Introduction
Cereal grains and other commodities are commonly contaminated in the field and in the storage by fungi such as Fusarium and Aspergillus species. These molds produce secondary metabolites known as mycotoxins that reduce the commercial and nutritional grain quality (Doko et al., 1996; Tequida-Meneses et al., 2002). Fusarium verticillioides (Sacc.) Nirenberg (= monili forme) and Fusarium proliferatum (Matsush.) Nirenberg have been reported as natural contaminants of cereals worldwide and are mainly found in corn and its by-products (Acuña et al., 2005; Marasas et al., 1996; Mazzani et al., 1999), sorghum and oat (Leslie et al., 1990; Bacon and Nelson, 1994), rice (Abbas et al., 1998), and wheat (Castoria et al., 2005; Shephard et al., 2005). F. verticillioides has also been isolated frequently from maize in several states of Mexico (Hernández-Delgado et al., 2007; Gallardo-Reyes et al., 2006; Cortez-Rocha et al., 2003; Robledo et al., 2001).
These fungi are important plant pathogens that produce a variety of mycotoxins, the major class of which is called fumonisin. Of the twenty-eight fumonisin analogues that have been currently described (Rheeder et al., 2002), three (FB1, FB2, and FB3) have been reported to occur naturally at significant levels in corn and corn-based products (Sydenham et al. 1990, Doko et al. 1996). FB1 is the most abundant and accounts for about 70% of the fumonisins in naturally contaminated corn samples. The presence of fumonisins in feeds has been implicated in outbreaks of equine leukoencephalomalacia, porcine pulmonary edema (Bezuidenhout et al., 1988; Norred and Voss, 1994). In humans F. verticillioides and fumonisins have been epidemiologically associated with esophageal cancer in areas of Transkei, South Africa (Sydenham et al., 1990; Marasas et al., 2001) and China (Yang, 1980), where FB1-contaminated corn was consumed as a dietary staple.
To reduce the associated problems with Fusarium and their toxins, it is necessary to prevent fungal growth on the grains, which can be achieved by the use of synthetic fungicides. The use of natural bioactive substances for the control of fungal infections has gained attention because of fungicide resistant strains, which increases food-borne pathogenic microorganisms, in addition to increasing the number of pesticides under observation or regulation (Rabea et al., 2003).
Plant extracts are generally assumed more acceptable and less hazardous than synthetic products and can be used as alternative antifungal treatment (Jobling, 2000, Ramírez-Chávez et al., 2000; Guerrero-Rodríguez et al., 2007). Aqueous plant extracts from garlic, creosote bush, and clove inhibited the growth of Fusarium oxysporum f. sp. lycopersici, Rhizoctonia solani, and Verticillium dahliae (López-Benítez et al, 2005). According to Verástegui et al. (1996), alcoholic extracts from Baccharis glutinosa and Larrea tridentata, may act against the growth of fungi, yeast and bacteria. In addition, Sánchez-Rangel et al. (2005) reported the inhibition of both the growth and mycotoxin production by Aspergillus flavus and Aspergillus parasiticus when exposed to ethanolic, methanolic, and aqueous extracts of Agave species. Also, extracts of Flourensia cernua caused more than 91% of reduction in the colony growth of Alternaria alternata, Penicillum digitatum and Colletrotichum gloeosporiodes, but they don ́t affected sporulation (Guerrero-Rodríguez et al., 2007). Methanolic extracts of Baccharis gluti nosa have been reported to contain antifungal activity against phytophathogenic molds (Suárez-Jiménez et al., 2007; Tequida-Meneses et al., 2002). A study by Cespedes et al (2006) reported that chloroform/methanol extracts of Tagetes lucida inhibited 89% of the colony radial growth of F. moniliforme. Krameria erecta is one of 17 species belonging to the Family Krameriaceae. It has been reported for this genus the presence of phenolic compounds like tannins and lignans and various biological activities such as hepatoprotective effect, antioxidant and antiinflammatory (Carini et al., 2002). Recently, Morán-Palacio et al. (2014) and Jimenez-Estrada et al. (2013) found that K. erecta has high antiproliferative activity, high flavonoids and total phenols content. In the study of Morán-Palacio et al. (2014), it was found that K. erecta possess five times the antioxidant activity of ascorbic acid and also they demonstrate high phenolic content that supports the beneficial properties attributed to these plants in traditional medicine. Torres-González et al. (2011) mentioned that K. ramosissima is used by traditional healers in the northeastern region of Mexico to protect against liver damage. The aim of this work was to study J. macrocarpa and K. erecta as a source of natural compounds for controlling F. verticillioides and its production of toxins. Effects of methanolic extracts were tested on spore germination, biomass production, and radial growth. The best extract was fractionated and its effects on fumonisin production was measured.
