Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Revista mexicana de micología
versão impressa ISSN 0187-3180
Rev. Mex. Mic vol.27 Xalapa Dez. 2008
Contribuciones
Methods of agar culture of myxomycetes: an overview
Métodos para el cultivo de myxomycetes en medios con agar: una visión general
Edward F. Haskins1, Diana Wrigley de Basanta2 *
1 Department of Biology, University of Washington, Seattle, WA 98195, USA.
2 Real Jardín Botánico de Madrid, CSIC. Plaza de Murillo, 2, 28014 Madrid, Spain.
* Autor para correspondencia: dwb@eresmas.net
Recibido 31 de enero 2006
Aceptado 12 de febrero 2008
Resumen
Hasta el momento, solo el 10% de las especies conocidas de myxomycetes se han logrado cultivar. Este trabajo expone la importancia que en la actualidad tienen los cultivos en medios con agar para muchas líneas de investigación, así como la de mantener material vivo en colecciones de cultivos, para generaciones futuras. Se da un breve repaso a su historia, se incluyen los métodos y materiales usados, las dificultades encontradas y la manera de superarlas, y fuentes bibliográficas donde encontrar información complementaria. Se sugieren algunas especies cuyo cultivo puede ser de especial interés.
Palabras clave: Eumycetozoa, germinación de esporas, ciclo de vida, cultivo xénico.
Abstract
Only about 10% of known Myxomycete species have been cultured from spore to spore. This paper reviews the work done to date and the importance of cultures for research. It provides a synopsis of successful culture methods, details on how to induce stasis in cultures and information on how to combat some possible sources of error. The purpose of the paper is to encourage other investigators to attempt agar culture of myxomycetes.
Key words: Eumycetozoa, spore germination, life cycle, xenic culture, target species.
Introduction
The plasmodial slime molds or Myxomycetes have a unique life cycle that differentiates them from all other groups. Named in 1833 by Link [22], they have been known to biology for over three hundred years [12], and yet there is still a pressing need to get a wide range of Myxomycetes into agar culture. Although Physarum and Didymium [1] have served as excellent model systems for study, they give only a partial view of the biology of the whole group of Myxomycetes. Agar cultures of key species can now provide material for DNA sequences to build phylogenies, to track changes in gene expression during life cycles and to determine phenotypic plasticity of characters in the sporophore (see) http://slimemold.uark.edu/educationframe.htm. For the latter reason spore to spore culture is already an important addition to the description of new species [17]. A taxonomically diverse array of myxomycete species in agar culture, would also permit evaluation of their reproductive systems, as has been shown by Clark et al. [7]. In addition some new initiatives such as the Physarum polycephalum genome project (http://genome.wustl.edu/genome), the Partnerships for Enhancing Expertise in Taxonomy (PEET) taxonomic revision project and the Planetary Biodiversity Inventories (PBI) (http://slimemold.uark.edu) in conjunction with the American Type Culture Collection (www.atcc.org) are now generating a need for cultures from a broad taxonomic range. These cultures in stasis, a dormant state or period of stability during which little change occurs, which are available for worldwide distribution, can form a permanent method of storage of type material and a genetic legacy for future work. The use of spore to spore cultures is equally important in the instruction of high school, undergraduate, graduate and post–graduate students, and to provide material for various research projects. In two groups of Eumycetozoa, the Dictyostelids and Protostelids, almost all known species have been cultured [28,29], but very few of the Myxomycetes have completed their life cycle in laboratory culture.
A comprehensive list of 55 Myxomycete species in the literature that had been cultured from spore to spore on agar, or other media, was given by Gray & Alexopoulus [12], and updated by Clark & Collins [4] and Collins [8] but more species have been cultured by other researchers since then and 98 are listed by Clark [2]. The few additional species that have been cultured since 1995 are Collaria arcyrionema [6], Licea succulenticola [26], Didymium megalosporum, Didymium laxifilum Clark et al. [7] and Didymium wildpretii Lado et al. [21]. In addition, the culture of Macbrideola cornea was described in some detail by Wollman [31] in her thesis, but has been omitted from later compilations.
