Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista mexicana de micología
versión impresa ISSN 0187-3180
Rev. Mex. Mic vol.40 Xalapa dic. 2014
Contributions
Antagonistic properties of micromycetes isolated from sinkholes of the Yucatán Península against fungal phytopathogens
Propiedades antagonistas de micromicetos aislados de cenotes de la península de Yucatán contra hongos fitopatógenos
Pablo Moreno-Pérez1, Marcela Gamboa-Angulo1*, Gabriela Heredia2, Blondy Canto-Canché1 Miguel Rosado-Vallado3, Irma L. Medina-Baizabat1 y Raúl Tapia-Tussell1
1 Unidad de Biotecnología, Centro de Investigación Científica de Yucatán, Metida 97200, Yucatán, México.
2 Red de Biodiversidad y Sistemática, Instituto de Ecología A.C., Km 2.5 antigua carretera a Coatepec No. 351, Xalapa 91070, Veracruz, México.
3 Centro de Investigación Regional "Hideyo Noguchi", Universidad Autónoma de Yucatán, México.
* Autor para correspondencia:
Marcela Gamboa-Angulo mmarcela@cicy.mx
Received 22 January 2014.
Accepted 30 June 2014.
Abstract
In the search for natural alternatives to control fungal diseases, antagonistic fungi are valuable sources to find new models. In the present study, a total of 41 tropical micromycetes were isolated from plant debris submerged in sinkholes of the Yucatán Península. All strains were tested in antagonist assays against four phytopathogenic fungi (Colletotrichum gloeosporioides, Corynespora cassiicola, Curvularia sp. and Fusarium sp.). Results of the antagonistic assays showed mycelial growth inhibition (MCI ≥50 %) by 17 isolates (41 %) against at least one of the targets tested. The highest inhibition was exhibited by the Hypocrea lixii OSN-37 (MCI=61-77%) and Rhizoctonia solani OSE-73 (MCI=55-64%) strains against all targets while Pestalotiopsis mangiferae OH-02 (51-59%) caused inhibition on three of four pathogen strains. These three strains were cultured in fermented rice to obtain their ethyl acetate and methanol extracts which were tested against C. gloeosporioides using the microdilution assay. Results showed H. lixii OSN-37 and R. solani OSE-73 to be producers of antifungal metabolites as one of their modes of action. In conclusion, three promising antagonistic native strains were isolated from plant debris submerged in the Yucatán sinkholes, representing a valuable contribution to the development of ecofriendly alternatives to control fungal diseases in agriculture crops of the tropical regions.
Keywords: antagonist, Hypocrea lixii, micromycetes, Pestalotiopsis mangiferae, sinkhole, Rhizoctonia solani.
Resumen
En la búsqueda de alternativas naturales para el control de enfermedades fúngicas, los micromicetos con propiedades antagónicas son considerados una fuente valiosa para detectar modelos novedosos. En el presente estudio, un total de 41 micromicetes tropicales se aislaron a partir de restos vegetales sumergidos en cenotes de la Península de Yucatán. Todas las cepas se evaluaron en ensayos antagonistas contra cuatro hongos fitopatógenos (Colletotrichum gloeosporioides, Corynespora cassiicola, Curvularia sp. y Fusarium sp.). Los resultados de este ensayo detectaron que 17 aislamientos (41 %) provocaron ≥ 50 % de inhibición del crecimiento micelial (MCI ≥ 50 %) de al menos uno de los patógenos evaluados. La inhibición más alta fue ocasionada por las cepas Hypocrea lixii OSN-37 (MCI = 61-77 %) y Rhizoctonia solani OSE-73 (MCI = 55-64 %) contra todos los objetivos, mientras que Pestalotiopsis mangiferae OH - 02 (51 -59%) causó la inhibición en tres de las cuatro cepas patógenas. Las cepas antagonistas con mejores resultados fueron cultivadas en arroz fermentado para obtener sus correspondientes extractos de acetato de etilo y metanol los cuales se evaluaron contra C. gloeosporioides en ensayo de microdilución. Los resultados indicaron que H. lixii OSN-37 y R. solani OSE- 73 son productores de metabolitos antifúngicos como uno de sus modos de acción. En conclusion, tres cepas nativas promisorias como antagonistas de fitopatógenos se aislaron de los cenotes de la península de Yucatán, lo que representa una valiosa contribución para desarrollar alternativas eco-amigables en el control de enfermedades fúngicas que afectan los cultivos agrícolas en regiones tropicales.
