Patel, 1998).
Potential of A. ustus to solubilize rock phosphate or tricalcic phosphate and enhance D. carota root growth
For rock phosphate treatments, the mean increase in the number (6.2) and length (109.0 mm) of branches in the inoculated RP treatment, after 30 days, of culture was significantly greater than the non-inoculated treatment (Table 1). This response indicates that A. ustus solublized RP and transferred P to the root resulting in increased branching and growth primarily in the root compartment where P was absent. The initial fungal colonization was very diffuse with low visibility. After roots were placed, the fungal colony increased in density and had a mean diameter of 15.8 mm at the end of the experiment. This indicates that the fungus utilized carbon from the root and which was transferred from the root compartment to the fungus through the root. A few tertiary branches were observed only in the inoculated roots.

Regarding to tricalcic phosthate, the mean number and length of branches, 11.0 and 205.3 mm respectively, of inoculated roots was significantly greater than those of non-inoculated roots, 1.6 and 15.5 mm respectively. Most branching occurred in the root compartment where P was absent. The main non-colonized roots continued to elongate (39.0 mm), whereas the main inoculated roots did not (Table 2). The elongation of non-inoculated roots was attributed to cell elongation and not cell division. Once the main roots were inoculated they ceased to elongate, but substantial branching and lateral root growth occurred toward the basal portion of the root. This response indicated that A. ustus initiated root branching and growth, and supports the hypothesis that A. ustus enhances the solubilization and uptake of P from TCP.
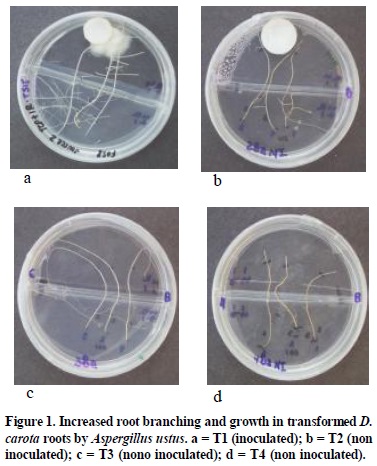
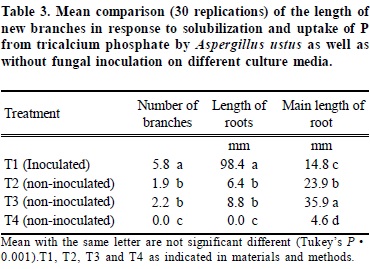
In the treatments where TCP disks were inoculated without roots, the fungus spread radially from the inoculation point and after 30 days it could not be observed. In contrast, the fungal colony adjacent to the TCP disk in inoculated roots increased in density and had a mean of diameter of 24.0 mm after 30 days. Because there was no carbon in the fungal compartment, A. ustus utilized carbon from the root which it mobilized from the root compartment to the fungus by the root. Both inoculated roots and fungi had a healthy white appearance. This experiment demonstrates a mutual growth dependency and a positive relationship between A. ustus and D. carota roots. Significant increases in root branching and length were observed in inoculated D. carota roots T1(98.4 mm) compared to controls T2 (6.4 mm) T3 (8.8 mm) and T4 (0.0 mm) (Figure 1) (Table 3). Roots colonized by fungi were thicker than non- colonized regions (Figures 2a, b), while roots in T2 and T3 were thinner and meristems desiccated as they grew aerially away from the agar. In the T4 only the meristem (x = 6 mm) remained active and the nonmeristem region turned brown. On the TCP side of the petri dish the non-inoculated roots, T3 were longer (x = 35.9mm) than in the T2 (x = 23.9 mm), T1 (x = 14.8 mm) and T4 (x = 4.6 mm). Dark septate fungal endophytes are a widely recognized group of undefined fungi that colonize many plant species whose function is still unknown. Their study and importance have been minimized because they do not conform to conventional mycorrhizal morphology (Jumpponen, 2001; Trappe, 1981; Barrow, 2003) therefore they are sometimes considered as pathogenic fungi by some observers. This study revealed that A. ustus, previously considered to be an exclusive resident of the rhizosphere also non-destructively colonized the root and supplied P in a fashion similar to mycorrhizal fungi. This was verified by transparent septate hyphae observed in the cortex of the D. carota roots which were similar to those observed in native A. canescens (Barrow, 2003).