The purpose of this paper is to summarize the culture methods used successfully by the first author over the last 50 years and results obtained, with the aim of encouraging others to attempt these methods and so increase the number of species that can be cultured from spore to spore. These methods were used recently as the basis for three workshops to train researchers on how to get field collections of myxomycetes into agar culture and their subsequent laboratory maintenance. The workshops took place in Fayetteville, Arkansas, USA (October 2004), Madrid, Spain (May 2005), and lastly in Tlaxcala, Mexico in August 2005, as part of the 5th International Congress on the Systematics and Ecology of Myxomycetes.
Material and methods
The following is a synopsis of the most useful methods employed to culture myxomycetes. Specific methods for each species can be found in the original publications.
Source of specimens. Clean sporocarps selected from field and moist chamber culture collections, free from fungal contaminants, were isolated from all other Myxomycete spores in closed wrapped containers, such as small boxes, Petri dishes or Eppendorf tubes. These exsiccata were labelled with the collection number and details. None of the specimens had been frozen, heated to dryness or fumigated.
Cultivation from spores. Germination cultures were set up on sterile 0.75% water agar (WA), weak Malt Yeast (wMY) agar, or Yucca bark extract agar (See materials below). A fine marking pen was used to divide the Petri dish bottom into quadrants. An alcohol flamed #5 jewellers forceps was used to pick up spore material and inoculate the surface of the agar in each of the quadrants by using a gentle slashing motion to make sure some of the spores were submerged and others were left on the surface (Figure 1). Individual spores were well–separated on the substratum. The areas of spore deposit were circled on the bottom of the dish, in order to confirm that the spores were from the specimen sampled, and to check the spores at intervals to watch for germination. Parafilm was used to seal the dishes.
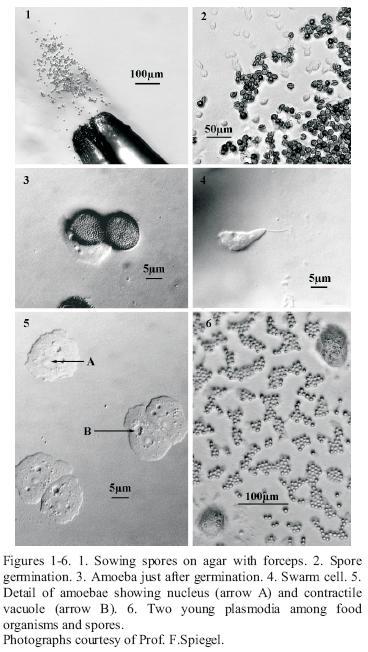
Spore germination (Figure 3) occured in hours to days (up to one month). Spore germination was observed by inverting the agar plate, still closed, on the stage of a compound microscope from which the clips or mechanical stage had been removed. A 5X or 1 0X objective were used with 1 0X or 15X oculars to view the spores, amoebae (Figure 2), swarm cells (Figure 4) and plasmodial phases at 50X–150X. A dissecting microscope provides less resolution at a comparable magnification. After germination occured, amoebae and plasmodia often flourished on the microbial melange (bacteria and/or yeasts) that developed from the original spore inoculum. All too often there was also a subsequent development of filamentous fungi and/or mycelial Actinomycetes.
Cleaning the culture. Small populations of amoebae or plasmodia were separated from these contaminants and food organisms by sub–culturing on small squares of agar, which were cut out and lifted with a sterile spatula to a new culture dish of the same medium. If the amoebae were not on clean areas of the agar, but mixed with the contaminants, the block was inverted, and carefully pushed from one side of the new plate to the other, in an attempt to separate the amoebae from the contaminants. This sub–culturing often had to be repeated several times to clean the culture. The bacterium Escherichia coli or the yeast Cryptococcus laurentii were inoculated as food organism replacements, but in minute quantities, since the food organisms have a shorter generation time than the myxomycetes and can over run the culture. Some species (Echinostelium arboreum) would only grow on the original melange.