Palabras clave: antagonista, Hypocrea lixii, micromicetos, Pestalotiopsis mangiferae, cenote, Rhizoctonia solani.
Introduction
The control of pathogenic fungi in agricultural crops using synthetic agrochemicals has been well documented and proven to have adverse side effects on the environment (Alwathnani et al., 2012). Therefore, there is an urgent need to find effective new strategies to replace them. One alternative is the use of antagonistic fungi that have demonstrated valuable abilities to inhibit the growth or development of fungal pathogen populations, with the additional advantage of reduced environmental and human health impacts (Whipps and Lumsden, 2001). For example, Muscodor sp. produces a mixture of volatile compounds with activity against bacteria, fungi, insects and nematodes (Strobel et al., 2012), Sphaerodes mycoparasitica is an antifungal agent with wide spectra against members of the genera Fusarium, Pythium, Rhizoctonia and Sclerotinia (Vujanovic, 2012) and Emericella rugulosa is a biocontrol agent of Fusarium wilt of tomato (Sibounnavong et al., 2012), among others.
In México, relatively few contributions dealing with the evaluation of antagonistic fungal native species for the control of phytopathogenic fungi have been published. Some examples are those of Quiroz-Sarmiento et al. (2008), Michel-Aceves et al. (2009), Galindo-Flores et al. (2005) and Cuervo-Parraera/.(2011).
In this context, our research group has been carrying out antimicrobial screening with extracts from tropical fungi of the southeast of México against very important phytopathogens belonging to the Alternaría, Colletotrichum and Fusarium genera, among others (Gamboa-Angulo et al., £ 2012; Reyes-Estebanez et al,. 2011). These previous studies ψ included isolation and identification of saprophytic fungal strains from sinkholes (cenotes in Spanish, from the Mayan word dzonot), which were cultured in fermented rice, and organic extracts tested against a set of bacterial and fungal phytopathogens. These investigations showed a broad antimicrobial spectrum in 53% of the saprophytic strains, and eight of which were also effective against fungal phytopathogens (Gamboa-Angulo et al., 2012). The sinkholes are special freshwater ecosystems where the low availability of nitrogen and phosphorus sources induces strong competition for survival (Schmitter-Soto et al., 2002). Around 2241 sinkholes have been reported in the state of Yucatán (www.seduma.yucatan.gob.mx), most of them have not been investigated for their microbiota, and of course, in their antimicrobial abilities.
With these antecedents, the aim of the present work was to isolate native micromycetes from two different sinkholes of Yucatán to enrich our collection of fungal strains and to evaluate their antagonistic abilities against Colletotrichum gloeosporioides, Corynespora cassiicola, Curvularia sp. and Fusarium sp., four pathogenic fungi of agricultural importance.
Materials and methods
Isolation of saprophytic fungi
Two semi-closed cylindrical sinkholes named Oxola (N 20°40'41", W 89°14'30") and Kikal (in the Yalahau reserve, Ν 20°34'59", W 89°10'49") were selected. Both are located in the center of the state of Yucatán and are far from agricultural activities and human settlements. In order to detect a wider range of fungal species, besides collecting plant debris submerged in the study áreas, litter traps (mesh bags of 20 x 30 cm, 1-mm mesh size) were installed with poles buried within the sinkholes. A set of litter traps was filled with non-sterile material gathered around the sinkholes, and a second one consisted in litter traps with plant debris sterilized and collected in the botanical gardens of the Scientific Research Center of Yucatán (CICY for its acronym in Spanish). In both sinkholes, after 7, 14 and 21 days the submerged bags were collected. Samples were washed in laboratory with a slow water flow and then plant debris were gently dried with sterile paper towels and incubated (25 ± 2°C) in damp chambers, made with glass Petri dishes with wet sterile filter paper. Every day the chambers were ventilated and monitored. With the aid of a stereo microscope structures of fungi were detected and transferred to Petri dishes containing PDA medium (Krug, 2006).