Bi-directional transfer of carbon and P between D. carota root and A. ustus
Lateral root induction length was greatest in the sucrose supplemented compartment. The inoculated D. carota roots (T1) induced a greater number of branches and length of branches than the T2, T3 and T4 respectively (Figure 1) (Table 3). In T1, only the more distal of the roots were colonized (x = 14.8 mm). The colonized portions of the roots remained healthy and the mycelium stayed thick and white in color. However, the control roots stayed very thin without showing the swelling distal portion (Figure 2c) as was observed in the colonized roots (Figure 2a, b). These results support the hypothesis that A. ustus colonized the D. carota and promoted branching and increased the length of branches as a result solubilization and uptake of P from insoluble TCP. It was presumed that P was transferred to the roots via non-destructive hyaline hyphal interfaces with the roots and A. ustus had greater density than hyphae without root contact, indicating it utilized carbon supplied by the root (Figure 2). Such a response is similar to mycorrhizal fungi where they solubilize and transfer P to the host in exchange for carbon.
These results indicate a mutualistic association between A. ustus and D. carota roots which A. ustus colonizes root tissue and render the host more competitive and better able to survive over the barrier of the divided petri dishes. On T1, the main roots were thicker along the root, healthy and enhanced yield of root biomass which means P translocation compares with the rest of the treatments where the P was limited. The increased root biomass in the inoculated TCP treatments compare with the control indicated that A. ustus is one of the best P solubilizers among the rizhosphere inhabiting fungi of Atriplex canescens in arid ecosystems. This fungus penetrated the pore of the screen and had the ability to dissolve inorganic P from TCP and transferred to the D. carota root which promoted the extensive branched roots (Figure 1 and 2).
Microscopic analysis showed that the physiologically active D. carota roots on T1 were internally colonized by A. ustus. The atypical fungal structures like protoplasts, without visible wall or hyaline hyphae made it difficult to quantify their presence. However, the greater extra-radicle production and the internal fungal structures of D. carota root suggest that A. ustus non-destructively colonized the root cortex (Figure 2e) and transported soluble P to the D. carota roots. The great performance of A. ustus on TCP and root association indicates its natural range in no-host D. carota roots and potential as endophytes of solubilizing Ca-P compounds common to arid zones. The dual culture experiments also determined the biotrophic nature of the fungus, in that the fungal colony increased in density only in the presence of roots, when no carbon was available in the fungus compartment. These responses may be defined as an interspecific interaction involving net mutual benefits where members of two species experience higher fitness when they occur together than they occur alone, which means mutualism (Bronstein, 1998). It is also consistent with mycorrhyzal fungi where the plant exports carbon to the fungus and the fungus supplies P to the host (Ezawa et al., 2000).
The complex cellular relationship between roots and fungus for morphological integration and reciprocal functional compatibility depend on finely tuned recognition process between the two partners. The tendency of A. ustus hyphae goes toward root or roots go toward the fungal colonization could be a signal to recognize each other for nutrient exchange. Signal exchange and recognition between host plants and VA fungi initiate before they come into physiological contact (Gianinazzi-Pearson, 1996). These exchanges affect the regulation of genes whose products participates in the metabolic and structural changes leading to the symbiosis (Gianinazzi et al., 1995). Signal or receptor molecules involved in this dialogue could be metabolites exuded by host roots which specifically enhance fungal growth, as compared with root exudates from non-host plants which do not exert this effect (Dannenberg et al., 1992; Gianinazzi-Pearson, 1996; Kennedy, 1998). The distal portion of the inoculated D. carota roots with A. ustus presented a swelling area and as compared with non-inoculated roots which did not exert this phenomena. The main non-colonized root continued to elongate, whereas the main inoculated roots did not. VA mycorrhiza induces morphogenetic modifications in the root system of the host plant which has a significance of functional changes, as related to nutrient absorbing strategies (Berta et al., 1993). For example in ectomycorrhizae, the root tip morphology is profoundly modified and root growth slowed, compared to uninfected roots, apical meristem and caps are smaller. Also, modifications in root system architecture were observed in Ericoid and vesicular mycorrhizae (Berta et al., 1988; Schellenbaum et al., 1991) and root branching in inoculated Bouteloua eriopoda by fungal endophytes (Lucero et al., 2006, 2008). Because A. ustus improved the fitness of the D. carota roots and although the carbohydrate flow was not observed directly for A. ustus, it can be inferred that the fungal partner had a biotrophic behavior. A. ustus asymptomatically colonized an atypical host D. carota roots it means that it does not have narrow host preference and is similar to other mycorrhizae in native grasses and shrubs; therefore we assumed that A. ustus could be participating in the nutrition and survival of Atriplex canescens in the desert rangelands.