Materials and methods
Aerial parts of Jacquinia macrocarpa and Krameria erecta were collected in the area of Los Arrieros, Sonora (Latitude N 28° 20.538' W 111° 08.911' altitude 280 feet and latitude N 28° 19.526' W 111° 08.828' altitude 227 feet) during august 2010. A voucher sample of each plant was deposited at the Herbarium of the Scientific Research and Technology Department of the University of Sonora (DICTUS) in Hermosillo, Sonora (Mexico) to confirm its identification. Plant material was dried at room temperature in the dark for 2 weeks and finely ground with a Wiley mill (200 μm mesh). Six grams of powdered aerial parts of each plant were extracted with 94 ml of 70% methanol, stirred during 1 h, and stored at room temperature for 10 days at darkness. The extracts were filtered first through Whatman filter paper No. 1. The methanolic extracts (crude extracts) were evaporated to dryness at 45 °C under reduced pressure. Crude extracts were evaluated for antifungal activity.
A strain of Fusarium verticillioides (ATCC 52539) was activated in PDA agar media (DIFCO, USA) and incubated at 25 ± 2 °C for 10 days using a 12 h light/dark cycle (Precision Low temperature Illuminated Incubator 818, USA). Spores were harvested by pouring a sterile solution of 0.1% (v/v) Tween 20 into the flask and stirring with a magnetic bar for 5 min. The spore concentration of the suspension was determined using a Neubauer chamber.
Kinetics of radial extension growth
Petri dishes of solid PDA media containing 500, 1000, and 2,000 mg/mL of each crude extract were centrally point-inoculated with 1x105 spores/mL from 7-day-old cultures of F. verticillioi des (ATCC 52539). Petri dishes with PDA and methanol and a blank with only PDA media were included as controls. All Petri dishes were incubated at 25 °C using a 12 h light/dark cycle. The colony diameters were measured with a caliper every 24 h and compared to the control media until the control reached the plate border. The extract concentration that delayed 50% of colony radial extension (CI50) was determined at 95% of confidence intervals, using a Probit analysis with NCSS 97 statistical program (NCSS Inc., USA). All determinations were carried out in triplicate.
Kinetics of spore germination
PDA plates centrally point-inoculated with 1x105 spores/mL from 7-day-old cultures of F. verticillioides (ATCC 52539) were added with the estimated CMI (1, 882 mg/L) of J. macrocarpa crude extract and incubated at 25 °C using a 12 h light/dark cycle. Petri dishes with PDA and methanol and a blank with only PDA media were included as controls. Samples were taken at different times and 200 spores (germinated and non-germinated) were randomly counted using a light microscope. The number of germinated spores per plate was determined. A spore was considered germinated when the length of its germinal tube reached one-half of the spore-diameter (Plascencia-Jatomea et al., 2003). All determinations were carried out in triplicate.