Identification of saprophytic fungi
Fungi were identified according to their morphological characteristics using taxonomic keys (Barnett and Hunter, 1998; Carmichael et al, 1980). The fungal strains that showed antagonistic activity were submitted to molecular analysis. Genomic DNA (gDNA) was obtained according to Conde-Ferráez, (2008). The internal transcribed spacer regions, including the 5.8S rDNA, were amplifíed using universal primers ITS1 and ITS4 (White et al, 1990). PCR products were analyzed by gel electrophoresis and the DNA bands carefully cut and purified with the Gene Clean II kit (Roche 1001-400). Samples were sequenced using 20 ng of PCR product and ITS1 primer. Sequences were obtained by Macrogen Inc, Korea. Alignment and edition were done with the BioEdit Program ν 7.0.5 (Hall, 2005), and manually corrected. The sequences obtained were compared against those available in the GenBank database (GenBank, http://www.ncbi.nlm.nih.gov).
Target strains
Colletotrichum gloeosporioides Penz. & Sacc. (CICY-05) were isolated from Carica papaya L., Corynespora cassiicola Berk. & M. A. Curtís (ITC-03) from Capsicum anuum L., Curvularia sp. (ITC-01) from Zea mayz L. and Fusarium sp. (CICY-04) from Lycoperscum esculentum. These phytopathogenic fungi were obtained from the CICY and Conkal Technological Institute (ITC) culture collection, and were identified by morphological characteristics using taxonomic keys (Barnett and Hunter, 1998).
Antagonistic trial
Antagonistic activity against the phytopathogenic fungi was carried out by the in vitro dual culture assay (Asran-Amal et al, 2010). All phytopathogenic and saprophytic fungal strains were maintained on potato dextrose agar (PDA), with natural light at 25 ± 2°C. Amycelial disk of a seven day-old culture of the saprophytic strain (5 mm in diameter) was placed on one edge of the Petri dish (6 cm in diameter), while a mycelial disk of a seven day-old culture of the target (5 mm in diameter) was transferred to the opposite edge. For control, each target plant pathogen was confronted with a sterile disk of PDA (5 mm in diameter), and the mycelial growth was measured (negative control of inhibition); a PDA disk (5 mm in diameter) with antifungal Mirage® (Prochloraz 900 μg/mL) was also used as positive control of inhibition; experiments were performed in quadruplicate. All dishes were incubated at 25 ± 2°C with continuous light, and growth was evaluated at the sixth day by measuring the radius of each mycelium, from the outer edge of the disk with the pathogen to the edge of the growth of the antagonist, using a Truper® millimeter gauge (CALDI-6MP model). Results were reported as percentage of mycelium growth inhibition (%MGI) of each pathogen, determined by Abbott's formula [(C-T/C) x 100] (Abou et al, 2002) where C is the hyphal extension of the target strain in single culture and Τ is the target fungus growth in dual culture.
Fungal extracts
Antagonistic strains Hypocrea lixii OSN-37, Pestalotiopsis mangiferae OH-02, and Rhizoctonia solani OSE-73 were inoculated into in fermented rice (160 g of rice was fermented with distilled water overnight and sterilized); these were then incubated at 25 ± 2°C, using a light-dark photoperiod (12/12 h). After 40 days, cultures were frozen and lyophilized. They were then fragmented and subsequently extracted with ethyl acetate (three times, 50 mL, 24 h at 25°C, each) and methanol (50 mi, 24 h at approximately 50°C). The solvents were eliminated by reduced pressure to obtain the corresponding fungal organic extracts (Soman et al., 2001). These were dissolved in acetonitrile and defatted with hexane (three times, 2:1,1:1,1:1, v/v) obtaining the hexane and acetonitrile fractions and a precipitate. All organic samples were tested in broth microdilution bioassay.
