Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista mexicana de micología
versión impresa ISSN 0187-3180
Rev. Mex. Mic vol.33 Xalapa jun. 2011
Contribuciones
Nematophagous fungi (Orbiliales) capturing, destroying and feeding on the histotrophic larvae of Haemonchus contortus (Nematoda: Trichostrongylidae)
Hongos nematófagos (Orbiliales) capturando, destruyendo y alimentándose de larvas histiotrópicas de Haemonchus contortus (Nematoda: Trichostrongylidae)
Ivonne Carolina Alfaro Gutiérrez1,3, Pedro Mendoza de Gives1, Enrique Liébano Hernández1, Ma. Eugenia López Arellano1, Rosa Ofelia Valero Coss1, Víctor Manuel Hernández Velázquez2
1 INIFAP, Carretera Federal Cuernavaca–Cuautla, No 8534, Col. Progreso, Jiutepec, Morelos, CP 62500, México.
2 Centro de Investigaciones en Biotecnología, Universidad Autónoma del Estado de Morelos (UAEM).
3 Facultad de Ciencias Biológicas, (UAEM), Av. Universidad 1001, Col. Chamilpa, Cuernavaca, Morelos, CP 62209, México.
Autor para correspondencia:
Pedro Mendoza de Gives
pedromdgives@yahoo.com
Recibido 3 de julio 2010;
aceptado 29 de marzo 2011.
Resumen
Este trabajo investigó el comportamiento depredador de tres hongos nematófagos (HN) contra larvas histiotróficas (L4) de Haemonchus contortus, usando a larvas infectantes (L3) como patrón de comparación. Los hongos fueron aislados de suelo e identificados como Arthrobotrys oligospora, A. musiformis y Monacrosporium thaumasium. Se utilizaron dos series de placas de agar conteniendo los tres HN. En cada placa de las series 1 y 2, se depositaron 200 larvas histotrópicas, e infectantes; respectivamente y se mantuvieron a temperatura ambiente. La reducción larval se estimó comparando las medias de larvas recuperadas en ambas series. Los tres hongos capturaron y se alimentaron de ambos tipos de larvas de la siguiente manera: Las larvas infectantes se redujeron por acción de A. oligospora en 90.3%, A. musiformis 97% y M. thaumasium 62.3% (P>0.0001). Para el caso de larvas histiotrópicas, los hongos redujeron la población de la siguiente manera: A. oligospora 63.3%; A. musiformis 73.2% y M. thaumasium 69.2% (P>0.0003). La más baja actividad de captura se observó con A. oligospora y A. musiformis sobre larvas histiotrópicas.
Palabras clave: hongos atrapadores de nemátodos, Arthrobotrys; Monacrosporium.
Abstract
This research was aimed at investigating the predatory behavior of three nematophagous fungi against the histotrophic larvae of H. contortus (L4). Infective larvae (L3) of H. contortus were used for comparison. Three nematophagous fungi were isolated from soil and identified as Arthrobotrys oligospora, A. musiformis and Monacrosporium thaumasium. Two series of agar plateswere used to grow the three NF. Two hundred histotrophic or infective larvae of H. contortus were added to series 1 plates and series 2 plates, respectively, and kept at room temperature. The reduction in larval population was estimated by comparing the mean number of larvae recovered from both series. Capture rates of infective larvae were as follows: A. oligospora 90.3%, A. musiformis 97%, and M. thaumasium 62.3% (P>0.0001). In comparison, the predatory capture rates of t the histotrophic larval populations were as follows: A. oligospora 63.3%; A. musiformis 73.2% and M. thaumasium 69.2% (P>0.0003). The lowest capture rate of histotrophic larvae was observed with A. oligospora and A. musiformis.
Keywords: nematode–trapping fungi; Arthrobotrys; Monacrosporium.
Introduction
Nematophagous fungi have been evaluated as possible biological control agents of both animal and plant parasitic nematodes (Tahseen et al., 2005, Mendoza de Gives et al., 1999). In previous studies, some fungi have been evaluated for their capacity to capture infective larvae of Haemonchus contortus (L3) under both in vitro and in vivo conditions (Mendoza de Gives et al., 1994, 2006, Arroyo Balán et al., 2008, Casillas Aguilar et al., 2008). The capture of nematodes by nematophagous fungi is determined by a recognition mechanism that is regulated by glycoprotein receptors present in both the cuticle of nematodes and on the fungal cells (Barron, 1977). Previous studies reported differences in the cuticle antigenic mosaic during the different developmental stages of H. contortus (Fetterer, 1989). The protein constitution of the parasitic nematode's cuticle varies among the different developmental stages, i.e., H. contortus adult stages contain higher amounts of basic amino acids than do the larval stages (Cox et al., 1989). Furthermore, the different surface–protein patterns suggest differences between the exposed antigens of infective (L3) and histotrophic (L4) larvae. These differences between the L3 and L4 larvae are perhaps attributable to the totally different environments in which they normally inhabit. For example,the first endo–parasitic stage of the L4 larvae occurs in sheep where there is no possible contact with the external environment.
