INTRODUCTION
Fungi are not only important to human society; they also develop close relationships with other organisms (Moreira-Arana et al., 2007). In this sense, mutualistic associations benefit both organisms in such a way that they are usually indispensable or necessary for their survival (Herrera and Ulloa 1998). Therefore, many fungi with symbiotic relationships with plants, algae, cyanobacteria and animals have been reported (Campbell and Reece, 2007). The most widespread and well-characterized symbiotic associations of fungi are with algae in lichens symbiosis) and with vascular plants in mycorrhizae. In regard to the symbiosis or mutualistic relations with animals it can be mentioned anaerobic fungi of the phylum Chytridiomycota in the rumen of herbivores (Ho and Barr, 1995), truffles with rodents (Frank et al., 2006) and associations of fungi with insects from orders Coleoptera, Homoptera, Hymenoptera and Isoptera. The latter associations could be explained by the fact that many insects and fungi share their habitat (Herrera and Ulloa, 1998). A good example of this kind of relationship is the one between the Basidiomycete fungus of the Agaricaceae family, Leucoagaricus gongylophorus (A. Møller) Singer and leaf-cutter ants of the genus Atta (Silva-Pinhati et al., 2004). This association is crucial for both organisms’ life cycles, due to: (a) the fact that L. gongylophorus lost the ability to produce sexual spores (Stevens, 1983), and it had been probably asexually cultivated by ants for over than 23 million years (Chapela et al., 1994); (b) without L. gongylophorus as a food source of the colony, the leaf-cutter ants will die (Fisher et al., 1996). However, the sexual structures of L. gongylophorus were also reported (Fisher et al., 1994b; Pagnocca et al., 2001; Mueller et al., 2001), indicating on sexual reproduction possibility (Doherty et al., 2003).
The understanding of this mutual association between the fungus and the ant is not clear mainly due to the lack of taxonomic information and the evolutionary history of the cultivated mushrooms species (Currie, 2001). The main difficulty has been related with reluctance of the fungus L. gongylophorus to produce basidiomes, which are structures required for conventional taxonomic identification (Loeck et al., 2004). The ants species belong exclusively to Attini tribes (Myrmicinae), which cultivate fungi in their nests and their diet is almost completely dependent on mycelia (Herrera-Salazar, 2009). The cultivated fungus cannot survive without the ants caring the fungal garden, and reciprocally the ants need the fungus to survive (Van-Bael et al., 2011). This ancient mutualism is based on the hygienic behavior of the ants that protects the fungal garden from bacteria and mycoparasites, by the application of antimicrobial substances secreted by them, which eliminates among 75 and 90 percent of the microorganisms (Fernández-Marín and Wcislo, 2010). Due to that there are no reports about the in vitro isolation of the fungus cultivated by Atta mexicana, this work is focused in the in vitro isolation and identification of Leucoagaricus gongylophorus from a fungal garden of Atta mexicana.
MATERIALS AND METHODS
Biological material
The fungal garden of A. mexicana ant was prepared at Instituto de Ecología, A.C. of Mexico (INECOL). The ant and the fungus were kept in artificial terrarium (nests), adapted to their survival and reproduction conditions at temperature of 25 ± 2 °C with white light, except for the fungal garden area that was kept in darkness. Three of twelve nests were randomly chosen, and were used as a source for the fungus. Mycelium fragments and some ants were taken from each of the selected nests and transferred to previously sterilized jars for their transportation to the Laboratorio de Alta Tecnología de Xalapa (LATEX) where the isolation and purification of Leucoagaricus gongylophorus was carried out.
Culture medium
For in vitro isolation of Leucoagaricus gongylophorus from the fungal garden of A. mexicana, first, the growth was induced in a moisture plate and then subsequently on malt extract agar (MEA, DIBICO) and potato dextrose agar (PDA, DIBICO) medium. From three fungal gardens, small fragments of the mycelium were taken and disinfected with 4% sodium hypochlorite solution and 70% ethanol for 3 minutes. Each disinfected fragment was inoculated in moisture plates as well as in MEA and PDA in 5 replicas and then inoculated plates were incubated at 25 ± 2 °C for 7 days. After the incubation period or until fungal colony developed, all isolates were purified by single-spore culture cloning technique.
Morphological identification
Morphological identification was carried out through the observation of gongylidias under a microscope after 21-day incubation period at 25 ºC on MEA plates. Characteristic reproductive structures of this genus from the fungal garden of Atta mexicana were analyzed (DeFine Licht, 2014).
Molecular identification
The fungus strain was genetically identified based on ITS-rDNA nuclear sequence analysis. Isolations of genomic DNA from the mycelium were performed according to the protocol proposed by Yu et al. (2011) with modifications. ITS5- ITS4 primers were employed to amplify the ITS-rDNA nuclear region through direct PCR. The amplification products of the PCR were purified using Wizard kit (Pro-mega®, USA) following the manufacture instructions and were sequenced by an Applied Biosystems (model 391) sequencer at Instituto de Biotecnología, Universidad Nacional Autónoma de México (UNAM). The nucleotides sequences were edited employing BioEdit v7.0.5.3 software and were compared through BLAST search with the NCBI GenBank (http://www.ncbi.nlm.nih.gov/) to confirm the species identity. For the phylogenetic analysis, the sequences were aligned using Clustal W algorithm from the software MEGA6 (Tamura et al., 2011). The nucleotide alignments were tested on jModeltest (Darriba et al., 2012; Guindon and Gascuel 2003) to define an evolutionary model; the phylogenetic tree was constructed using Maximum Likehood (TPM3+G) Method of MEGA6 software. The analysis was performed with boostrap value of 1000.