Plasmodia. With small typically corticolous species of the genera Echinostelium and Licea it was difficult to recognise the early protoplasmodia at 100X magnification, and opening the plate to allow greater magnification at this stage is unwise. There are some useful differences between them and the surrounding bacteria and yeasts. First, the amoebae or plasmodia move very slowly or change shape. Observations could take up to an hour to see this. Both these stages also have contractile vacuoles (Figure 5), and these "appear and disappear" as they open and close. They also have a different refractive index from bacteria, and are multinucleate. The nuclei are dark at 100X magnification and surrounded by a clear area (Figures 5 & 6). If growth of plasmodia in xenic culture was sparse, plasmodia were transferred successively from weaker to more nutrient rich media, for example from 1.5% WA to half strength Bacto Corn Meal Agar CM/2, or a few grains of sterile "Old Fashion" Quaker oat flakes or oat flake flour were added. Some Myxomycetes (ex. Stemonitis, Comatricha, Lamproderma, Trichia elaterensis and in some cultures Licea biforis) required the presence of a thin water film for amoebal growth and subsequent plasmodial development, which was encouraged by adding about 5 ml of sterile, glass distilled water to the surface of a 15 mm X 100 mm agar Petri dish. If soft (0.75%) water agar was used for germination, this may not be necessary.
Sporulation. Some species crawled to the sides or lids of the container to sporulate. Those listed above, sporulated when the fluid was drained from the Petri dish and the dish exposed to light. In other cases, as with Physarella oblonga (Berk. & M. A. Curtis) and Comatricha laxa Rostaf. it was necessary to cut a block of agar bearing the plasmodia and transfer it to 1.5% water agar and expose it to fluorescent light. In this case sporulation may have been induced by starvation and a light cue. Another method involving making a paper cone for the plasmodium to climb is described in Venkataramani et al. [30].
To prepare living cultures for stasis. The International Code of Botanical Nomenclature, recommendation 8 B.1. [13], states that, whenever possible, living cultures be prepared of holotype material of new taxa and deposited in a culture collection such as the American Type Culture Collection (ATCC). The following methods have been used as simple alternative stop–gap stasis approaches in contrast to liquid N2 stasis employed by the ATCC or other international microbial archives. None of the methods described below guarantee long–term survival of myxomycete cultures. The first author has been able to store and revive cultures after 40 years using a simple lyophilization method [18]. This technique requires the availability of a machine shop to build the lyophilization apparatus.
It is critical to have simpler methods available for longer term storage of cultures. Some cultures preserved 10 years earlier by the following method have been revived successfully, but be advised that the viability of the stasis stocks must be checked from time to time. A number of No. 1 or No. 1–1/2, 22 mm2 glass cover slips were placed in a glass Petri dish and sterilized in a drying oven for 2 hr or more at ca 160°C. After sterilization and cooling, several sterile cover slips were placed into a 100 X 15 mm sterile plastic dish. Using a flame sterilized and cooled flat–ended spatula, 1 cm2 agar blocks of actively growing Myxomycete cultures were cut out. These were placed one block on each cover slip, culture side up. The Petri dish was labelled with the name, and other data of the culture, and closed. It took up to 1 month for the agar block to dry down to a thin film. When dehydration had occurred, the dish was sealed with Parafilm to avoid fungal, etc. contamination and stored at 18–25°C in the dark. To revive a culture, a drop of sterile distilled water was added to the top of the agar fragment, and after 10–20 minutes the film was lifted with a sterilized spatula. The agar fragment was place cell–side down on the surface of an appropriate agar plate and resumed growth was looked for in 1 –14 days. When growth occurred, food micro–organisms were added. A second air–drying method was used for four years and is still being assessed for its effectiveness. All details of handling the culture blocks, dried agar fragments and revival methods are the same. However, in the second method about 12, 1 cm2 agar blocks of culture material were cut out aseptically and placed cell side up on the bottom of the 100 X 15 mm sterile plastic Petri dish. The dish was labelled, dehydrated for up to a month, sealed with Parafilm, and stored as described. Some cultures stored this way were revived after four years.
Agar cultures of Myxomycetes wrapped in parafilm strips and stored at 5°C in a refrigerator can survive for 1–2 months as amoebae or microcysts and occasionally up to a year.