Broth microdilution assay on Colletotrichum gloeosporioides
The minimum inhibitory concentrations (MIC) of the extracts were determined by broth microdilution techniques according to the National Committee for Clinical Laboratory Standards (NCCLS, 2004) with slight modifications. Each organic extract, fraction, and precipitate was dissolved in dimethyl sulfoxide (DMSO) (40 μg/μL) and 10 μΕ were transferred to microwells containing medium Roswell Park Memorial Institute (RPMI-1640, 90 μΕ, www.sigmaaldrich.com). A conidial suspension of C. gloeosporioides (100 μΕ) was then added to each microwell to a final concentration of 2.5 x 104 conidia/mL, 2000 μg/mL extracts and 5% DMSO. Hexane, acetonitrile fractions and precipitate of P. mangiferae OH-02, T. lixii OSE-37, and R. O solani OSE-73, were tested at final concentrations of 2000, 1000 and 500 μg/mL, with 5, 2.5 and 1.25% of DMSO, respectively, while 25% DMSO was used as positive control, and negative controls were the spore suspension, RPMI-1640, 5% DMSO, and a blank of fermented rice. Tests were carried out in triplicate. The microdilution plates were incubated at 25 ± 2°C in the dark for 96 hours, after which hyphal growth (HG) was, determined visually with a microscope (50x) following NCCLS standards (2004).
Statistical analysis
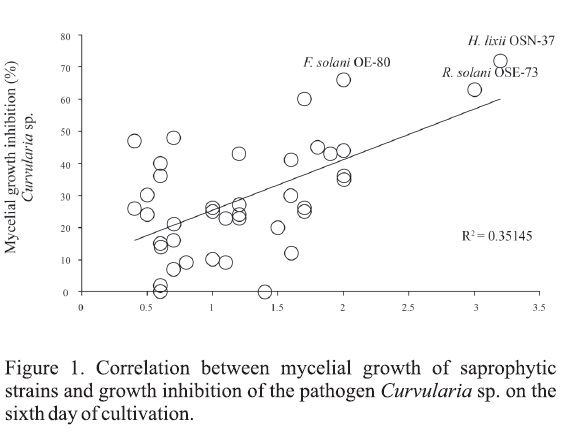
Inhibition data were analyzed with t-Student statistical package SPSS. 13.0, compared to negative control (P< 0.05). Subsequently, a linear correlation analysis was performed between percentage of mycelial growth of fungal saprophytic strains and fungal phytopathogen in dual culture (Figure 1).
Results
Isolation and Identification of strains
Forty-one fungal isolates were obtained from submerged native plant debris and from the litter traps installed in the two sinkholes of Yucatán. Most of them were captured by litter traps containing ex situ sterilize plant debris (44%). Among these, 18 (44%) were identified as belonging to the genera Bionectria, Clonostachys, Fomitopsis, Fusarium, Hypocrea, Monodictys, Penicillium, Pestalotiopsis, Pseudorobillarda, Rhizoctonia and Scopulariopsis, and ten were identified at species level (Table 1). The remaining 23 strains (46%) were not taxonomically identified because they did not sporulate in the culture media used. On the other hand, since they were inactive in antagonistic assays, they were not analyzed by molecular techniques (Table 1).
Antagonistic trial
Results of the antagonistic evaluation of fungal isolations against four fungal phytopathogens in dual culture assay showed statistical differences (P < 0.05) after six days (Table 1). Seventeen of the 41 saprophytic strains were able to inhibit mycelial growth (≥ 50%) of at least one target tested, and six of them displayed good antagonistic properties (MGI values of > 60%). Half of these antagonistic fungi were obtained from the litter traps with ex situ sterilized material. The active fungi were Fusarium solani OE-80, Hypocrea lixii OSN-37 (teleomorph of T. harzianum), P. mangiferae OH-02, and R solani OSE-73. The highest antagonist effect was produced by H. lixii OSN-37 (61-77%) and R. solani OSE-73 (55-64%), both were successful against all target phytopathogens (Table 1). Furthermore, H. lixii OSN-37 caused the highest inhibition of C. gloeosporioides, Curvularia sp. and Fusarium sp. (MGI values of 67,72 and 77%, respectively), and was notably active against C. cassiicola (MGI values of 61%). Other attractive antagonist strain was P. mangiferae OH-02 that showed good inhibitory properties against C. cassiicola, Curvularia sp. and Fusarium sp. (MGI values of 51-59%) (Table 1).
