Introduction
Rust fungi form an important group of plant pathogens that cause rust diseases in a diverse group of hosts, which include crops and trees of ecological and economic importance. These fungi have complex life cycles and are obligate biotrophs. Because of these features, they are very difficult to study under laboratory conditions. In general, the problems with rust fungi in the field tend to increase with intensive and extensive monoculture, causing serious losses in commercial plantations by interfering with the physiology of the host and thereby reducing productivity (Zazzerini et al., 2005; Twizeyimana and Hartman, 2010; Kobayasti et al., 2011; Johnson, 2012; Helfer, 2014).
The species known as Physic nut (Jatropha curcas L.) belongs to the Euphorbiaceae family and is a native crop of Mexico and Central America. It has been considered worldwide an important source of seed oil for the production of biofuel; however, this crop faces several phytosanitary problems, among which the effects caused by fungi infections, especially rust, are the major constraint in obtaining expected yields (Anitha and Varaprasad, 2012; Machado and Pereira, 2012).
The agent of Physic nut rust was recorded for first time in 1915 in Puerto Rico, and referred to as Uredo jatrophicola Arthur. In 1937, Cummins described the fungus under the name of Phakopsora jatrophicola (Arthur) Cummins, which technically refers only to an anamorph. In 1994, a new name for this species was published, P. arthuriana (Buriticá and Hennen), but recently it was stated that the current name of this species is P. jatrophicola (Index Fungorum, 2015). This fungal species has been reported in every country where Jatropha curcas is grown, from southern Texas in the United States of America to Brazil, including the Antilles (Cummins, 1937; Buriticá, 1999; Hennen et al., 2005).
Few studies have been conducted on this species, all of which have focused on the morphological characters of the fungus examined by standard light microscopy (LM), and all of them agree on the size and shape of reproductive structures (Buriticá, 1999; Hennen et al., 2005; Sotão et al., 2006; Vieira et al., 2009; Kobayasti et al., 2011; Nolasco et al., 2013).
One particular advantage of Scanning electron microscopy (SEM) over LM, among others, is that it allows us to study details of fungal taxonomy, as well as several aspects of morphology such as surface details and fungi parasitism, and to date, has produced a wealth of knowledge on fungi and their interactions (Alves et al., 2013).
Since there are no previous studies at ultramicroscopic morphology level of the reproductive structures of P. jatrophicola, in this work we conducted an SEM examination of uredospores, uredia and telium of this species, in order to obtain bi-dimensional images of them and more useful information for further studies on the interaction of P. jatrophicola and J. curcas. We also developed a bioassay in order to examine the viability and infectivity of P. jatrophicola urediniospores after inoculation on J. curcas detached leaves.
Materials and methods
Sample collection
From March 2013 to January 2015, samples of J. curcas leaves with typical symptoms of rust were collected in commercial plantations, in three different municipalities (Tizimin, Merida and Conkal) of Yucatan, Mexico.
Morphological characterization
For the microscopic examination of the fungus, permanent preparations in wax rings in glycerin were made with transversal sections of the structures present in the abaxial surface of the leaves. A morphometric analysis of 33 uredinia, 60 urediniospores, 13 uredinial paraphyses, 33 telia and 23 teliospores, randomly selected, was done with a light microscopy (Zeiss, Axiostar plus) at 40X magnification. The identification of the fungus genus and species was done with the use of specialized identification keys (Buriticá, 1999; Cummins and Hiratsuka, 2003).
Scanning electron microscopy (SEM) analysis
Selected specimens were also examined by SEM (Jeol, Jsm6360LV). For this purpose, leaf material with rust fungi was hand cut with a razor blade into small pieces of approximately 5 x 5 mm. The specimens were fixed in 2.5% glutaraldehyde in 0.02 M sodium phosphate buffer (pH 7.1) for 48 h (24 h at room temperature and 24 h at 4 °C). This was followed by six 30 min washes in 0.02 M sodium phosphate buffer at 4 °C. The specimens were then dehydrated in graded ethanol series (30, 40, 50, 60, 70, 85, 95 and 100% v/v, two times for 30 min). For the SEM analysis, the samples were critical point dried in CO2 after fixation and dehydration, mounted onto carbon stubs and coated with 15 nm gold. SEM analysis was carried out using an acceleration voltage of 20 kV (Alves et al., 2013).
Inoculation of detached Jatropha curcas leaves with Phakopsora jatrophicola
Forty seeds of J. curcas were embedded for 24 hours in Procloraz (1 mL/L), after which they were scarified in order to promote germination and placed in trays with moistened sterile cotton at 25 °C, in darkness for 3 days. The Physic nut seedlings obtained were planted in bags with sterile substrate and grown under controlled conditions in a greenhouse in order to obtain healthy plants.