Biomass production
The biomass production was daily quantified as the mycelium dry weigh. Petri dishes of solid PDA media containing 1, 882 mg/L of J. macrocarpa crude extract were centrally point-inoculated with 1x105 spores/mL from 7-day-old cultures of F. ver ticillioides (ATCC 52539). Petri dishes with PDA and methanol and a blank with only PDA media were included as controls. All Petri dishes were incubated at 25 °C using a 12 h light/dark cycle. The agar gel with the produced biomass was separated from the plate, poured into a glass beaker containing 200 mL of water, and heated until complete dissolution of the agar. The solution was vacuum filtered using a previously weighted Whatman No. 40 filter paper and washed with distilled water. Finally, the filter containing the mycelium was dried at 105 °C for 2 h and the colony dry weight was expressed in mg/cm2, corresponding to mg of mycelium per plate area (Larralde et al., 1997). All determinations were carried out in triplicate.
Partition of the crude methanolic Jacquinia macrocarpa extract
The crude extract of J. macrocarpa was evaporated to dryness at 40 °C, resuspended in water, and sequentially partitioned with hexane, ethyl acetate, and n-butanol. The crude extract and the partitioned extracts were evaluated for their antifungal activity in the radial growth using 100, 500 and 1,000 mg/L as described previously. Controls were prepared using the different solvents except n-butanol because it totally inhibited the fungal growth.
Fumonisin B1 (FB1) production
The methanolic and butanolic extracts of J. macrocarpa were analyzed for their possible effects on FB1 production in F. verti cillioides-inoculated corn grain. Fifty mg of each extract were dissolved in 500 μL de MeOH and appraised to 10 mL with sterile water. FB1 production was carried out using healthy maize as substratum. Corn grain (50 g) portions, free from FB1, were placed in 500 mL Erlenmeyer flasks, adjusted at 40% humidity, and sterilized for two consecutive days in an autoclave for 15 minutes at 121 °C. Autoclaved maize was separately treated with the extracts. Control flasks were prepared following the same procedure with no extract added, only MeOH. Each treatment was inoculated with 1x106 spores of F. verticillioides. Flasks were incubated for 30 days at 25 ± 2 °C using a 12 h light/ dark cycle (Precision low temperature illuminated incubator 818, USA). Three replicates for each treatment were performed. For separation and purification of FB1 the cultures were ovendried overnight at 50 °C. Extraction procedure and quantification of FB1 were based on the quantitative Fumonitest® Immunoaffinity Column method from VICAM (Fumonitest manual).
Statistical analysis
Statistics on a completely randomized design were determined using the one-way analysis of variance (ANOVA) procedure with JMP software (JMP version 5.0, SAS Institute Inc., USA), at a level of significance set at P = 0.050. Means for groups in homogeneous subsets were determined using the Tukey multiple comparisons test (Tukey's posthoctest), at 95% confidence interval. All data were presented as mean value with their standard error indicated (mean ±SE).
Results and discussion
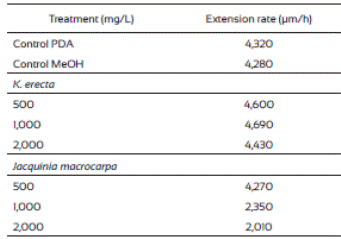
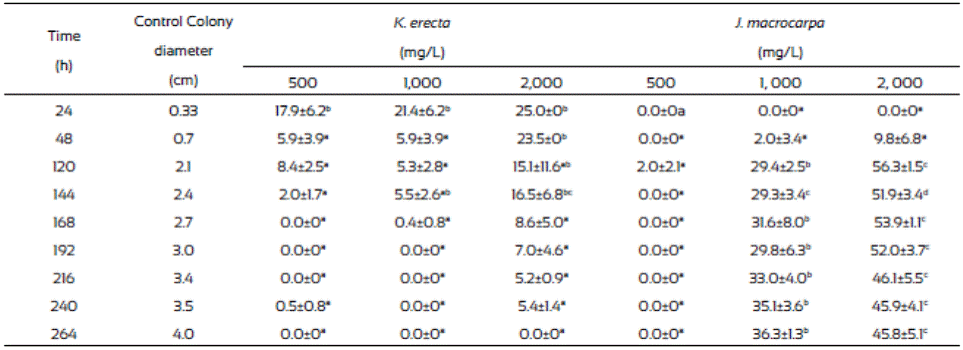
Results from radial extension growth inhibition (%) are presented on Table 1. K. erecta crude extract at the three concentration tested showed low inhibition effect during 144 h. The application of 2,000 mg/L of the extract exhibited the higher inhibition (25%) at the first 24 h, however this effect diminished after this time, even more, the radial growth was higher than in the methanol control since the plate were completely covered with the mycelium after 144 h of incubation, except for the 2,000 mg/L treatment. J. macrocarpa crude extract did not affected mycelial growth during the first 24 h but after this time it happens. Radial growth was delayed for 264 h only with the 1,000 and 2,000 mg/L. An inhibition range from 29.4 36.3% was observed at 264 h of incubation when 1,000 mg/L was added to the medium. The highest reduction of radial growth (56.3%) was reached with 2,000 mg/L of crude extract at 120 h of incubation.