In this study, the most sensitive pathogen was C. cassiicola, showing inhibition by 16 fungal saprophytes (MGI ≥ 50). On the other hand, C. gloeosporioides was the most resistant target, being affected only by H. lixii OSN-37 and R. solani OSE-73 at 67 and 62 % (MGI ≥ 60%) respectively. Curvularia sp. was inhibited (MGI ≥ 60%) by Ε solani OE-80, H. lixii OSN-37, P. mangiferae OH-02, Pseudorobillarda sojae OSN-36 and R. solani (Figure 1). Finally, Fusarium sp. was affected only by H. lixii OSN-37, P. mangifeare OH-02 and R solani OSE-73 (MGI values of 55-77%).
Antifungal assay of extracts on Colletotrichum gloeosporioides
The three most active antagonistic strains detected were H. lixii OSN-37, P. mangiferae OH-02, and R. solani OSN-73, all of them were cultured on fermented rice. Two extracts (ethyl acetate and methanol) and three fractions (Hexane, acetonitrile and precipitate) were obtained from each strain, and all were assessed against C. gloeosporioides.
Results showed that all samples of H. lixii OSN-37 were active, with the exception of the polar methanol extract and the acetonitrile fraction of R. solani OSE-73 (Table 2). The lowest MIC was displayed by the acetonitrile fraction of H. lixii OSN-37 with values of 1000 μg/mL.
Discussion
The serial collections led to the isolation of 27 saprophytic fungal strains from Oxola (66%) and 14 (34%) from Kikal. Among these, strains belonging to the Bionectria, Fomitopsis, Hypocrea, Monodictys, Pestalotiopsis, Pseudorobillarda and Scopulariopsis genera corresponded to the first fungal contributions of Yucatán, México. The genus Fusarium was predominant in the sinkholes studied, with seven strains isolated, which is congruent with previous reports from other sinkholes (Gamboa-Angulo et al., 2012).
All the strains were tested against four recognized fungal phytopathogens, the most active were H. lixii OSN-37, P. mangiferae OH-02, and i?, solani OSE-73. Hypocrea lixii OSN-37 showed the most active and broadest antagonistic effect against the targets tested. Although H. lixii is widely distributed in the world, (Druzhinina et al., 2010), this is the first report from the state of Yucatán.
The antagonistic properties of Trichoderma harzianum, the asexual stage of Hypocrea lixii, have been extensively documented (Elgorban et al., 2013; Asran-Amal et al, 2010). At present several commercial formulations are available to control diverse fungal plant pathogens, such as Trianum® by Koppert Biological Systems (Trichoderma harzianum T22,), Trichodex® by AMC Chemical (T. harzianum T39) and Tusal® by NewBiotechnic (T. harzianum and T. viride) (Reino et al., 2008; Xu, et al., 2010), among others. On the other hand, it is widely known that Trichoderma possess several antagonistic mechanisms. In particular, it was observed that our native strain H. lixii OSN-37 displayed overgrowth on four phytopathogens tested herein, which suggests mycoparasitic action due to its rapid growth and abundance. This effect could be regulated by the production of enzymes such as chitinase, β-glucanase, cellulose and protease, and also by secondary metabolites (Harman, 2006; Reino et al, 2008).
Therefore, the ability of H. lixii OSN-37 to produce antimicrobial metabolites was confirmed when this strain was cultured on fermented rice, and its ethyl acetate extract showed hyphal growth inhibition of C. gloeosporioides, as observed in dual culture (Table 2). This pathogen is one of the causal agents of anthracnose in various tropical crops in the world, such as Annona muricata, C. papaya, Coffea arabica, Fragaria vesca, Lycopersicum esculentum, Mangifera indica, Persea americana and Vitis vinifera (Beltrán-Cifuentes and García-Jaramillo, 2006). This extract was later partitioned and all corresponding fractions displayed antifungal effect; the most effective was the acetonitrile fraction with MIC values of 1000 μg/mL (Table 2). Thus, low and medium polarity compounds are implicated in the mechanism of action of H. lixii OSN-37. A great number of low molecular weight antibiotics with diverse structures have been isolated from several species of the Trichoderma genus (Reino et al, 2008). Chemical purification and identification of these active metabolites, therefore, would be highly recommended.