The infectivity of rust spores of P. jatrophicola was assessed using an in vitro detached leaf assay (Park et al., 2008) with some modifications which are described below.
Uredinidiospores were collected from leaves with symptoms of rust using a sterile needle; these spores were re-suspended in deionized water containing 0.01% Tween 20. Spore concentration was determined using a hemacytometer and adjusted to 1 x 105 spores/mL of water. Inoculum (200 μL) containing 20 000 spores was applied evenly to the adaxial surfaces of detached J. curcas leaves, harvested from greenhouse plants. These leaves had been previously disinfected with sodium hypochlorite 1% for 1 minute, washed three times with deionized water containing 0.01% Tween 20 and finally dried on sterile brown paper with dry air under a laminar flow cabinet. The inoculated leaves were placed adaxial surface up on filter paper soaked with sterile water in petri dishes and incubated at 20 ± 2 °C, with a 12 h photoperiod. Pustule formation was determined by visual inspection daily. High moisture (75%) inside the petri dishes was maintained by adding 4 mL of sterile deionized water every 7 days. Infection rate was determined by the percentage of leaves with visible pustules versus total number of inoculated leaves. Pustule density was defined as the average number of pustules per leaf 13 days after inoculation. This experiment was conducted twice with four replicates. Each replicate consisted of 24 detached J. curcas leaves, half of them inoculated with rust urediniospores and the other half with water containing 0.01% Tween 20. The data from two repeated experiments were combined to calculate the mean pustule densities.
Germination of urediniospores
The germination percentage of urediniospores from P. jatrophicola was assessed using the methodology reported for P. pachyrhizi by Park et al. (2008). With the aid of a sterile needle, spores were collected from leaves with symptoms of rust and placed in microcentrifuge tubes (1 mg/ tube) in 1 mL of deionized water containing 0.01% Tween 20; they were then allowed to germinate at 20 ± 2 °C with a 12-h photoperiod. Spore germination was assessed at 12, 24, 36, 48, 72 and 96 hours, using spore germination rate, which was defined as the percentage of spores germinated. For each assessment, the spore suspension was mixed and three subsamples of 20 μL were removed from the microcentrifuge tubes and examined with a light microscope. The percentage of spores germinated was determined based on the total number of germinated spores versus total number of spores counted from at least 24 different fields of view (at 40X magnification) for each sample. The germination percentage for each time point was the mean from three replicated samples. The means and standard deviations were calculated with the statistical software StatGraphic XVI (StatPoint Technologies Inc., 2010).
Results
It was observed a high incidence (30 to 70%) of rust in Physic nut commercial plantations located in three different municipalities of Yucatan, Mexico. The highest levels of rust, each year of the study, appeared from November to February, which are the months with lower average temperatures (16 a 23 °C) in Yucatan.
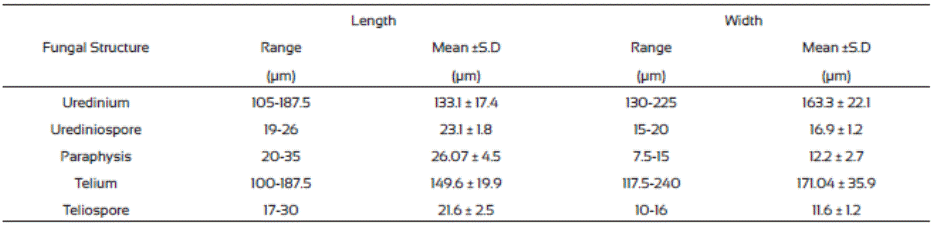
A total of 140 samples of J. curcas leaves with typical symptoms of rust were collected, the leaves presented irregular chlorotic halos on the adaxial surface, which later became necrotic, while in the abaxial area, reddish-brown erumpent pustules (uredia) were predominant, although dark brown telios were also observed (Figure 1).

Figure 1 Symptoms of physic nut rust (Phakopsora jatrophicola) in physic nut (Jatropha curcas) leaves. a. Chlorotic halos on adaxial surface of a leaf. b. Rust symptoms on abaxial surface of a leaf.
