Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Revista mexicana de micología
Print version ISSN 0187-3180
Rev. Mex. Mic vol.39 Xalapa Jun. 2014
Contribuciones
The antifungal effect of Jacquinia macrocarpa plant extracts on the growth of Aspergillus flavus, A. parasiticus and Fusarium verticillioides
Efecto antifúngico de los extractos de la planta Jacquinia macrocarpa en el desarrollo de Aspergillus flavus, A. parasiticus y Fusarium verticillioides
Daniel Fernando Valenzuela-Cota, Génesis Vidal Buitimea-Cantúa, Ema Carina Rosas-Burgos*, Francisco Javier Cinco-Moroyoqui, María Susana Yépiz-Gómez, Mario Onofre Cortez-Rocha, Maribel Plascencia-Jatomea, Armando Burgos-Hernández
Departamento de Investigación y Posgrado en Alimentos. Universidad de Sonora. Blvd. Luis Encinas y Rosales s/n. Col Centro. CP. 83000 Hermosillo, Sonora, México.
* Autor para correspondencia:
Ema Carina Rosas-Burgos ecrosas@guayacan.uson.mx
Received 7 May 2013;
Accepted 21 March 2014.
Abstract
The aim of this study was to evaluate the effect of fractions obtained from the plant Jacquinia macrocarpa against phytopathogenic fungi Aspergillus flavus, A. parasiticus and Fusarium verticillioides. The powder of the dried plant materials was extracted with methanol (70 % v/v) and partitioned with hexane, ethyl acetate, and n-butanol. The most active fraction against the radial growth of the fungi was further fractionated by silica gel chromatography. The fraction from J. macrocarpa partitioned in n-butanol (BF) showed the highest antifungal activity against the three fungi. Sub-fraction F5.4 completely inhibited the growth of F. verticillioides, whereas the radial growth of A. flavus and A. parasiticus was inhibited 70 % and 64 %, respectively. Spores germination of A. parasiticus and F. verticillioides was inhibited 7.8 % and 11.8%, respectively. The lengths and diameters of hyphae of the three fungi were smaller than those of controls. Spore diameters of the Aspergillus species were reduced, while spores of F. verticillioides were not affected. The spore viability of A. flavus, A. parasiticus and F. verticillioides was reduced 40.7 %, 44.8 % and 46.3 %, respectively. Fraction F5.4 significantly inhibited the development of the three fungi, mainly F. verticillioides.
Keywords: antifungal activity, maize pathogens, plant extracts.
Resumen
El objetivo de este estudio fue evaluar el efecto de fracciones obtenidas de la planta Jacquinia macrocarpa contra los hongos fitopatógenos Aspergillus flavus, A. parasiticus y Fusarium verticillioides. Polvos de la planta deshidratada fueron extraídos con metanol (70 % v/v) y particionados con hexano, acetato de etilo y n-butanol. La fracción con mayor actividad contra el crecimiento radial de los hongos fue adicionalmente fraccionada por cromatografía en sílica gel. La fracción particionada en n-butanol (BF) mostro la mayor actividad antifúngica contra los tres hongos. La sub-fracción F5.4 inhibió el crecimiento radial de F. verticillioides completamente, mientras que el de A. flavus y A. parasiticus fue inhibido en 70 % y 64 %, respectivamente. La germinación de esporas de A. parasiticus y F. verticillioides fue inhibida en 7.8 % y 11.8 %, respectivamente. La longitud y el diámetro de las hifas de los tres hongos fueron menores que los de los controles. El diámetro de las esporas de las especies de Aspergillus fueron reducidas, mientras que las esporas de F. verticillioides no fueron afectadas. La viabilidad de A. flavus, A. parasiticus y F. verticillioides fue reducida en 40.7 %, 44.8 % y 46.3 %, respectivamente. La fracción F5.4 inhibió el desarrollo de los tres hongos significativamente, principalmente a F. verticillioides.
Palabras clave: actividad antifúngica, patógenos del maíz, extractos de plantas.
Introduction
The growth of mycotoxigenic fungi during culture and storage of corn causes serious economic losses and human and animal health problems in producer and consumer communities (Nelson, 1992; Nichols, 1983). Aflatoxins, produced mainly by Aspergillus flavus Link and A. parasiticus Speare (Dorner et al., 1984) and fumonisins, produced mainly by Fusarium verticillioides (Sacc) Nirenberg (synonym: Fusarium moniliforme J. Sheldon) [teleomorph: Gibberella fujikuroi (Sawada) Wollenw or Gibberella moniliformis Wineland] (Nelson, 1982), cause acute and chronic toxicity in animals of economic importance and are associated to cancer in humans (Eaton and Groopman, 1994; Constable et al., 2000; Marasas et al, 1984). Aflatoxin B1 is classified as potent carcinogenic agent, whereas fumonisin B1 has been considered as possible carcinogenic to human (IARC, 2002).