RESULTS
Morphological identification
From three analyzed nests (the artificial terrariums) of A. mexicana, three isolates of L. gongylophorus were obtained. L. gongylophorus was isolated and maintained on MEA at 25 ± 2 °C for 60 days of incubation. After the incubation time, white cottonlike aerial colonies were produced by the mycelium at the center, and young hyaline hyphae were observed towards the edge of the colony. Later, the cultures produced secretion droplets in the aerial part of the colony and, at the edge, lumpy farinaceous area was observed, that was formed mainly by gongylidias. From these cultures and from the fungal garden of A. mexicana, abundant gongylidias of size of 10-15 µ and 25-30 µ respectively were observed under the light field microscopy at 100X using fresh preparation stained with lactophenol blue (Figure 1).

Figure 1 A) Fungal garden from Atta mexicana nest. B) Group of gongylidias stained with lactophenol blue at 100X direct from the fungal garden. C) Mycelial isolate of L. gongylophorus in MEA. D) Presence of secretions on the edges of L. gongylophorus colony. E) and F) Gongylidias stained with lactophenol blue at 100X from a isolate in MEA of L. gongylophorus.
Molecular identification
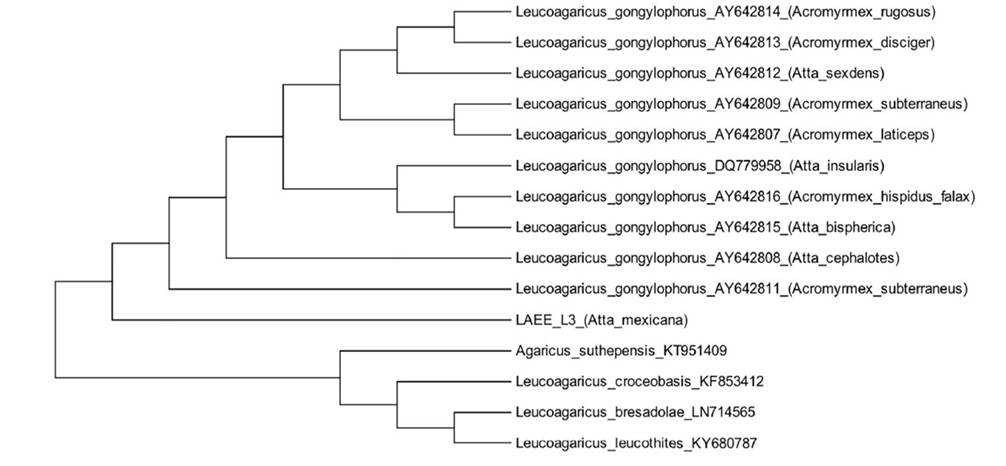
PCR product of approximately 700 bp was obtained using the primers ITS5-ITS4 for the isolate L3 of the symbiotic fungus of the ant A. Mexicana fungal garden. A total of 549 bp were used for the analysis of nucleotide sequences; the comparison with BLAST determined a similarity of 99% with the basidiomycete species L. gongylophorus. The phylogenetic analysis revealed the fungal isolate L3 within the cluster of Leucoagaricus gongylophorus species (Figure 2).
DISCUSSION
In vitro isolation of Leucoagaricus gongylophorus, as well as the study on the mutualistic relationship between ants of the genus Atta and the fungus, has been carried out for several species of the genus Atta , among them are ant species A. cephalotes (Mohali, 1998), A. capiguara, A. laevigata (Silva-Pinhati et al., 2005), A. sexdens (Miyashira et al., 2010), A. texana and A. mexicana (Sánchez-Peña, 2005), the latter only reported aspects of the fungus-growing ant symbiosis of A. mexicana. Nevertheless, there are no studies on in vitro isolation and identification of the fungus cultured by ant species A. mexicana. The molecular analysis placed the isolated fungus L. gongylophorus particularly with those reported by Pereira et al. (2015) and Silva-Pinhati et al. (2004) for symbiotic fungi of several species of Atta and Acromyrmex originated from Brazil. Interestingly, L. gongylophorus isolates associated with the ant genera Atta and Acromyrmex were separated from other representatives of L. gongylophorus cultured by Myrmicocrypta ednaella on the dendrogram (Figure 2). Likewise, L. gongylophorus isolates from Atta and Acromyrmex fungal gardens were clearly separated from other species such as L. bresoldae and L. croceobasis, which were reported as free-living fungi and not cultured by ants.
Thus, our data confirm that the species A. mexicana, as well as other species of Attini tribe, are able to cultivate fungi of the same species L. gongylophorus, even when some intraspecies differences could be observed, that possibly come from co-evolution of the fungus with their respective ant species, as well as because of nutritional differences as pointed by Pereira et al. (2015). Additionally, it was also reported that the fungus species L. gongylophorus is characterized by diverse morphological traits and different physiological behavior that depends on the genus of ant that cultivates the fungus. Morphological cultural characteristics of the fungus L. gongylophorus are also related with the culture medium composition, pH and in vitro cultivating temperature (Borba et al., 2007).
This work reported for the first time in vitro isolation and identification of basidiomycete fungus Leucoagaricus gongylophorus from the fungal garden of Atta mexicana that was confirmed by employing morphological and molecular characteristics. The ant species A. mexicana was shown to be able to co-exist with the fungus L. gongylophorus, as well as the other Atta species such as A. cephalotes, A. capiguara, A. laevigata, A. sexdens and A. texana are also able to develop symbiotic relationship with this fungus. The aforementioned observation demonstrates that the basidiomycetes L. gongylophorus is the symbiotic fungus cultured by A. mexicana ant.











 nova página do texto(beta)
nova página do texto(beta)