The biochemical structure of these cuticle proteins has an important biological role, such as evading the immune defenses of the host (Cox et al., 1989). Previously, it was unknown if differences in the antigenic mosaic between the infective and histotrophic larvae of H. contortus influenced the predatory behavior of nematophagous fungi. The present paper tests the hypothesis that histotrophic larvae of H. contortus are less efficiently trapped by nematophagous fungi than are the infective larvae of the parasite. Specifically, this study was aimed at investigating the in vitro predatory activity of three nematophagous fungi against H. contortus histotrophic larvae (L4) using infective larvae (L3) for comparison.
Materials and methods
Location
This work was conducted at the National Centre for Disciplinary Research in Veterinary Parasitology, (CENID–PAVET–INIFAP), located at Jiutepec Municipality, State of Morelos, Mexico.
Nematophagous fungi
Fungi were isolated from mossy soil, decaying plant material (a rotten trunk) and the soil containing Brahea palm roots.
The "soil sprinkle" technique developed by Barron (1977) was used to capture the nematophagous fungi. The addition of nematode "baits" was used to stimulate the fungal growth. Fungal structures typical of nematophagous fungi, i.e., conidiophores, conidia, trapping devices and trapped nematodes, were observed under a microscope. Such structures were transferred to Petri dishes containing sterile water agar. After 14 days of growth, fungi were subcultured to sterile water agar plates to obtain pure cultures of the fungal isolates. Fungal isolates were taxonomically identified using the keys for nematophagous fungi published by Cooke and Godfrey (1964) and Rubner (1996).
Haemonchus contortus infective larvae
A 6–month–old Pelibuey, male lamb, was experimentally infected with 350 H. contortus infective larvae (L3) per kg of body weight. After 21 days of prepatent period (or the incubation period into the animal), the McMaster coprological technique (Anonymous, 1971) was carried out to determine the presence of the infection based on number of nematode eggs eliminated per gram of feces (epg). Once the lamb was shown to be infection positive, it was kept in a metabolic cage and the total volume of feces was collected on a plastic bowl. Feces were then ground and mixed with small pieces of polyurethane. Water was added to obtain a suitable medium for incubating the nematode eggs and supporting a maximum number of hatched larvae. After 5–7 days of incubation, infective larvae were recovered from fecal cultures and purified by density–gradient centrifugation, using a 50% sucrose solution. Recovered larvae were then rinsed in penicillin–streptomycin solution (200 UI and 260 (µg/ml; respectively) and re–suspended in sterile water.
Haemonchus contortus histotropic larvae
A H. contortus in vitro development technique was conducted to remove the larval sheaths (Ramírez Vargas et al., 2006). This technique was performed in two stages: a) larvae unsheathing, using 6% sodium hydrochloride. (Note: double dilutions were performed until achieving a 0.187% final concentration). The time needed for larvae unsheathing after exposure to the established sodium hydrochloride concentration was observed under the microscope. The loosening of the larvae sheath was facilitated by strong lateral movements of the larvae. To support development of unsheathed infective larvae to histotrophic stages, larvae were put on a medium containing Hank's Solution (49.2 ml), antibiotic–antimycotic supplements (246 of 10,000 units/ml. Penicillin G Sodium, 10,000 µg/ml Streptomycin sulfate, 25 µg/ml amphotericin B ) and sheep's red blood cells (8µl). Larvae were incubated in this medium at 37°C with 95% CO2 95% and 5% air for 14 days. After this period, a light microscope was used to observe the typical features of the fourth larval (L4) stage of the parasite, including the formation of their oral cavity and perfectly defined intestine (Ramírez Vargas et al., 2006).
Quantification of Haemonchus contortus larvae
To estimate the number of H. contortus larvae in a known volume of a larval suspension, ten 5–µl (vortex) aliquot drops were deposited on a slide and the larvae were counted using a light microscope. The mean number oflarvae in these aliquots was used to estimate the total number of larvae contained in the total volume of the larval suspension.