Media. These are the most useful media mentioned above and what they were used for. Details of other media and media preparation can be found on www.slimemold.uark.edu
1). 0.75% Water Agar (0.75% WA) Bacto agar 7.5 g, Glass distilled water 1 L [excellent for spore germination]
2). Weak Malt Yeast (wMY) Agar (courtesy of Prof. F.W Spiegel) Malt extract 0.002 g, Yeast extract 0.002 g, Dibasic potassium phosphate 0.75 g, Bacto agar 15 g, Glass distilled water 1 L [for spore germination and amoebal growth]
3). 1.5% Water Agar (1.5% WA) Bacto agar 15 g, Glass distilled water 1 L [for growth of amoebae and protoplasmodia]
4). Half Strength Bacto Corn Meal Agar (CM/2) Bacto corn meal agar 8.5 g, Bacto agar 12.5 g, Glass distilled water 1 L [for plasmodial growth and growth of amoebae]
5). Yucca bark extract agar 25 g of outer bark pieces of Yucca spp. soaked in 1 L glass distilled water for 24 h. Filter the supernatant through cheesecloth, and then filter again through Whatman #1 filter paper. Bring the final volume to 1 L with additional distilled water. To this liquid add: Bacto agar 17.5 g, Bacto corn meal agar 8.5 g [for spore germination and growth of corticolous spp]
Myxomycete nomenclature in this paper follows Lado [19], and Gams [11].
The dissecting microscope images were taken with a Nikon SMZ 1600 and the light micrographs were taken with a Zeiss Axioskop II. All are montages from through–focus series of images that were processed using Auto Montage (Syncroscopy).
Results and discussion
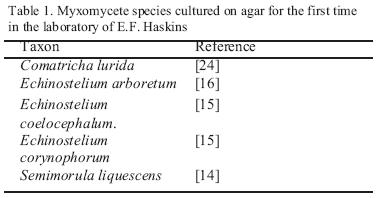
At least fourteen species of myxomycete have completed their life cycle in spore to spore agar culture done by the first author using these methods. Of these, the species reported in culture for the first time by him appear in Table 1. The others (Didymium iridis, Echinostelium minutum, Trichia elaterensis, Licea biforis, Comatricha laxa, Badhamia gracilis, Perichaena vermicularis, Lamproderma scintillans, Stemonitis flavogenita) had all been cultured previously (12; 8; 2). Some of these species have been cultured many times by the author.
An isolate of E. minutum (isolate Ca 6 [5]) was cultured from spore to spore and found to be a different biological species somewhat similar to E. paucifilum but with extremely scanty capillitial development, and another heterothallic E. minutum isolate (isolate Az 1 [5]), deposited by Haskins at the ATCC, which has been repeatedly cultured from spore to spore. A detailed list of all the different reproductive systems within the other species complexes included here, can be found in [3], and would further increase the number of biological species cultured. For instance, isolates of the morphospecies Didymium iridis were found to be from at least nine biological species.
In addition, during the recent workshops, Macbrideola oblonga, Licea rugosa, Didymium difforme, D. iridis, and D. vaccinum, were all germinated on agar several times without difficulty. The latter completed its life cycle and sporulated, and a Licea species and Trichia species germinated and made plasmodia. These are being maintained on agar with the hope of completing their spore to spore cycle. Other parts of the life cycle were observed during the workshops by inducing sporulation on agar of plasmodia derived from sclerotia. Physarum melleum sporulated from sclerotia on leaf litter of Cassia tomentosa collected in the field in Peña Miller (Querétaro, Mexico), and Didymium iridis from sclerotia produced on liana bark from Pinal de Amoles (Querétaro, Mexico), in moist chamber culture. With viable spores available, subsequent agar cultures can be established from these.
The total number of myxomycetes that have completed their life cycle in culture to date amounts to just over one hundred, which is still approximately only 10 % of all described species [19]. This is a lower percentage than reported by Gray & Alexopoulos [12], since many species have been described since then, but few have been cultured on agar from spore to spore. Of those cultured, more than 60% belong to the Physarales, which appear to complete their cycle more readily on agar than others.
Germination has been accomplished in many more taxa than those that have completed the full life cycle (see above, and (12, pp. 26–27), also Cribraria zonatispora [20]). Similarly some species such as Cribraria violacea [25], Kelleromyxa fimicola, [9], have been grown on agar from sclerotia and plasmodia, but without germination from spores. Cribraria fragilis [10] has been grown on columnar cactus remains from plasmodia, and Hemitrichia imperialis completed its cycle on rotten wood from spores [23].