Synthetic fungicides have been employed for controlling pre- and postharvest diseases in plants (Sommer, 1985). However, in recent years, there has been a strong interest on biologically active plant compounds as potential alternatives to synthetic hazardous fungicides as that many microorganisms develop resistance to synthetic fungicide compounds (Cowen and Steinbach, 2008). In addition, use of synthetic fungicides has been increasingly restricted in many countries due to their entrance into the food chain (Soković and Van Griensven, 2006). Plants are generally assumed to be safer alternative than synthetic compounds (El-Ghaouth, 1997) and can be used as alternative antifungal treatments (Jobling, 2000; Rahman et al., 2010).
Jacquinia macrocarpa Cav. (San Juanico) is a plant used in the traditional medicine by the native people of Mexico (López-Estudillo and Hinojosa, 1988). The antimicrobial activity of some species of Jacquinia genus has been studied. An ethanolic extract of Jacquinia ruscifolia petals showed antifungal activity against ten of twelve pathogenic molds evaluated (Sharma et al., 2008). The methanolic crude extract from roots of Jacquinia flammea showed moderate antifungal activity against dermatophytes and very strong antifungal activity against Colletotrichum gloeosporioides. In that study the compound sakurasosaponin was reported as the main metabolite responsible for the antifungal activity (García-Sosa et al., 2011). Okunade and Wiemer (1985) found that the jacquinonic acid extracted from Jacquinia pungens showed ant repellent activity. Despite of all those studies, there is no research on the antimicrobial activity of Jacquinia macrocarpa specie and their affect against micotoxigenic fitophatogenic molds. The aim of this study was to evaluate the effect of J. macrocarpa crude and purified extracts against A. flavus, A. parasiticus and F. verticillioides.
Materials and methods
Plant materials
Aerial parts of the wild plant Jacquinia macrocarpa were collected in Los Arrieros Ranch, Guaymas, Sonora, México, a rural community located at the southwestern area of the state ofSonora (geographic coordinates: 28°17'00"N, 11°02'00" W). Identification of the plant species was carried out at the Herbarium of Departamento de Investigaciones Científicas y Tecnológicas de la Universidad de Sonora (DICTUS) in Hermosillo, Sonora, México (Voucher USON2008-6). Once in the laboratory, the plant samples were cut into small pieces and dried at room temperature (approximately 30 °C) during 15 days, in the dark.
Fungal cultures
Aspergillus flavus Link (NRRL 55210) and A. parasiticus Speare, anamorph (ATCC 16992) were inoculated on potato dextrose agar (PDA, Difco Laboratories; Detroit, MI) and incubated in the dark at 27 °C for 7 days until sporulation. Fusarium verticillioides (Sacc) Nirenberg (syn. Fusarium moniliforme J. Sheld, anamorph (ATCC 52539) was grown at the same conditions, except that the incubation temperature was 25 °C. Spores were harvested, counted using a Neubauer counting chamber and stored at 4 °C.
Preparation of plant extracts
Sixty grams of powdered aerial parts of J. macrocarpa were extracted with 1L of 70 % methanol, stirred for 1 h, and stored at room temperature for 72 h in the dark. After that, the extract was filtered first through Whatman filter paper No. 1 and then through micropore glass filter. The methanolic extract (crude extract) was evaporated to dryness at 40 °C under reduced pressure ( 19 % of yield was obtained), re-suspended in water, and sequentially partitioned with hexane, ethyl acetate and n-butanol (Koketsu et al, 1996), yielding 48.0 %, 6.4 %, 5.0 % and 27.0 % of solids, respectively. The crude and partitioned extracts were evaluated in their antifungal activity.