Another antagonistic strain was R. solani OSE-73 which showed overgrowth on all target strains. This effect was similar to that displayed by H. lixii OSN-37, suggesting a mycoparasitism effect. Rhizoctonia solani is classified as a soil fungal pathogen, although it presents saprophytic and symbiotic associations (Bateman, 1970; Masuhara et al, 1993). About organic extract and fractions of this fungus, only the acetonitrile fraction was able to inhibit the growth of C. gloeosporioides. This fact indicated the diffusion of medium polarity and low molecular weight compounds into the medium, as one of its strategies against fungal pathogens. In the literature, only one report was found dealing with metabolic fingerprinting studies of sclerotial extracts from R. solani (Aliferis and Jabaji, 2010).
In contrast, the P. mangiferae OH-02 isolate produced a notable inhibition zone without contact with the mycelium surrounding of C. cassiicola, Curvularia sp. and Fusarium sp. This would suggest an antibiosis phenomenon in which could be involved enzyme secretion, release of volatile compounds or diffusion of metabolites into the medium, inhibiting the development of other fungi. Pestalotiopsis mangiferae occur on multiple hosts (Yang et al., 2012) and more recently, Subban et al. (2013) have reported a novel compound with antibacterial and antiyeast effect, named 4-(2,4,7-trioxa-bicyclo[4.1.0.]heptan-3-yl) phenol. As expected, the organic extract and fractions of P. mangiferae OH-02 showed no effect against C. gloeosporioides.
Finally, a correlation analysis was carried out between mycelial growth of saprophytic strains (cm) and the percentage of MGI obtained from each of the fungal phytopathogen studied in dual culture, on the sixth day of cultivation. With the exception of H. lixii OSN-37, results showed lower correlation in the interactions. This suggests that the inhibitory effect is not due to direct contact among antagonists and phytopathogen mycelia, or competition for substrate or nutriente. In particular, this mechanism is presented by the P. sojae OSN-36 and F solani OE-80 strains only against this pathogen (Figures 1 and 2).
In conclusion, H. lixii OSN-37, P. mangiferae OH-02 and R. solani OSE-73 are the most promising antagonistic saprophytic strains isolated in this work. These strains represent alternatives to control fungal diseases in tropical regions. Furthermore, H. lixii OSN-37 and R. solani OSE-73 produced metabolites effective against C. gloeosporioides. These saprophytic native fungal strains require further research to determine their active principles, mechanisms of action and to establish formulations that will allow the application of fungal extracts at greenhouse and field levels in the near future to protect crops in the tropical regions.
Acknowledgements
Elmer Medina, Raúl Manzanilla for their valuable help in locating the sinkholes; Miguel Tzec, Sergio Pérez and Narcedalia Gamboa for their technical support. Jairo Cristóbal Alejo and Andrés Quijano Ramayo for giving us phytopathogens. This work was supported by CONACYT project No. 2009/131256 and by graduate student fellowship to P.M.P. (CVU No. 209062).
References
Abou, J.Y., Η. Sobh, A. Salemh, 2002. Antimycotic activities of selected plant flora, growing wild in Lebanon, against phytopathogenic Fungi. Journal of Agricultural and Food Chemistry 50: 3208-3213. [ Links ]
Alwathnani, H.A., K. Perveen, R. Tahmaz, S. Alhaqbani, 2012. Evaluation of biological control potential of locally isolated antagonist fungi against Fusarium oxysporum under in vitro and pot conditions. African Journal of Microbiology Research 6:312-319. [ Links ]