Morphological characterization
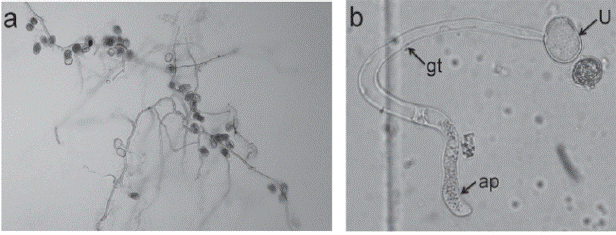
Light microscopy observations showed that uredinia of P. jatrophicola (Figure 2a) were hypophyllous, and occasionally epiphyllous. They were in small groups on leaf spots framed by leaf veins, brownish, ellipsoid, subepidermal, erumpent, and opening by a pore. Telia (Figure 2b) were hypophyllous, suberpidermal, not erumpent, yellowish to brownish, consisting of crusts of laterally adherent teliospores, 6-10 spore layers deep, covered by the epidermis. There were numerous peripheral, incurved, claviform, not septate, unusually dorsally thick-walled (5 μm) paraphyses (Figure 2c) surmounting peridial tissue, that project outside the host, with colorless to yellowish walls. Urediniospores (Figure 2d) were mostly obovoid, ranged from 15 to 20 μm wide, sessiles, 0.5-1 μm uniformly thick echinulate wall, colorless to brownish; and the germ pores were not visible. Teliospores (Figure 2e) were sessile, range from 17 to 30 μm length, irregularly arranged, 1-celled, and ellipsoid to polygonal, the wall was usually brown or brownish. The data of morphological characteristics of P. jatrophicola isolated in Yucatan is presented in Table 1.
Figure 2 Morphological structures of Phakopsora jatrophicola on Jatropha curcas observed at Light microscopy at 40X magnification. a. Uredinium (U) subepidermal, erumpent, opening by a pore surrounded by paraphysis (P) and with urediniospores (U) inside. b. Telia (T) subepidermal, not erumpent with teliospores (Ts). c. Incurved, dorsally thick-walled (Tw) paraphysis. d. Urediniospores appear sessile, obovoid. e. Teliospore.
Scanning electron microscopy analysis
The detail of uredinia erumpent, with an open pore (25 to 35 μm of diameter) containing numerous urediniospores and peripheral paraphyses surmounting peridial tissue were observed (Figure 3a). Uredinia erumpent associated with telium were also observed embedded in J. curcas tissue, telia were subepidermal, not erumpent, consisting of crusts of laterally adherent teliospores, closely around the uredinia (Figure 3b). SEM images of P. jatrophicola also showed the surface sculpturing of spores to be echinulate (Figure 3c and Figure 3d). The leaf tissue with several erumpent uredinia is presented in Figure 3e.
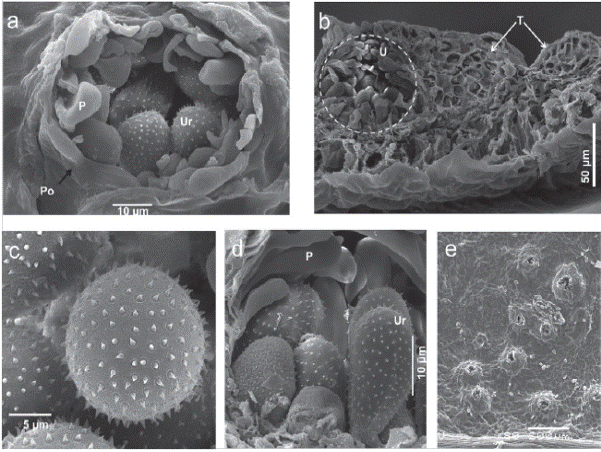
Figure 3 Scanning electron micrographs of Phakopsora jatrophicola inside plant tissue. a. Cross section of Jatropha curcas leaf with uredium of the rust fungus P. jatrophicola, detail of a pore (Po) opening with peripheral paraphyses (P) and urediniospores (Ur). b. Cross section of leaf with uredium (U) and telio (T) with teliospores. c. Top view of echinulate surface sculpture of urediniospores. d. Cross section of uredium with Paraphyses (P) and lateral view of urediniospores (Ur). e. Top view of erumpent uredinium in a Jatropha curcas leaf.
Phakopsora jatrophicola spore infectivity in a detached leaf assay
Detached Jatropha leaves were able to remain green up to 25 days after incubation under the detached-leaf assay conditions. Spores harvested from Physic nut leaves with rust symptoms retained their infectivity and were able to produce new pustules when inoculated onto healthy detached J. curcas leaves. Pustules were observed 13 days after inoculation on 50% of the inoculated leaves. Infectivity was retained up to 30 days after inoculation. In addition, pustule density in inoculated leaves was recorded at 14 days after inoculation (Figure 4), with an average of 181.16 ± 19.5 pustules/leaf observed on the abaxial side. An average of 15.5 ± 2.3 lesions/leaf and 12.63 ± 2.4 pustules/lesion were also recorded.
Germination of Phakopsora jatrophicola spores assay
Phakopsora jatrophicola rust spores were recovered from J. curcas leaves collected in commercial plantations. The highest number of spores seen in a hemacytometer field was 43 and the lowest number was 13, with an average of 20.45 ± 6.4 spores per hemacytometer field.