Chromatography in silica gel
The antifungal fraction obtained after partition with butanol (BF) was purified via column chromatography (2.5 x 100 cm) using silica gel 60 (Sigma-Aldrich Chemical Co., St. Louis, MO, particle size 70-230 mesh) and further fractionated by continuously washing the column with 500 mL of a solvent mixture containing butanol:acetic acid:water (4:1:5). Fractions of 5 mL were collected using a fraction collector. With the aim to identify and discard those fractions containing compounds of the preceding band, aliquote of 5 µL of every second fraction were analyzed by thin layer chromatography (TLC) using a precoated Kieselgel 60 F254 TLC plates (Merck Co., Darmstadt, Germany) and developed with the same solvent system used in the column separation. Spots in the TLC plates were visualized under UV light. The number of spots visualized on TLC indicated that five fractions eluted from the column and were identified as Fl to F5 according to their order of elution. All these fractions were evaporated to dryness at 40 °C under reduced pressure, dissolved in methanol, and assayed for antifungal activity. Fraction F5, which showed the highest antifungal activity, was further fractionated by TLC using 2 mm thickness plates (DC-Fertigplatten Si G-200 UV254) using the same solvent system employed in the column separation (Buitimea-Cantúa et al, 2010). Five fractions were obtained and identified as F5.1 to F5.5.
Radial extension growth
Petri dishes with potato dextrose agar (PDA) media containing 5 mg mL-1 of solids from plant extracts were centrally point-inoculated with 1 χ 104 spores mL-1 from 7-day-old cultures of A. flavus, A. parasiticus, and F. verticillioides. Two types of controls were prepared, one contained PDA media plus aliquote of each solvent used to dissolve the solids of the antifungal fraction, and the other one contained only PDA media. The inoculated Petri dishes were incubated in the darkness at 27 °C to grow Aspergillus species, or at 25 °C for F. verticillioides. The colony diameters were measured every 24 h during 336 h with the help of a caliper. The experiment was run in triplicate (Suarez-Jiménez et al, 2007).
To determine the most appropriate concentration of the antifungal fractions to be used in subsequent analyses, the following experiment was made. The partitioned fraction that showed the highest inhibition activity (n-butanol fraction) was evaporated to dryness under vacuum at 45 °C and re-dissolved in methanol. Petri dishes containing PDA media plus 1.0, 2.0, 3.0, 4.0 and 5.0 mg mL-1 of the extract were inoculated with 1 χ 104 spores mL-1 from 7-day-old cultures of each fungus. Measurement of colonies radius was carried out to determine the extract concentration that caused the maximum radial growth inhibition (MGI), the minimum inhibitory concentration that inhibited 50 % of radial growth (MIC50), and the minimum lethal concentration (MLC) that inhibited 100 % of radial growth and that did not succeed when it was transferred to fresh culture media containing no antifungal fraction (Rosas-Burgos et al., 2009). The experiment was run in triplicate.
The radial growth inhibition percentages were calculated using the following formula:

Where Rc is the mean value of colony radius of control PDA and Ri is the colony radius value of colonies grown in PDA media containing plant extracts taking into account the solvent effect (Plascencia-Jatomea et al., 2003).
Kinetics of spores germination
PDA plates containing 5 mg mL-1 of solids of the antifungal fraction F5.4 were inoculated by spreading 20 µL of a spore suspension containing 1 χ 105 spores onto the agar surface. The plates were incubated at 27 °C to grow Aspergillus species or 25 °C to grow F. verticillioides. The number of germinated spores per plate was determined every 3 h by counting 200 spores (germinated and non-germinated) using a light microscope. A spore was considered as germinated when the length of its germinal tube reached one-half of the spore diameter (Paul et al., 1993, Plascencia-Jatomea et al., 2003). Each germination assay was made in duplicate. Two types of controls were prepared, one contained PDA media plus methanol, and the other one contained only PDA media. The inhibition of spore germination was calculated using the following equation:

Where Se represents the percentage of germinated spores in samples treated with the antifungal fraction F5.4, and Sc is the percentage of germinated spores observed in the control containing methanol.
Hyphal diameter and length
Petri dishes with potato dextrose agar (PDA) containing 3.0 mg mL-1 of the antifungal fraction F5.4 was centrally point-inoculated with 1 χ 104 spores mL"1 from 7-day-old cultures of the fungi. After 72 h of incubation, one hundred measurements of diameters and lengths of apical hypha were made on mycelia. The measurements were carried out using the Image-Pro Plus version 6.3 software (Media Cybernetics, Inc.) as reported by Larralde et al. (1997) and Cox et al. (1998). One hundred measurements were also made on mycelia grown in the control plates. The experiment was repeated twice.