Table 1 Inhibition of Fusarium verticillioides radial growth (%) after 264 hours of incubation in media with Krameria erecta and Jacquinia macrocarpa methanolic extracts.
Values are the average of triplicates ± the standard error of the mean. Different letters mean different statistical groups. Tukey test p < 0.05.
The radial extension rate, U (µm/h), of F. verticillioides was estimated from the radial growth results (Table 2). The lowest radial extension rate (2,010 µm/h) was observed with the 2,000 mg/L of J. macrocarpa crude extract and 2,350 µm/h with 1,000 mg/L. Both results agree to those of radial growth because they delayed it. The values from the radial extension rate of the PDA and PDA-methanol controls (4,320 μm/h and 4,280 μm/h, respectively) were higher than those from the J. macrocarpa methanolic extract exhibiting effects in F. vertici llioides.
Krameria erecta at the low amount (500 and 1,000 mg/L) used in the study did not affect F. verticillioides development and weak effects were noticed when exposed to 2,000 mg/L. In accordance with our study, several authors have mentioned that fungi species reacts to plant extracts in different ways (Fokialakis et al., 2006; Hernández-Albiter et al., 2007; Tequida-Meneses et al., 2002). Jiménez-Estrada et al. (2013) reported that methanolic extract of K. erecta has high antioxidant and antiproliferative activities; however, it did not affect the energy process in the fungus development. Also, Morán-Palacio et al. (2014) reported the presence of polyphenols and terpenes in a methanolic extract of K. erecta from Sonora, Mexico. These authors found that K. erecta has five times greater than ascorbic acid and a high phenolic content. It has been reported that this kind of compounds have antimicrobial and antifungal activity, however they had no effect in fungus species treated. Due to the low growth inhibition of F. verticillioides exerted by K. erecta, we proceed to evaluate only the effects of J. macrocarpa extract on the spore germination, biomass and FB1 production, using the estimated MIC (1,882 mg/L).
The spore germination inhibition percentages of F. vertici llioides are shown in Table 3. We observed that J. macrocarpa extract delayed spore germination. The crude extract was most effective in controlling spore germination of F. verticillioides at the first hours after the treatment and this effect decreased as the time passed. This phenomenon might probably be due to presence of certain resistance compounds such as enzymes in the mold, or to mold adaptation to the extract present in the medium. This is in accordance with Trione (1981), who mentioned that it is possible that molds such Aspergillus flavus and Fusarium moniliforme have enzymatic mechanisms to inhibit the effects of plant metabolites and grow in their presence, which was observed in our study.
Table 3 Inhibition of Fusarium verticillioides spores germination in media supplemented with Jacquinia macrocarpa (1,882 mg/L) methanolic extract
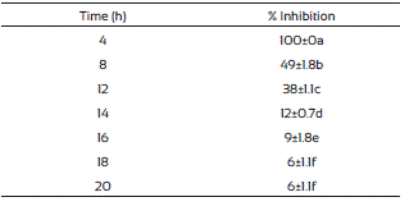
Values are the average of triplicates ± the standard error of the mean. Different letters mean different statistical groups.