CONCLUSIONS
- Success of an in vitro dual culture system depended upon meeting several requirements. Both roots and fungi must be precultured to reduce P tissue concentrations. It was necessary to separate carbon available only to the root and recalcitrant P sources available only to the fungus. Residual P must be eliminated by using P free agarose agar. The fungus was restricted to the fungus compartment by the physical plastic barrier and a fungicide strip. Dancus carota roots grew well on plant available P (KH2 P04) but did not respond to either rock phosphate (RP) or tricalcium phosphate (TCP).
- Aspergillus ustus grew best on TCP>P>RP and no response was obtained in the absence of P. D. carota roots were independently able to utilize P from KH2P04 and required colonization and association with A. ustus to solubilize and transport P for enhanced root branching and growth. Morphological differences were observed in non-inoculated D. carota roots when carbon and P were separated compared to inoculated roots subjected to RP and TCP. In the case of plant available P root branching occurred in the P (fungus) compartment, whereas in inoculated roots, branching occurred in the carbon compartment. The inorganic P solubilization by A. ustus decreased the pH from 7.0 to 6.5 and increased it from 5.5 to 6.5 in the culture medium. This suggests that Aspergillus ustus uses a different mechanism, other than organic acid production which acidifies the medium for P solubilization.
- These results demonstrated a mutualistic association between A. ustus and D. carota roots, in that, the fungus enhanced root branching and growth of roots while the fungus increased biomass and activity in the presence of the roots.
LITERATURE CITED
Ahlich, K. and T. N. Sieber. 1996. The profusion of dark septate endophytic fungi in non ectomycorrhyzal fine roots of forest trees and shrubs. New Phytol. 132: 259-270. [ Links ]
Bacon, C. W., P. C. Lyons, J. K. Porter, and J. D. Robbins. 1986. Ergot toxicity from endophyte-infected grasses: a review. Agron. J. 78: 106-116. [ Links ]
Bago, B., P. E. Pfeffer, D. D. Douds, J. Brouillete, G. Becard, and Y Sachar-Hill. 1999. Carbon metabolism in spores of the arbuscular mycorrhizal fungus Glomus intraradices as revealed by NMR spectroscopy. Plant Physiol. 121: 263-271. [ Links ]
Bago, B., P. E. Pfeffer, and Y. Sachar-Hill. 2000. Carbon metabolism and transport in arbuscular mycorrhizas. Plant Physiol. 124: 949-958. [ Links ]
Barrow, J. R., K. M. Havstad, J. Hubstenberger, and B. D. McCaslin. 1997a. Fungal root endophytes in fourwing saltbush, Atriplex canescens, on arid rangelands of Southwestern USA. Arid Soil Res. Rehab. 11: 177-185. [ Links ]
Barrow, J. R., K. M. Havstad, J. Hubstenberger, and B. D. McCaslin. 1997b. Seed borne fungal endophytes on fourwing saltbush, Atriplex canescens. Arid Land Res. Manage. 11: 307-314. [ Links ]
Barrow, J. R. and R. E. Aaltonen. 2001. Evaluation of the internal colonization of Atriplex canescens (Pursh) Nutt. roots by dark septate fungi and the influence of host physiological activity. Mycorrhiza 11: 199-205. [ Links ]
Barrow, J. R. and P. Osuna. 2002 Phosphorus solubilization and uptake by dark septate fungi in fourwing saltbush, Atriplex canescens (Pursh) Nutt. J. Arid Environ. 51: 449-459. [ Links ]
Barrow, J. R. 2003. Atypical morphology of dark septate fungal root endophytes of Bouteloua in arid southwestern USA rangelands. Mycorrhiza 13: 239-247. [ Links ]
Becard, G. and J. A. Fortin. 1988. Early events of vesicular-arbuscular mycorrhiza formation on RiT-DNA transformed roots. New Physiol. 108: 211-218. [ Links ]