Spores diameter
Spores diameter was determined according to the procedure of Harris (1999). Coverslips were placed in Petri plates and covered with 10 mL of potato dextrose broth (PDB) containing 5.0 mg mL-1 of F5.4. Two types of controls were prepared, one contained PDB plus a volume of methanol equal to that used to dissolve the solids of the antifungal fraction, and the other one contained only PDB. The plates were inoculated with 20 µL of a 1 χ 105 spore suspension of A. flavus, A. parasiticus, or F. verticillioides, and their development was monitored until they germinated. One coverslip containing spores was removed at random every 3 h from the plates and 100 measurements of spore diameter were carried out using the Image-Pro Plus version 6.3 (Media Cybernetics, Inc.). For F. verticillioides spores, the measurements were made wide- and length-wise. The experiment was repeated twice.
Spores viability
Spores viability analysis of the fungi was made according the methodology described by Granjo et al. (2007). Discs of sterilized cellophane measuring 2.5 cm in diameter were distributed with sterile forceps on the surface of a Petri dish containing solidified PDA medium plus 5 mg mL-1 of F5.4. Two types of controls with no cellophane discs were prepared, one contained PDA media plus a volume of methanol equal to that used to dissolve the solids of the antifungal fraction, whereas the other one contained only PDA media. The cellophane discs were inoculated with 50 µL of a 1 χ 105 spore suspension of each fungus and incubated at 27 °C to grow Aspergillus species or at 25 °C to grow F. verticillioides. The cellophane discs were incubated by 0,12,18,24,48,72 y 96 h. At the end of each incubation time, each cellophane disc was removed from the Petri dish and placed over a microscope slide and covered with equal volumes of final working solutions of FDA [3,6' diacetyl fluorescein-FDA (Sigma Chemical Co., St. Louis, MO, USA); 5 mg mL-1 acetone and diluted 2500 times in phosphate-bufered saline (PBS), pH 7.4 at the time of use] and EB [2.7-diamino-10-ethyl-9-phenyl phenanthridine (Sigma Chemical Co.); 1 g mL-1 in PBS and diluted 20 times in PBS at the time of use] (Granjo et al., 2007). The treated slides were incubated at 25 °C for 30 min, covered with a coverslip, and observed under a fluorescence microscope (Leica DM 2500). One hundred spores were analyzed for each incubation time and the proportion of the green-colored viable spores and the red-colored non-viable spores was determined. The experiment was repeated twice.
Statistical analysis
Analysis of data was carried out by ANOVA using a factorial design (plant and extraction solvent). Comparison of means was performed by the Tukey test (Ρ<0.05) using the SAS program (SAS, 2005).
Results
Radial extension growth
The crude methanolic extract from J. macrocarpa (CE) and every partitioned fraction showed radial growth inhibition against the three fungi (P<0.05), with major inhibition effect against F. verticillioides (Table 1). The n-butanol fraction (BF) showed the highest radial growth inhibition against the three fungi. F. verticillioides did not show statistical differences (P<0.05) in inhibition growth by BF and CE. Similarly, no statistical differences (P<0.05) were also observed in inhibition growth of A. parasiticus by AEF and WF, whereas the radial growth inhibition values of A. flavus by CE and all fractions differed significantly (P<0.05). The antifungal fractions obtained in the different stapes of purification (F5 and F5.4) showed high radial growth inhibition activity against the three fungi in comparison to those of the partitioned fractions and CE. The radial growth of F. verticillioides was totally inhibited when it was inoculated on PDA containing fractions F5 and F5.4.
MGI, MIC50 and MLC of BF
The three fungi showed gradual radial growth inhibition when the BF concentration in the PDA media was increased (Table 2). The MGI for A. flavus and A. parasiticus was 5.0 mg mL-1, which caused that the fungi did not reach 50 % of radial growth inhibition. F. verticillioides showed MGI and MIC50 values of 4.0 mg mL-1 and 1.0 mg mL-1, respectively. In the presence of BF, the radial growth inhibition of A. flavus and A. parasiticus were 70 % and 64 %, respectively. However, when F. verticillioides was inoculated on PDA media containing the fractions obtained from BF, its radial growth was completely inhibited (Table 1) although was able to grow when it was transferred to PDB containing no antifungal fraction. The influence of the variable concentration of the antifungal fraction BF over the three fungi is shown in Figure 1.