Aliferis, K.A., E.S. Jabaji, 2010. Ή NMR and GC-MS metabolic fingerprinting of developmental stages of Rhizoctonia solani sclerotia. Metabolomics 6:96-108. [ Links ]
Asran-Amal, S., M. Moustafa-Mahmoud, K.K. Sabe, O.H. Banna, 2010. In vitro antagonism of cotton seedlings fungi and characterization of chitinase isozyme activities in Trichoderma harzianum. Saudi Journal of Biological Sciences 17:153 -157. [ Links ]
Bateman, D.F., 1970. Pathogenesis and diseases. In: Parmeter, J.R. (ed.), Rhizoctonia solani, Biology and Pathology. Berkeley, CA: University of California Press.pp. 161. [ Links ]
Barnett, H.L., B. Hunter, 1998. Illustrate Genera of Imperfect Fungy, 4* Ed. The American Phytopatological Society. St Paul, Minnesota, USA. 218p. [ Links ]
Beltrán-Cifuentes, M.C., D.J. García-Jaramillo, 2006. Colletotrichum gloeosporioides fitopatógeno asociado a la nutrición humana. Red de Revistas Científicas de América Latina, el Caribe, España y Portugal 13 73-80. [ Links ]
Carmichael, J.W., W.B. Kendrick, L. Conners. L. Sigler, 1980. Genera of Hyphomycetes. p. 386. Alberta, Canadá, University of Alberta Press. [ Links ]
Conde-Ferráez, L., R. Grijalva-Arango,A.C. James-Kay, 2008. Arapid DNA extraction method from mycelium which is suitable for PCR. Revista Latinoamericana de Microbiología 50:3-4. [ Links ]
Cuervo-Parra, J.A., M. Ramírez-Suero, V. Sánchez-López, M. Ramírez-Lepe, 2011. Antagonistic effect of Trichoderma harzianum VSL291 on phytopathogenic fungi isolated from cocoa (Theobroma cacao L.) fruits. African Journal of Biotechnology 10:10657-10663. [ Links ]
Druzhinina, I.S., CP. Kubicek, M. Komon-Zelazowska, T.B. Mulaw, J. Bissett, 2010. The Trichoderma harzianum demon: complex speciation history resulting in coexistence of hypothetical biological species, recent agamospecies and numerous relict lineages. BMC Evolutionary Biology 10:94 -108. [ Links ]
Elgorban, A.M., M.A. Abdel-Wahab, A. Bahkali, B. Al-Sum, 2013. Biocontrol of Meloidogyne javanica on tomato plants by Hypocrea lixii (the Teleomorph of Trichoderma harzianum). CLEAN-Soil, Air, Water 41:1-6. [ Links ]
Galindo-Flores, H., J.C. Martínez-Álvarez, E. Nava-Pérez, RS. García-Estrada, LE. Maldonado-Mendoza, 2005. A saprotrophic fungal isolate from northern Sinaloa, México, with homology to members of the Chaetomiaceae behaves as an antagonist of phytopathogenic fungi in vitro. Revista Mexicana de Fitopatología. 23:130-139. [ Links ]
Gamboa-Angulo, M., S.C. De la Rosa-García, G. Heredia-Abarca, I. Medina-Baizabal, 2012. Antimicrobial screening of tropical microfungi isolated from sinkholes located in the Yucatán peninsula, México. African Journal of Microbiology 6: 2305 -2312. [ Links ]
Hall,T,2005. Bio Edit v7.0.5. http://www.mbio.ncsu.edu/BioEdit/ [ Links ]
Harman, G.E, 2006. Overview of mechanisms and uses of Trichoderma spp. Phytopathology 96:190-194. [ Links ]
Krug, J.C, 2006. Moist chamber for the development of fungi. In: Mueller G.M., G.F. Bills, M.S. Foster (eds.), Biodiversity of Fungi Inventory and Monitoring Methods, Part III Apéndice I. USA, Elsevier Academic Press, pp. 589-594. [ Links ]
Masuhara, G., K. Katsuya, K. Yamaguchi, 1993. Potential for symbiosis of Rhizoctonia solani and binucleate Rhizoctonia with seeds of Spiranthes sinensis var. amoena in vitro. Mycological Research 97:746-752. [ Links ]
Michel-Aceves, A.C., M.A. Otero-Sánchez, L.Y Solano-Pascacio, R. Ariza-Flores, A. Barrios-Ayala, A. Rebolledo-Martínez, 2009. Biocontrol in vitro con Trichoderma spp. de Fusarium subglutinans (Wollenweb. y Reinking) Nelson, Toussoun y Marasas y F. oxysporum Schlecht., agentes causales de la "Escoba de Bruja" del mango (Mangifera indica L.). Revista Mexicana de Fitopatología. 27:18 - 26. [ Links ]