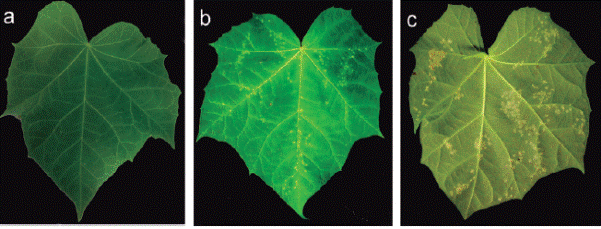
The high germination value was obtained at 96 hours (18%), but this value was not different from those obtained in the remaining measurements (15% at 12 and 24 h, and 17% at 36, 48 and 72 h). All germinated urediniospores showed the germ tube and the appressorium (Figure 5).
Discussion
In this study, we found that the P. jatrophicola specimens obtained from different commercial plantations of Physic nut, when examined under light microscope, showed reproductive structures and ranges of spore dimensions similar to those described in previous studies of P. jatrophicola in J. curcas (Cummins, 1937; Viégas, 1945).
Additionally, the present study also examined the morphological features of this rust via scanning electron microscopy (SEM) for the first time, and we are able to provide clear evidence of a cross section of a J. curcas leaf with uredia and their peripheral paraphyses and urediniospores, where the echinulate surface sculpture of urediniospores can be observed in detail. Furthermore, the SEM images showed the telium and teliospores on a J. curcas leaf and a cross section of the leaf with the uredium and telium together.
Until recently, Physic nut was used only as a living fence, and intensive cultivation is relatively new, because of this, little information is available on crop management. In order to establish adequate rust disease control, phytopathologists need to understand the pathogen biology, host-pathogen interactions, and environmental conditions that favor the establishment and development of rust. Additional research, similar to the present study, has been conducted in other pathogens of Phapkopsora genus in order to obtain this valuable information (Park et al., 2008; Twizeyimana and Hartman, 2010). It has been reported that the natural defoliation of J. curcas leaves can be confused with the symptoms of rust disease due to the fact that, when the infection is severe, P. jatrophicola causes complete defoliation in J. curcas plants (Vieira et al., 2009). The behavior of rust disease in many Physic nut varieties cultivated in Yucatan in order to obtain biofuel was similar to that reported. Nolasco et al. (2013) reported the presence of P. arthuriana in an noncommercial field (120 plants) in Puebla, Mexico and pointed out that the phytosanitary and economic importance of leaf rust in J. curcas in Mexico lies in the high levels of incidence and severity which can occur when environmental conditions are favorable, such as humid temperate climate with rainfall throughout the year; and they also hypothesized that in J. curcas commercial orchards, located in places where a sub-humid warm climate prevails with summer rains (such as the Yucatan peninsula), it is possible that this pathogen has not become established. Our results and observations made in the Physic nut commercial fields of the Yucatan Peninsula contradict this assumption, given that 30 to 70% of the 3000 hectares cultivated in this region of Mexico are affected by rust.
Phakopsora jatrophicola is an obligate pathogen; therefore it is very difficult to maintain the specimens in order to work, for example, on the genetic improvement for rust resistance of Physic nut, through artificial inoculations. In this sense, the possibility of having viable spores for a considerable period of time it is very important. The detached-leaf assay which has been used in culturing rust pathogens (Park et al., 2008; Twizeyimana and Hartman, 2010; Chang et al., 2014) provides a method that overcomes the time and space limitations of greenhouse and field evaluations. As Park et al. (2008) pointed out, a critical aspect in using the detached-leaf assay is to maintain healthy leaf tissue for the period of time that is required for disease development, and in this case, also for P. jatrophicola reproduction. This test proved the usefulness of this technique, not only for determining spore infectivity in a short time but also in maintaining live and other fungi contamination-free P. jatrophicola spores under in vitro conditions. Since it has been reported that rust may be parasitized by other pathogens (Ward et al., 2011; Nolasco et al., 2013), this approach would also enable the sequencing of different genic regions in order to developed specific primers for the molecular diagnostic for this species.
Park et al. (2008) found that the average germination rate of urediniospores of P. pachyrhizi, freshly harvested from soybean field, varies greatly (from 93% to 15%), depending on the time of harvest and the microenvironment to which the spores were exposed before harvest. The P. jatrophicola spores used in this study to measure germination rate had low germination percentage under the conditions established, these values match with the lower range reported in other works. However, there are no previous reports on germination percentages for this species. This study is the basis for further research to evaluate different ranges of temperature and obtain a better understanding of the biology of this pathogen.
Before this study there were no reports about this technique neither for P. jatrophicola nor for Jatropha curcas. To the best of our knowledge it has not been published any work on molecular studies in this host-pathogen-relationship. The findings of this study provide new information on the morphology and biology of this host-pathogen interaction, and will serve as the basis for further studies on Physic nut improvement for disease resistance.











 nueva página del texto (beta)
nueva página del texto (beta)