Evaluation of the predatory activity of nematophagous fungi against Haemonchus contortus infective and histotropic larvae
For the purposes of this study, two series of 40 water agar plates were used. Plates were divided into groups of 10 plates, each with 10 replicates per group. The first three groups of each series were designated as groups 1, 2 and 3, which contained 10–day–old fungal cultures of Arthrobotrys oligospora, A. musiformis or Monacrosporium thaumasium, respectively. Plates in group 4 constituted the control group and contained only water agar. Plates of the series 1 and 2 were supplied with 20 |l of an aqueous suspension with a known number of either infective larvae (184.3±64) or histotrophic larvae (107±37); respectively. All the plates were incubated at room temperature (25–30°C) for 7 days. After this period, the predatory activity of the fungi was observed under a compound microscope using magnifications of 10, 40, and 100X.
At the conclusion of the interaction period between fungi and larvae, the contents of every plate was put in separate Baermann funnels to measure the total of number of non–captured larvae, assuming that the captured larvae were destroyed by the fungal action. The estimation of the reduction in the larval population (A) on each plate was calculated using the following formula provided by Aguilar et al. (2008).

where:
A=Reduction percentage of the larval population
X control= Number of larvae recovered from the control group.
X treated= Number of larvae recovered from the fungal treated group.
Statistic analysis
Data were analyzed by a two factorial analysis of variance (factor 1, H. contortus, L3 or L4; factor 2, three nematophagus fungi) in a completely randomized design with ten replicates using Proc GLM (SAS statistical program, version 9.1). The Tukey honestly significant difference (Tukey HSD) multiple comparison test was also employed to determine significant differences between means in the event of a significant F value for ANOVA. Further separation of each fungal species on the reduction percentage of the L3 or L4 was performed using t test (Zar, 1999).
Results
Predatory activities of the three nematophagous fungi against both Haemonchus contortus developmental stages (L3 and L4) were visualized under the microscope (Figures 1, 2, 3). The number of H. contortus larvae of both development stages recovered from plates of the two series is shown in Table 1. The reduction percentage of the number of larvae of either infective (L3) or histotropic (L4) larval stages is shown in Table 2. The reduction percentages of the H. contortus infective larvae (L3) populations by Arthrobotrys oligospora, A. musiformis and Monacrosporium thaumasium were 90.3 (±8), 97 (±3.9) and 62.3% (±18.5), respectively. Meanwhile, these three nematophagous fungi reduced the histotropic larvae (L4) populations by 59.7 (±17.5), 75 (±13.9) and 69.2% (±14); respectively. The statistical analysis revealed that in both cases significant differences were found. When the predatory activity of A. oligospora against larvae of both developmental stages H. contortus was compared, the "t" test showed that larvae of the third stage (L3) of the parasite were more efficiently captured and destroyed compared to histotrophic larvae (L4) (P<0.0001).
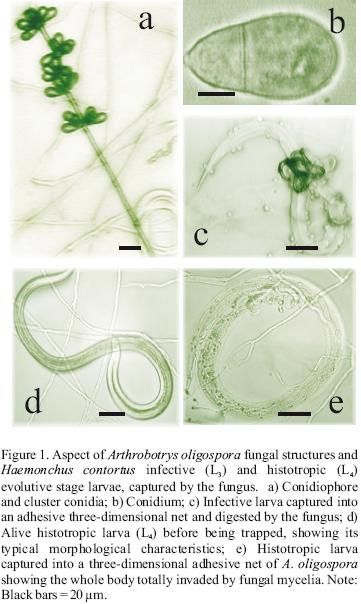
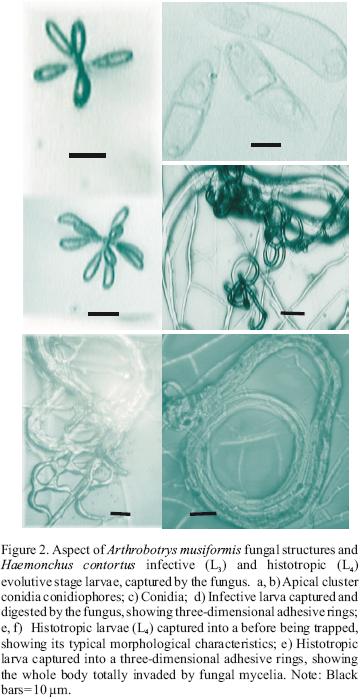

Similarly, A. musiformis also reduced the third stage (L3) larval population of the parasite more efficiently than the fourth larval stage (L4) (P<0.0051). In contrast, no statistically significant difference was found in the ability of M. thaumasium to capture either infective (L3) or histotropic (L4) larvae (P<0.515), and the predatory activity of M. thaumasium was lower than the two other Arthrobotrys species tested.