National Committee for Clinical Laboratory Standards, 2004. Method for antifungal disk diffusion susceptibility testing of yeasts. Approved guidance. Document M44-A. Wayne, PA: NCCL. [ Links ]
Quiroz-Sarmiento, V.F., R. Ferrera-Cerrato, A. Alarcón, M.E. Lara-Hernández, 2008. Antagonismo in vitro de Aspergillus y Trichoderma hacia hongos filamentosos que afectan el cultivo del ajo. Revista Mexicana de Micología 26:27-34. [ Links ]
Reino, J.L., R.F. Guerrero, R. Hernández-Galán, I.G. Collado, 2008. Secondary metabolites from species of the biocontrol agent Trichoderma. Phytochemistry Reviews 7:89 -123. [ Links ]
Reyes-Estebanez, M., J. Cristóbal-Alejo, E. Herrera-Parra, G. Heredia-Abarca, B. Canto-Canché, I.L. Medina-Baizabal, M. Gamboa-Angulo, 2011. Antimicrobial and nematotoxic properties of anamorphic fungi isolated from plant debris of tropical areas in México. African Journal of Microbiology 5:1083-1089. [ Links ]
Reyes, Y, B. Martínez, D. Infante, 2008. Evaluación de la actividad antagónica de trece aislamientos de Trichoderma spp. sobre Rhizoctonia sp. Revista de Protección Vegetal 23 (2): 112-117. [ Links ]
Schmitter-Soto, J.J., F.A. Comin, E. Escobar-Briones, J. Herrera-Silveira, J. Alcocer, E. Suárez-Morales, M. Elias-Gutiérrez, V. Díaz-Arce, L.E. Marín, B. Steinich, 2002. Hydrogeochemical and biological characteristics of cenotes in the Yucatán Península (SE México). Hydrobiologia 467:215-228. [ Links ]
Sibounnavong, P, S. Kanokmedhakul, K. Soytong, 2012. The role of Emericella rugulosa as a bio-control agent for controlling Fusarium wilt tomato. African Journal of Agricultural Research 7:4782-4789. [ Links ]
Soman, A.G., J.B. Gloer, RE Angawi, D.T. Wicklow, PE Dowd, 2001. Vertilecanins: new phenopicolinic acid analogues from Verticillium lecanii. Journal of Natural Products 64:189 -192. [ Links ]
Strobel, G., D.C. Manker, J. Mercier, 2012. Novel endophytic fungi and methods of use. PatentNo.; US 2012/0114610 Washington D.C: The United States of America. [ Links ]
Subban, K., R. Subramani, M. Johnpaul, 2013. A novel antibacterial and antifungal phenolic compound from the endophytic fungus Pestalotiopsis mangiferae. Natural Product Research 27: 1445-1449. [ Links ]
Vujanovic, V., 2012. Fusarium and other pathogenic fungi and mycotoxin biocontrol. Patent No.: US 2012/0156173 Washington DC: The United States of America. [ Links ]
Whipps, J.M., D.R Lumsden, 2001. Commercial use of fungi as plant disease biological control agents: Status and prospecte. In: Butt, T.M., C.W. Jackson, N. Magan (eds.), Fungi as biocontrol agents: Progress, problems and potentials. Wallingford, CABI Publishing.pp.9-22. [ Links ]
White, T.J., T. Bruns, S. Lee, J. Taylor, 1990. Amplification and direct sequencing of fungal ribosomal RNA genes forphylogenetics. In: Innis, M., D. Gelfand, J. Sninsky, T.J. White (eds.), PCR protocols, a guide to methods and applications. Academic Press, New York. pp. 315-322. [ Links ]
www.sigmaaldrich.com [http://www.sigmaaldrich.com/life-science/cell-culture/classical-media-salts/rpmi-media.html] Access date: 17-07-2014
www.seduma.yucatán.gob.mx. Secretaría de Desarrollo Urbano y Medio Ambiente. Cenotes y grutas [En Línea] http://www.seduma.yucatan.gob.mx/cenotes-grutas/censo-cenotes.php Access date: 17-06-2014. [ Links ]
Xu, X., J. Robinson, M. Jeger, P. Jeffries, 2010. Using combinations of biocontrol agents to control Botrytis cinérea on strawberry leaves under fluctuating temperatures. Biocontrol Science and Technology 20:359-373. [ Links ]
Yang, X., J. Zhang, D. Luo, 2012. The taxonomy, biology and chemistry of the fungal Pestalotiopsis genus. Natural Product Reports 29:622 -641. [ Links ]