Although only germination on media in Petri dishes has been described here, other methods may be useful. Some workers inoculate and germinate spores on agar slices on sterile microscope slides or cover slips, agar drops on cover slips set as hanging drops, or hanging drops of sterile liquid medium on sterile cover slips. These can then be transferred to agar plates to complete the cycle.
Contaminants such as Acanthamoeba [27], filamentous fungi, Ascomycetes and even food organisms can cause serious difficulty in culturing, and increase the cost in both time and money by making frequent sub–cultures necessary. The strict use of aseptic techniques, and close and frequent observation of the cultures can limit this. It is important to start with only one species, and to keep the number of cultures down in order to facilitate close control. However, it is also important to set up multiple cultures once germination has occurred, in order to keep a clean "stock" culture of amoebae, but also have others to try out different media and conditions until the optima for completing the cycle for that species have been found.
Clark [2] commented that most major genera have at least one species cultured and "thus a reasonable and fairly representative accumulation of culture studies for the myxomycetes" have been done. However there are some obvious genera where spore to spore culture work is almost totally lacking, such as Cribraria, Licea, Lycogala. Hemitrichia, Trichia, Lamproderma, Colloderma, Listerella, Barbeyella, Tubifera and Brefeldia.
Certain species of these genera, with importance for taxonomic and genomic studies, should be considered to be target species, and attempted first. These should include the type species of the different genera such as Licea pusilla Schrad., although the age of spores in the actual type specimens may preclude their germination, and in these cases other collections would have to be used. All future species descriptions should be accompanied wherever possible by spore to spore culture [17]. It is also important to have descriptions of all life cycle stages, including microcyst development and details of plasmodial and sporocarp development as Collins [8] suggested.
Taxonomic or phylogenetic studies, whether traditionally done or with molecular techniques, that are based on morphospecies, which may include clonal populations or ecotypes, or may be species complexes, are susceptible to error. These and other lines of investigation need the complete base research of each species to underpin the accuracy of their systems. For this, spore to spore culture, clarification of biological species by investigation of mating systems, and the deposit of authentic, uncontaminated living material for research by future generations is therefore urgently needed.
Acknowledgements
We are grateful to Professor Fred Spiegel for his help and suggestions, his critical reading of the manuscript and the photographs; to Dr. Carlos Lado for use of his outstanding reprint library, his critical reading of the manuscript and his help with composing the photographic plate. The PEET grant [NSF DEB03–29102] and the PBI grant [NSF DEB03–16284] to Professor F. Spiegel and Dr. S. Stephenson at the University of Arkansas supported travel to, and funded the workshops. Grant CGL2005–00320/ BOS to Dr. Carlos Lado supported culture materials. We dedicate this paper to the late Professor Henry Aldrich who has contributed substantially to the study of Eumycetozoans.
1. Aldrich, H.C., J.W. Daniel, eds., 1982. Cell Biology of Physarum and Didymium. 2 vols., Academic Press, New York, London. 373 p. [ Links ]
2. Clark, J., 1995. Myxomycete reproductive systems: additional information. Mycologia 87:779–786. [ Links ]
3. Clark, J., 2004. Reproductive systems and taxonomy in the myxomycetes. Systematics and Geography of Plants 74:209–216. [ Links ]
4. Clark, J., O.R. Collins, 1976. Studies on the Mating Systems of Eleven Species of Myxomycetes. American Journal of Botany 63: 783–789. [ Links ]