Kinetics of spores germination
The spores of the three fungi inoculated on the PDB controls completely germinated within 30 h. Although spores germination of A. flavus was delayed when they were grown on PDA containing the antifungal F5.4 fraction, all spores were able to germinate at the end of the experiment (Figure 2). Similar results were observed for A. flavus, A. parasiticus and F. verticillioides spores, which were delayed in growth on PDA containing the F5.4 fraction (Figure 2). However, the spores of the two last fungi showed significant (P<0.05) germination inhibition at the end of the incubation time (7.8 % and 11.8 % for A. parasiticus and F. verticillioides, respectively).
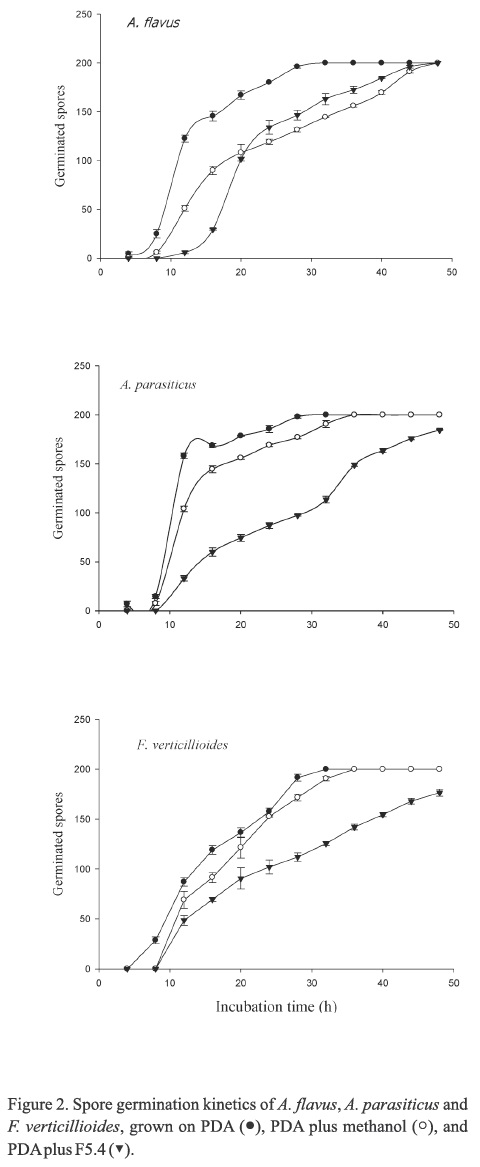
Hyphal diameter and length
The three fungi significantly (P<0.05) underwent a reduction in diameter and length of apical hyphae when they were grown on PDA agar media containing the F5.4 fraction in respect to the PDA and methanol controls. A. flavus apical hyphae showed a reduction in diameter and length in 20.8±1.8 % and 43.8±2.1 %, whereas those for A. parasiticus were reduced in 18.5±0.8 % and 40.1±1.7 %, respectively. The decrease in diameter and length of F. verticillioides apical hyphae was of 18.2±1.6 % and 41.4±2.6 %, respectively. The three fungi showed the higher (P<0.05) reduction percentage in length than in diameter.
Spores diameter
Diameter of spores of each fungi were measured every 3 h of incubation. In both Aspergillus species, the spores diameter increased when they were incubated in PDB and PDB containing methanol, however the diameter of spores of Aspergillus species were reduced in PDB containing the antifungal fraction F5.4 (Figure 3). Spores of F. verticillioides showed no significant differences (P<0.05) in length or diameter throughout the incubation time (Figure 3).
Spores viability
Spores of three fungi treated with the antifungal fraction F5.4 showed a significant viability reduction (P<0.05) after 96 h of incubation. The spores viability of A. flavus, A. parasiticus and F. verticillioides was reduced in 40.7±1.8 %, 44.8±1.9 % and46.3±0.9 %, respectively.
Discussion
Fungal growth inhibition of few Jacquinia species has been reported. Sharma et al. (2008) studied an ethanolic extract of petals of J. ruscifolia and reported growth inhibition of some fungi such as A. flavus and F. moniliforme (syn. F. verticillioides). García-Sosa et al. (2011) reported antifungal activity of a methanolic extract of J. flammea against phytopathogenic fungi different to those evaluated in the present study. Although, extracts from other plants have been tested in their antifungal activity against A. flavus, A. parasiticus and F. verticillioides (Dabur et al., 2005; Rosas-Burgos et al., 2009; Sánchez et al., 2005; Satish et al., 2007; Suárez-Jiménez et al., 2007; Tequida-Meneses et al., 2002; Vargas-Arispuro et al., 2005), no studies on the antifungal activity of J. macrocarpa have not been reported before. Results of the radial growth inhibition test for A. flavus were similar to those reported by Tequida-Meneses et al. (2002), although they differed to those reported by other authors (Cowen and Steinbach, 2008; Rosas-Burgos et al., 2009; Sanchez et al., 2005; Satish et al., 2007; Vargas-Arispuro et al., 2005).