The percentage of spores germinated at 12 and 24 h are presented in Table 4. The spores placed on the control with methanol and PDA control germinated after 24 h of incubation. There was a significant difference (p<0.05) in the percentage of spore germination inhibition between those exposed to the J. macrocarpa extract and the controls. In addition, there was a statistical difference between the biomass produced by F. ver ticillioides in presence of J. macrocarpa crude extract and the control. Other authors have reported the effect of plant extracts on the germination process. Suárez-Jiménez et al., 2007 reported that 5.6% (v/v) methanolic extracts of Larrea tridentata, Baccharis glutinosa, Ambrosia confertiflora, and Azadirachta indica caused 68 88% inhibition of spore germination of F. verticillioides after 100 h of incubation, which agrees with our findings since they followed the same trends. In addition, Abou-Jawdah (2004) reported 90 to 100% of inhibition of spore germination of Fusarium oxysporum by the application of extracts of nine Lebanese wild plants. Hernández-Albíter et al. (2007) reported similar results when studied the effect of extracts from forty plants on the germination of Colletotrichum gloeosporioides spores. They found variation in the effects by the type of plant and place of plant collection.
Table 4 Spore germination of Fusarium verticillioides with methanolic extract of Jacquinia macrocarpa (1,882 mg/L) and biomass production

Values are the average of triplicates ± the standard error of the mean. Different letters mean different statistical groups.
Antifungal activity of partitioned extract
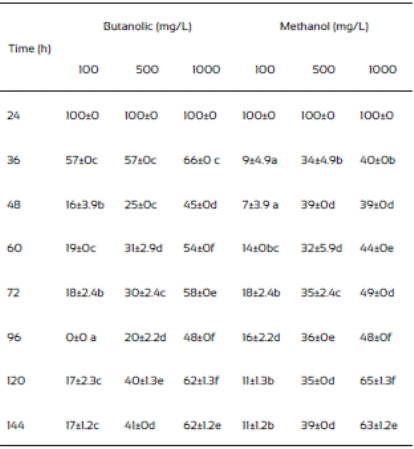
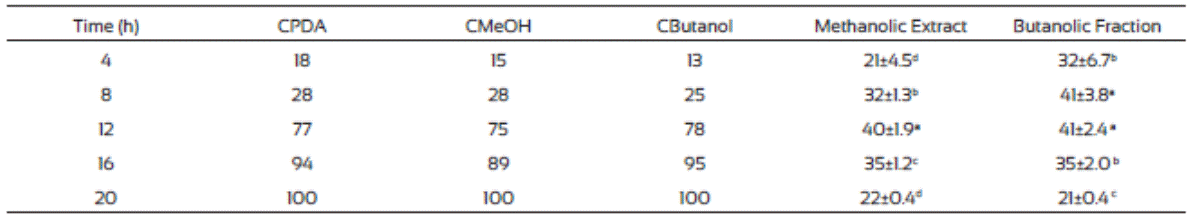
The hexane and ethyl acetate fraction of J. macrocarpa did not inhibit the mycelial radial growth of F. verticillioides. Ethyl acetate fraction promoted mycelial radial growth. This effect could be due to the presence of alellochemicals that stimulate the spore germination reported in other plant species (Montes-Belmont and García-Licona, 1997). Two concentration (500 and 1000 mg/mL) of the methanolic extract of J. macrocarpa and its butanolic fraction of J. macrocarpa highly affected negatively the radial mycelial growth (Table 5). Both fractions were more effective in inhibiting the growth of the fungus at both concentrations, causing growth delayed in the first 36 h. According to these results, we can assume the possible presence of flavonoids, phenols and alkaloids in both fractions of J. macrocarpa.