Belesky, D. P. and D. P. Malinoski. 2000. Abiotic stresses and morphological plasticity and chemical adaptations of Neotyphodium-infected tall fescue plants. pp. 455-484. In: C. W. Bacon and J. F. White (ed.). Mycrobial Endophytes. Marcel Dekker, New York, NY, USA. [ Links ]
Berta, G., V. Gianinazzi-Pearson, G. Gay, and G. Torri. 1988. Morphogenetic effects of endomycorrhyza formation on root system of Calluna vulgaris (L.) Hull. Symbiosis 5: 33-34. [ Links ]
Berta, G., A. Fusconi, and A. Trotta. 1993. VA mycorrhyzal infection and the morphology and function of root systems. Environ. Exper. Bot. 33: 159-173. [ Links ]
Bills, G. F. 1996. Isolation and analysis of endophytic fungal communities from woody plants. pp. 31-65. In: S. C. Redlin and L. M. Carris (eds.). Endophytic fungi in grasses and woody plants. APS Press. Saint Paul, MN, USA. [ Links ]
Bronstein, J. L. 1998. The contribution of ant-plant protection studies to our understanding of mutualism. Biotropica 30: 150-161. [ Links ]
Campbell, N. A. 1993. Biology. Benjamin/Cummings Publishing. Redwood City, CA, USA. [ Links ]
Clay, K. 1990. Fungal endophytes of grasses. Annu. Rev. Ecol. Syst. 21: 275-97. [ Links ]
Cunningham, J. E. and C. Kuiack. 1992. Production of citric and oxalic acids and solubilization of calcium phosphate by Penicillium bilaii. Appl. Environ. Microbiol. 58: 1451-1458. [ Links ]
Chriqui, D., A. Guivarc'h, W. Dewitte, E. Prinsen, and H. Van Onkelen. 1996. Rol genes and root initiation and development. Plant Soil. 187: 47-55. [ Links ]
Dannenberg, G, C. Lotus, W. Zimmer, B. Hundeshagen, H. J. Schneider-Poetsch, and H. Bothe. 1992. Influence of vesicular-arbuscular mycorrhiza on phytohormone balances in maize (Zea mays L.). J. Plant Physiol. 141: 33-39. [ Links ]
Ezawa, T., S. E. Smith, and F. A. Smith. 2000. Differentiation of polyphosphate metabolism between the extra-and intraradical hyphae of arbuscular mycorrhizal fungi. New Phytologist 149: 555-563. [ Links ]
Gasoni, L. and B. Stegman. 1997. The endophite Cladorrhinum foecundissimum in cotton roots: phosphorus uptake and shoot growth. Mycol. Res. 101: 867-870. [ Links ]
Gianinazzi-Pearson, V. 1996. Plant cell responses to arbuscular mycorrhizal fungi: getting to the roots of the symbiosis. Plant Cell 8: 1871-1883. [ Links ]
Gianinazzi-Pearson, V,. A. Gollotte, J. Lherminier, B. Tisserant, P. Franken, E. Dumas-Gaudot, M. C. Lemoine, D. Van Tuinen, and S. Gianinazzi. 1995. Cellular and molecular approaches in the characterization of symbiotic events in functional arbuscular mycorrhizal associations. Can. J. Bot. 73: 526-532. [ Links ]
Guri, A. Z. and K. N. Patel.1998. Compositions and methods to prevent microbial contamination of plant tissue culture media. US Patent 5750402. [ Links ]
Habte, M. 1997. Use of benlate to obtain soil free of arbuscular mycorrhizal fungal activity for greenhouse investigations. Arid Land Res. Rehab. 11: 151-161. [ Links ]
Hierrel, M. C., H. Mehravaran, and J. W. Gerdemann. 1978. Vesicular-arbuscular mycorrhizae in the Chenopodiaceae, Cruciferae,: Do they occur. Can. J. Bot. 56: 2813-2817. [ Links ]
Jumpponen, A. and J. M. Trappe. 1998. Dark septate endophytes; a review of facultative biotrophyc root-colonizing fungi. New Phytol. 140: 295-310. [ Links ]
Jumpponen, A. 2001. Dark septate endophytes-are they mycorrhizal. Mycorrhiza 11: 207-211. [ Links ]
Kennedy, A. 1998. The rhizosphere and spermosphere. pp. 389-407. In: D. M. Sylvia, J. F. Fuhrmann, P. G. Hartel, and D. A. Zuberer (eds.). Principles and applications of soil microbiology. Prentice-Hall. Upper Saddle River, NJ, USA. [ Links ]