Radial growth inhibition of A. parasiticus and F. verticillioides by methanolic plant extracts have also been observed by Sánchez et al. (2005) and Suárez-Jiménez et al. (2007), respectively. The radial growth inhibition value of F. verticillioides was the highest observed in all fungi, which is in agreement with other authors (Tequida-Meneses et al., 2002; Rosas-Burgos et al., 2009). Sharma et al. (2008) reported a similar inhibition effect against F. moniliforme and A. flavus of ethanolic petals extracts of J. ruscifolia. In the present study, the fractions obtained after the chromatographic purification steps (F5 and F5.4) from BF, showed the higher antifungal activity over the three fungi than that of the crude extracts and partitioned fractions. It was observed that F. verticillioides was the most inhibited mold when it was inoculated on PDA containing the fraction F5 or F5.4. The concentration of BF that caused the maximum radial growth inhibition of both Aspergillus species was significantly higher (P<0.05) than that for F. verticillioides, which confirms that this last fungus is very sensible to the antifungal fraction of J. macrocarpa. The results of this study indicate that J. macrocarpa contains antifungal compounds that are active against the growth of the fungi tested in the present study, especially against F. verticillioides.
Retarded spore germination observed in the present study was also reported in F. verticillioides by methanolic extracts of Baecharis glutinosa (Suárez-Jiménez et al., 2007). More recently, Rosas-Burgos et al. (2011) reported retarded germination of spores of A. flavus, A. parasiticus and F. verticillioides by a purified fraction of B. glutinosa. Rosas-Burgos et al. (2011) observed lower spore germination inhibition in A. parasiticus than those observed in the present study. However, those authors reported higher inhibition of F. verticillioides spores, which was not observed in this work. The high spore viability inhibition of A. parasiticus and F. verticillioides (P<0.05) coincided with high spore germination inhibition, whereas the opposite was observed in A. flavus, that is, at lower number of viable spores, the lower spores number with germination capacity.
Reduction in diameter and length of apical hyphae observed in the three fungi treated with a purified fraction of B. glutinosa was previously observed by Rosas-Burgos et al. (2011), although these authors observed a more pronounced antifungal effect of the plant extract. In the present study, spores of Aspergillus species incubated in PDA controls increased in size, while the spores incubated in the presence of the antifungal F5.4 fraction showed a reduction. Rosas-Burgos et al. (2011) reported that in A. flavus and A. parasiticus the spores diameter increased when they were incubated in media controls and media containing the B. glutinosa antifungal fraction, although the increase observed was smaller with the antifungal treatment in comparison of that of the controls.
A study reported by Ha et al. (2006) indicated that Fusarium solani f. sp. pisi, resistant to caspofungin acetate, which target is the fungal wall, decreased its resistance when FsFKS1 that encodes the ß(1,3)-D-glucan synthase was reduced, leading to a reduction in spore viability and causing lysis of spores and hyphae. The radial growth inhibition, delay of spores germination, size reduction of both hyphae and spores and spores and viability observed in the present study, might be due to the loss of cell wall integrity.
It is possible that some compounds such as sakurasosaponin and jacquinonic acid obtained from other Jacquinia species (J. flammea and J. pungens, respectively), which have been reported to show antifungal or repellent activity (García-Sosa et al., 2011; Okunade and Wiemer, 1985), might be responsible of the antifungal activity of J. macrocarpa fractions used in this study.
The results of this study demonstrated that J. macrocarpa contains antifungal compounds that could be used as an alternative method to treat corn and other cereals grains to control phytopathogenic molds, especially F. verticillioides.
Acknowledgments
The authors are grateful to the National Science Council and Technology (CONACyT)) for granting funding for this study (Ref. 58249).