Spore germination in partitioned extracts
The results of the mycelial radial growth were used for Probit analysis to estimate the MIC of J. macrocarpa methanolic extract and its butanolic fraction. The MIC values were 1,408 and 1,883 mg/L, respectively) and were used for the spore germination analysis. Table 6 indicates that both fractions delayed the germination process compared to controls, which agrees to our results on radial growth. Also, both fractions exhibited the highest inhibition at 12 h of incubation (60 and 59%, respectively) but the effect decreases with the time.
Biomass production in partitioned extracts
The F. verticillioides mycelium production was low in plates where methanolic extract and the butanolic fraction of J. macro carpa were added compared to those from the controls (Table 7). Also, after 48 h incubation the radial growth change to apical growth, probably due to presence of the plant extracts. Butanolic fraction had the same effects than the methanolic extract did. Gomez et al. (2007) found that Fagara monophylla methanolic extract had antifungal activity against nine fungi species whereas the butanolic fraction was only effective in three of them, Aspergillus flavus, Penicillum digitatum, and Candida albicans.
Table 7 Fusarium verticillioides biomass production (mg/cm2) in PDA medium amended with Jacquinia macrocarpa methanolic extract and its butanolic fraction
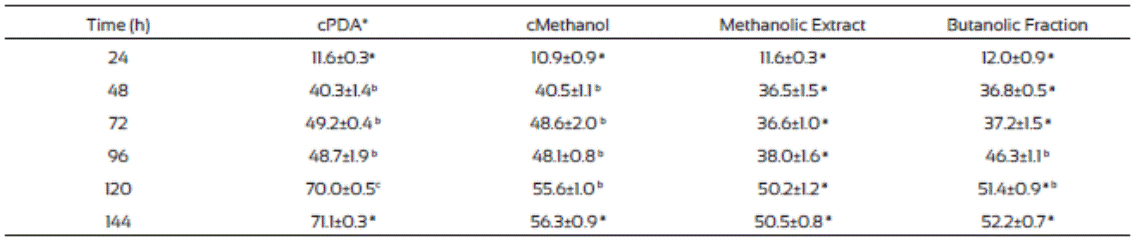
Values are the average of triplicates ± the standard error of the mean. Different letters mean different statistical groups. Tukey test p < 0.05.
Fumonisin production
Results showed that the fumonisin production is not influenced by the J. macrocarpa extracts (Table 8). The obtained values were not significantly different (P> 0.05) among control and plant extract, ranging from 6.57 to 11.93 μg kg-1. This results differs from those in study by Suárez-Jiménez et al (2007), they reported that Baccharis glutinosa and Larrea tridentata methanolic extracts increased the fumonisin B1 production in corn grain compared to methanolic control.
Tabla 8 Fumonisin B1 producend by fusarioum verticillioides in corngrain treated with Jacquinia macrocapa methalonic extract and its butanolic fraction

Values are the average of triplicates ± the standard error of the mean. Different letters mean different statistical groups. Tukey test p < 0.05.
Also, Rosas-Burgos et al (2011) reported that one fraction from B. glutinosa (fraction F-6) obtained by chromatographic purification and dissolved in methanol considerably increased the production of mycotoxins such as fumonisin B1 by Fusarium verticillioides and aflatoxins by Aspergillus flavus and A. niger.
The results obtained from this study indicate that K. erecta do not have any antifungal activity against F. verticillioides. Butanolic fraction from methanolic extract of J. macrocarpa caused 66% of inhibition of radial growth and 41% reduction in spore germination. J. macrocarpa contain chemical constituents that inhibited radial growth, inhibit spore germination and reduction in the mycelium production of F. verticillioides. Fumonisin production was not affected by the methanolic and butanolic J. macrocarpa extracts. According to the polarity of the extraction solvents used sequentially, a diversity of polar compounds could be present, which will be under investigation.











 text new page (beta)
text new page (beta)