Lucero, M. E., J. R. Barrow, P. Osuna, and I. Reyes. 2006. Plant fungal interactions in arid and semi-arid ecosystems: Large-scale impacts from microscale process. J. Arid Environ. 65: 276-284. [ Links ]
Lucero, M. E., J. R. Barrow, P. Osuna, I. Reyes, and S. E. Ducke. 2008. Enhancing native grass productivity by co-cultivating with endophyte-laden calli. Rangeland Ecol. Manage. 61: 124-130. [ Links ]
Mortimer, P. H., P. W. Young, and M. E. Di Menna. 1984. Perennial ryegrass research - an overview. Ruakura Animal Research Station, Mynistry of Agriculture and Fishers, Hamilton. Procc. N. Z. Soc. Anim. Prod. 44: 181-184. [ Links ]
O'dell, T., H. B. Massicotte, and J. M. Trappe. 1993. Root colonization of Lupinus latifolius Agardh. and Pinus contorta Dougl. New Pythol. 124: 93-100. [ Links ]
Pedersen, C. T. and D. M. Sylvia. 1997. Limitations to using benomyl in evaluating mycorrhizal functioning. Biol. Fertil. Soils 25: 163-168. [ Links ]
Pelaez, F., J. Collado, F. Arenal, A. Basilio, A. Cabello, M. T. Diez-Matas, J. B., García, A. González del Val, V. González, J. Gorrochategui, P. Hernández, I. Martín, G. Platas, and F. Vicente. 1998. Endophytic fungi from plants living on gossypium soils as a source of secondary metabolites with antifungal activity. Mycol. Res. 102: 755-761. [ Links ]
Pereira, J. O., M. L. Carneiro-Vieira, and J. L. Azevedo. 1999. Endophytic fungi from Musa acuminata and their reintroduction into axenic plants. World J. Microbiol. Biotechnol. 15: 37-40. [ Links ]
Perry, D. A., M. P. Amaranthus, J. G. Borchers, S. L. Borchers, and R. E. Brainerd. 1989. Bootstrapping in ecosystems. Bioscience 39: 230-237. [ Links ]
Pfeffer, P. E., D. D. Douds, G. Becard, and Y. Sachar-Hill. 1999. Carbon uptake and the metabolism and transport of lipids in an arbuscular mycorrhiza. Plant Physiol. 120: 587-598. [ Links ]
Read, D. J. 1992. The mycorrhizal mycelium. pp. 102-133. In: M. F., Allen (ed.). Mycorrhizal Functioning. Champan and Hall. New York, NY, USA. [ Links ]
Ritz, K. 1995. Growth responses of some soil fungi to spatially heterogeneous nutrients. Microbiol. Ecol. 16: 269-279. [ Links ]
Saif, S. R., I. Ali, and A. Zaidi. 1977. Vesicular-arbuscular mycorrhizae in plants and endogonaceous spores in the soil of northen areas of Pakistan. III. Dir and Chitral. Pakistan J. Bot. 9: 129-148. [ Links ]
Schardl, C. L. and F. T. Huei. 1992. Molecular biology and evolution of the grass endophytes. Nat. Toxins 1: 171-184. [ Links ]
Schellenbaum, L., G. Berta, F. Ravolanirina, B., Tisserant, S., Gianinazzi, and A. H., Fitter. 1991. Influence of endomycorrhyzal infection on root morphology in a micropropagated woody plant species (Vitis vinifera L.) Ann. Bot. 67: 135-141. [ Links ]
Tarafdar, J. C. and A. V. Rao. 1996. Contribution of Aspergillus strains to acquisition of phosphorus by wheat (Triticum aestivum L.) and chick pea (Cicer arietinum Linn) grown in a loamy sand soil. Appl. Soil Ecol. 3: 109-114. [ Links ]
Trappe, J. M. 1981. Mycorrhizae and productivity of arid and semiarid rangelands. pp. 581-593. In: J. T. Manassa and E. J. Briskey (eds.). Advanced in food-producing systems for arid and semiarid lands part A. New York, NY, USA. [ Links ]
White, J. F., B. H. Taylor, G. A. Huffman, M. P. Gordon, and E. W. Nester. 1995. Molecular and genetic analysis of the transferred DNA regions of the root inducing plasmid of Agrobacterium rhizogenesis. J. Bacteriol. 166: 33-44. [ Links ]
Williams, S. E., A. G. Wollum, and E. F. Aldon. 1974. Growth of Atriplex canescens (Pursh) Nutt. improved by formation of vesicular-arbuscular mycorrhizae. Soil Sci. Soc. Am. Proc. 38: 962-965. [ Links ]