References
Buitimea-Cantúa, G.V., E.C. Rosas-Burgos, M.O. Cortez-Rocha, J.C. Gálvez-Ruiz, R.I. Sánchez-Maríñez, 2010. Aislamiento biodirigido y análisis químico de un extracto con actividad antifúngica de Jacquinia macrocarpa (San Juanico). VII Congreso del Noroeste y III Nacional en Ciencias Alimentarias y Biotecnología, Universidad de Sonora, Hermosillo, México, pp 1025-1040. [ Links ]
Constable, P.D., G.W. Smith, GE. Rottinghaus, W.M. Hascheck, 2000. Ingestion of fümonisin Β,-containing culture materials decreases cardiac contractility and mechanical efficiency in swine. Toxicology and Applied Pharmacology 162:151-160. [ Links ]
Cowen, L.E., W.J. Steinbach, 2008. Stress, drugs, and evolution: the role of cellular signaling in fungal drug resistance. Eukaryotic Cell 7: 747-764. [ Links ]
Cox, P.W., G.C. Paul, C.R. Thomas, 1998. Image analysis of morphology of filamentous micro-organisms. Microbiology 144:817-827. [ Links ]
Dabur, R, A.K. Chhillar, V. Yadav, P.K. Kamal, J. Gupta, G.L. Sharma, 2005. In vitro antifungal activity of 2-(3,4-dimethyl-2,5-dihydro-lH-pyrrol-2-yl)-l-methylethyl pentanoate, a dihydropyrrole derivative. Journal of Medical Microbiology 54:549-552. [ Links ]
Dorner, J.W., R.J. Cole, U.L. Diener, 1984. The relationship of Aspergillus flavus and Aspergillus parasiticus with reference to production of aflatoxins andcyclopiazonic acid. Mycopathologia 87:13-15. [ Links ]
Eaton, D.L., J.D. Groopman, 1994. The toxicology of aflatoxins. Academic Press Inc., New York. [ Links ]
El-Ghaouth, A, 1997. Biologically-based alternatives to synthetic fungicides for the control of postharvest diseases. Journal of Industrial Microbiology and Biotechnology 19:160-162. [ Links ]
García-Sosa, Κ., A. Sánchez-Medina, SX. Alvarez, S. Zecchino, N.C. Veitch, P. Simá-Polanco, L.M. Peña-Rodríguez, 2011. Antifungal activity of sakurasosaponin from the root extract of Jacquinia flammea. Natural Products Research 25:1185-1189. [ Links ]
Granjo, CA., T.A. dos Reis, W. Gambale, Β. Correa, 2007. Morphogenesis and growth kinetics of Fusarium verticillioides. Mycopathologia 164:119-126. [ Links ]
Ha, Y., S.F. Covert, M. Momany, 2006. FsFKSl, the 1,3-ß-glucan synthase from the caspofungin-resistant fungus Fusarium solani. Eukaryotic Cell 5:1036-1042. [ Links ]
Harris, S.D., 1999. Morphogenesis is coordinates with nuclear division in germinating Aspergillus nidulans conidiospores. Microbiology 145:2747-2756. [ Links ]
IARC (International Agency for Research on Cancer), 2002. Fumonisin B1 IARC monographs on the evaluation of carcinogenic risks to humans 82:301-366. [ Links ]
Jobling, J., 2000. Essential Oils: a new idea for postharvest disease control. Good Fruit Vegetables Magazine 11:50. [ Links ]
Koketsu, M., M. Kim, T. Yamamoto, 1996. Antifungal activity against food-borne fungi of Aspidistra eliator Blume. Journal of Agricultural andFood Chemistry 44:301-303. [ Links ]
Larralde, C.C., L.F. López, G.G. Viniegra, 1997. Morphometry evaluation of the specific growth rate of Aspergillus niger grown in agar plates at high glucose levels. Biotechnology and Bioengineering 56: 287-294. [ Links ]
López-Estudillo, R., A. Hinojosa, 1988. Catálogo de Plantas Medicinales Sonorenses. Universidad de Sonora, Hermosillo. [ Links ]
Marasas, W.F.O.,N.P. Kriek, J.E. Fincham, S.J. van Rensburg, 1984. Primary liver cancer and esophageal basal-cell hyperplasia in rats caused by Fusarium moniliforme. International Journal of Cancer 34: 383-387. [ Links ]
Nelson, P.E., 1992. Taxonomy and biology of Fusarium moniliforme. Mycopathology 117:29-36. [ Links ]
Nichols, T.E., 1983. Economic impact of aflatoxins in corn. In: Diener, L., R. Asquith, J. Dickens (ed.), Aflatoxin and Aspergillus flavus in corn. Alabama Agricultural Experimental Station, Auburn, pp. 67-71. [ Links ]