A normal distribution of data was assumed and dependence between both series was reveled (P<0.0001) obtaining a variation coefficient of 16.74. The statistical analysis allowed a comparison of the capture percentages of infective larvae (L3) among the three fungi evaluated, and also showed a statistical significant difference between M. thaumasium and the other two fungi A. oligospora and A. musiformis; being M. thaumasium less active than the other two fungi. In contrast, no statistically significant differences were observed among the capture percentages of the three fungi against histotropic larvae (L4), and the variation coefficient recorded was = 17.84.
Discussion
Nematophagous fungi are capable of producing trapping devices especially designed to capture live nematodes in the soil, kill them, and use them for nutrition (Barron, 1977). At the beginning of the capture process by nematophagous fungi, a receptor–recognition process occurs. Such receptors are presents on the cuticle of the nematodes and they are composed of carbohydrates (Nordbring–Hertz et al., 1982, Rosenzweig, 1983). Differences in the protein profile between the infective and histotropic larvae of the sheep parasitic nematode Haemonchus contortus have been identified (Cox et al., 1989). The present paper shows evidence that the nematophagous fungal species Arthrobotrys oligospora and A. musiformis capture infective (L.) larvae of H. contortus more efficiently than histotropic (L4) larvae. This difference in capture efficiency is perhaps because the histotropic larvae do not possess the complementary molecules in their cuticles that allow for recognition by the nematophagous fungi. In nature, nematophagous fungi typically encounter infective (L3) larvae, but not histotropic (L4) larvae, in soil and animal feces. Because nematophagous fungi typically only encounter infective (L3) larvae in nature, it seems reasonable that the higher capture efficiency of infective larvae is likely due to an adaptation response in the protein profile of the fungi. Such adaptation would allow recognition of carbohydrates present on the nematode's infective larvae cuticle. In this manner, nematophagous fungi are prepared to play their biological role as predatory organisms that help to control nematodes in soil or in animal faeces. In the case of H. contortus the abomasum mucosa of infected sheep is the natural habitat for histotropic larvae (L4). Thus, a no direct relationship occurs between histotropic larvae (L4) and nematophagous fungi, which likely contributed to lower receptor compatibility. In contrast, Monacrosporium thaumasium showed a similar moderate predatory activity against both development stages of H. contortus. In contrast to our study, a previous work with some species of Monacrosporium found that fungi of this genus generally show a slow predatory activity and a low predatory efficiency against larvae of gastrointestinal parasitic nematodes (Morgan et al., 1997). Interpreting such complex interactions is complex, but it must be considered that the dynamic evolutionary processes of any organism in nature are dynamic. An organism's genome undergoes a continuous process of adaptation to the environment and selection pressures from numerous interacting organisms (Darwin, 2004). In this manner, the nematodes would be expected to develop mechanisms to evade the capture of nematophagous fungi, perhaps by probably modifying their antigenic mosaic. Similarly, nematophagous fungi are expected to respond by developing mechanisms that allow their continued capture and feeding on nematodes. This dynamic evolutionary process could help to understand why some isolates of the same species display variable predatory activity against the same nematode species. For example, in a previous study, one isolate of Arthrobotrys robusta showed a high in vitro capture efficiency (92.3%) against H. contortus infective larvae (Mendoza de Gives et al., 1994); while, another isolate of the same species showed a low capture efficiency (32.3%) against Strongyloides papillosus larvae (González Cruz et al., 1998). Our present study represents the first report on the in vitro predatory activity of nematophagous fungi against histotropic larvae. This study can provide baseline information toward understanding the complex interactions among nematophagous fungi and different developmental stages of nematode larvae. In addition, this approach can be adapted to study recognition mechanisms between nematophagous fungi and nematode larvae.
Acknowledgements
This research received financial support from SAGARPA–CONACYT, Project No.11990/2005.