Okunade, A.L., D.F Wiemer, 1985. Jacquinonic acid, an ant-repellent triterpenoid from Jacquinia pungens. Phytochemistry 24: 1203-1205. [ Links ]
Paul, G.C, CA. Kent, C.R. Thomas, 1993. Viability testing and characterization of germination of fungal spores by automatic image analysis. Biotechnology and Bioengineering 42:11-23. [ Links ]
Plascencia-Jatomea, M., G. Viniegra, R. Olayo, M.M. Castillo-Ortega, K. Shirai, 2003. Effect of chitosan and temperature on spore germination of Aspergillus niger. Macromolecular Bioscience 3: 582-586. [ Links ]
Rahman, Α., M.A. Hossain, S.C. Kang, 2010. Control of phytopathogenic fungi by the essential oil and methanolic extracts of Erigeron ramosus (Walt.) B.S.P. European Journal of Plant Pathology 128: 211-219. [ Links ]
Rosas-Burgos, E.C., M.O. Cortez-Rocha, F.J. Cinco-Moroyoqui, R.E. Robles-Zepeda, J. López-Cervantes, D.I. Sánchez-Machado, F. Lares-Villa, 2009. Antifungal activity in vitro of Baccharis glutinosa and Ambrosia confertiflora extracts on Aspergillus flavus, Aspergillus parasiticus and Fusarium verticillioides. World Journal of Microbiology and Biotechnology 25: 2257-2261. [ Links ]
Rosas-Burgos, E.C., M.O. Cortez-Rocha, M. Plascencia-Jatomea, F.C. Cinco-Moroyoqui, RE. Robles-Zepeda, J. López-Cervantes, D.I. Sánchez-Machado, F. Lares-Villa, 2011. The effect of Baccharis glutinosa extract on the growth of mycotoxigenic fungi and fumonisin B1 and aflatoxin B1 production. World Journal of Microbiology and Biotechnology 27:1025-1033. [ Links ]
Sánchez, E., N. Heredia, S. García S., 2005. Inhibition of growth and mycotoxin production of Aspergillus flavus and Aspergillus parasiticus by extracts of Agave species. International Journal of Food Microbiology 98:271-279. [ Links ]
SAS, Institute, 2005. SAS/STAT User's Guide, Version 8, SAS Publishing, N.C. [ Links ]
Satish, S., D.C. Mohana, M.P. Raghavendra, K.A. Raveesha, 2007. Antifungal activity of some plant extracts against important seed borne pathogens of Aspergillus sp. Journal of Agricultural Technology 3:109-119. [ Links ]
Sharma, RS., V. Mishra, R. Singh, N. Seth, C.R. Babu, 2008. Antifungal activity of some Himalayan medicinal plants and cultivated ornamental species. Fitoterapia 79:589-591. [ Links ]
Soković, M., L.J.L.D. Van Griensven, 2006. Antimicrobial activity of essential oils and their components against the three major pathogens of the cultivated button mushroom, Agaricus bisporus. European Journal of Plant Pathology 116:211-224. [ Links ]
Sommer,N.F., 1985. Strategies for control ofpostharvest diseases of selected commodities. In: A. Kader, R.F. Kasmine, J.F. Thompson (eds.), Postharvest Technology of Horticultural Crops. Cooperative Extension, Oakland, pp. 83-99. [ Links ]
Suárez-Jiménez, GM., M.O. Cortez-Rocha, E.C. Rosas-Burgos, A. Burgos-Hernández, M. Plascencia-Jatomea, F. J. Cinco-Moroyoqui, 2007. Antifungal activity of plant methanolic extracts against Fusarium verticillioides (Sacc.) Nirenb, and fumonisin B1, production. Revista Mexicana de Fitopatología 25:134-142. [ Links ]
Tequida-Meneses, M., M.O. Cortez-Rocha, E.C. Rosas-Burgos, S. López-Sandoval, C. Corrales-Maldonado, 2002. Efecto de extractos alcohólicos de plantas silvestres sobre la inhibición de crecimiento de Aspergillus flavus, Aspergillus niger, Pénicillium chrysogenum, Pénicillium expansum, Fusarium moniliforme y Fusariumpoae. Revista Iberoamericana de Micologia 19:84-88. [ Links ]
Vargas-Arispuro, I., R. Reyes-Báez, G. Rivera-Castañeda, M.A. Martinez-Téllez, I. Rivero-Espejel, 2005. Antifungal lignans from the creosotebush (Larrea tridentata). Industrial Crops and Products 22:101-107. [ Links ]