References
Anonymous, 1971. Helminthology. In: Ministry of Agricultura, Fisheries and Food (MAFF) (ed.), Manual of Veterinary Parasitological Laboratory Techniques. Bull. No. 18. Her Majesty's Stationery Office, London, pp. 6–7. [ Links ]
Arroyo–Balán, L.F., P. Mendoza–de Gives, M. E. López–Arellano, E. Liébano–Hernández, V.M. Vázquez–Prats, E. Miranda–Miranda, A.M. Ortiz de Montellano–Nolasco, 2008. Evaluación de un método combinado de control de la hemoncosis ovina bajo condiciones controladas. Técnica Pecuaria en México 46, 217–223. [ Links ]
Barron, G.L., 1977. The Nematode–Destroying Fungi. Canadian Biological Publications. Guelph, Ontario. [ Links ]
Casillas–Aguilar, J.A., P. Mendoza–de Gives, M.E. López–Arellano, E. Liébano–Hernández, 2008. Evaluation of Multi–nutritional Biopellets containing Duddingtonia flagrans chlamydospore for the control of ovine haemonchosis. Annals of the New York Academy of Science 1149: 161–163. [ Links ]
Cooke, C.R., B.E.S. Godfrey, 1964. A key to the nematode–destroying fungi. Transactions of the British Mycological Society 47: 61–74. [ Links ]
Cox, G.N., L.M. Shamansky, R.J. Boisvenue, 1989. Identification and preliminary characterization of cuticular surface proteins of Haemonchus contortus. Molecular and Biochemical Parasitology 36: 233–242. [ Links ]
Darwin, C., 2004. El origen de las especies. Libros en Red. Edición electrónica. http://www.librosenred.com/librosgratisclub.aspx. [ Links ]
Fetterer, R.H., 1989. The cuticular proteins from free–living and parasitic stages of Haemonchus contortus, Isolation and partial characterization. United States Department of Agriculture. Belville Biochem. Physioliology. USA 94, 338–383. [ Links ]
González, C.M., P. Mendoza–de Gives, H. Quiroz–Romero, 1998. Comparison of the trapping ability of Arthrobotrys robusta and Monacrosporium gephyropagum on infective larvae of Strongyloides papillosus. Journal of Helminthology 72: 209–213. [ Links ] Mendoza–de Gives, P., 1999. Interaction between nematodes and biocontrol agents with potential for use in biomanagement systems. PhD Thesis, University of Nottingham. 219 p. [ Links ]
Mendoza–de Gives, P., E. Zavaleta–Mejía, D. Herrera–Rodríguez, H. Quiroz–Romero, 1994. In vitro trapping capability of Arthrobotrys spp. on infective larvae of Haemonchus contortus and Nacobbus aberrans. Journal of Helminthology 68: 223–229. [ Links ]
Morgan, M., J.M. Behnke, J.A. Lucas, J.F. Peberdy, 1997. In vitro assessment of the influence of nutrition, temperature and larval density on trapping of the infective larvae of Helligmosomoides polygyrus by Arthrobotrys oligospora, Duddingtonia flagrans and Monacrosporiummegalosporum. Parasitology 115: 303–310. [ Links ]
Nordbring–Hertz, B., E. Friman, B. Mattiasson, 1982. A recognition mechanism in the adhesion of nematodos to nematode–trapping fungi. In: "Lectins–Biology, Biochemistry" Clinical Biochemistry 2: 83–90. [ Links ]
Ramírez–Vargas, G., Ma. E. López–Arellano, P. Mendoza–de Gives, E. Liébano–Hernández, V.M. Vázquez–Prats, 2006. Desarrollo de nematodos endoparásitos de Haemonchus contortus in vitro como modelo biológico de estudio. Asociación Mexicana de Parasitólogos veterinarios, A. C. VII Congreso Nacional de Parasitología Veterinaria, 27, 28 y 29 de Agosto del 2006. Acapulco, México. [ Links ]
Rosenzweig, W.D., D. Ackroyd, 1983. Binding Characteristics of Lectins Involved in the Trapping of Nematodes by Fungi. Applied and Environmental Microbiology 46: 1093–1096. [ Links ]
Rubner, A., 1996. Revision of predacious Hyphomycetes in the Dactylella–Monacrosporium complex. Studies in Mycology 39: 1–134. [ Links ]
Tahseen, Q., I.M. Clark, S.D. Atkins, R.R. Hirsch, B.R. Kerry, 2005. Impact of the nematophagous fungus Pochonia chlamydosporia on nematode and microbial populations. Communications in Agricultural and Applied Biological Sciences. Ghent University 70: 81–86. [ Links ]
Zar, H.J., 1999. Biostatistical Analysis. Fourth Edition. Prentice–Hall, Inc, Englewood Cliffs, New Jersey, USA. [ Links ]