5. Clark, J., E.F. Haskins, 1998. Heterothallic mating systems in the Echinostelium minutum complex. Mycologia 90:382–388. [ Links ]
6. Clark, J., E.F. Haskins, 2002. Reproductive systems of the myxomycetes Comatricha laxa and Lamproderma arcyrionema. Nova Hedwigia 75:237–240. [ Links ]
7. Clark, J., E.F. Haskins, S.L. Stephenson, 2004. Culture and reproductive systems of 11 species of Mycetozoans. Mycologia 96:36–40. [ Links ]
8. Collins R.O., 1979. Myxomycete Biosystematics. Some Recent Developments and Future Research Opportunities. Botanical Review 45:145–201. [ Links ]
9. Eliasson U., H.W. Keller, J.D. Schoknecht 1991. Kelleromyxa, a new generic name for Licea fimicola (Myxomycetes). Mycological Research 95:1201–1207. [ Links ]
10. Estrada–Torres, A., C. Lado, M. Rodríguez–Palma, 2001 .Two new species of Myxomycetes from a tropical deciduous forest of Mexico. Mycologia 93:744–750. [ Links ]
11. Gams, W., 2005. Report of the Committee for Fungi: 13. Taxon 54: 828–830. [ Links ]
12. Gray, W. D., C. J. Alexopoulos, 1968. Biology of the Myxomycetes. Ronald Press, New York. 288 p. [ Links ]
13. Greuter,W., J. McNeill, F.R. Barrie, H.M. Burdet, V. Demoulin, T.S. Filgueiras, D.H. Nicolson, P.C. Silva, J.E. Skog, P. Trehane, N.J. Turland, D.L. Hawksworth, 2000. International Code of Botanical Nomenclature (Saint Louis Code), Koeltz Scientific Books, Königstein, Germany. 474 p. [ Links ]
14. Haskins, E.F., M.D. McGuinness, C.S. Berry., 1983. Semimorula: New genus with myxomycete and protostelid affinities. Mycologia 75: 153–158. [ Links ]
15. Haskins, E.F., M.D. McGuinness., 1986. Comparative ultrastructural observations of spore wall structure in six species of Echinostelium and three species of Eumycetozoa. Mycologia 78: 613–618. [ Links ]
16. Haskins, E.F., M.D. McGuinness., 1989. Sporophore ultrastructure of Echinostelium arboreum. Mycologia 81:303–307. [ Links ]
17. Keller, H.W., 1996. Biosystematics of Myxomycetes: A Futuristic View. Plenary address. Second International Congress on the systematics and Ecology of Myxomycetes. Abstract volume. Real Jardín Botánico, CSIC. Madrid, Spain. 144 p. [ Links ]
18. Kerr, N.S., 1965. A simple method of lyophilization for the long–term storage of slime molds and small soil amoebae. Bio–Science 15: 469. [ Links ]
19. Lado, C, 2001. Nomenmyx. A Nomenclatural Taxabase of Myxomycetes. Cuadernos de Trabajo de Flora Micológica Ibérica 16:1–221. [ Links ]
20. Lado, C., J. Mosquera, E. Beltrán–Tejera, 1999. Cribraria zonatispora, development of a new myxomycete with unique spores. Mycologia 91: 157–165. [ Links ]
21. Lado. C., J. Mosquera, A. Estrada–Torres, E. Beltrán–Tejera, D. Wrigley de Basanta, 2007. Description and culture of a new succulenticolous Didymium (Myxomycetes). Mycologia 99:602–611. [ Links ]
22. Link, J.H.F., 1833.Handbuch zur Erkennung der nutzbarsten und am häufigsten vorkommenden Gewächse 3. Ordo Fungi, Subordo 6. Myxomycetes. 405–422; 432–433. Berlin. [ Links ]
23. Lister, G., 1929. A new species of Hemitrichia from Japan. Transactions of the British Mycological Society 14:225–227. [ Links ]
24. McGuinness, M.D., E.F. Haskins, 1985. Genetic analysis of the reproductive system of the true slime mold Comatricha lurida. Mycologia 77: 646–653. [ Links ]
25. McManus, M.A. 1966. Cultivation on agar of the plasmodia of Licea biforis and Cribaria violacea. Mycologia 58:479–483. [ Links ]
26. Mosquera, J., C. Lado, A. Estrada–Torres, E. Beltrán Tejera, D. Wrigley de Basanta, 2003. Description and culture of a new myxomycete, Licea succulenticola. Anales del Jardín Botánico de Madrid 60: 3–10. [ Links ]
27. Page, F.C. 1988. A New Key to Freshwater and Soil Gymnamoebae. Freshwater Biology Association Ambleside, UK. 122 p. [ Links ]
28. Raper, K.B. 1984. The Dictyostelids. Princeton University Press. Princeton, NJ. 453 p. [ Links ]
29. Spiegel, F.W.1990. Phylum Plasmodial Slime Molds Class Protostelida. In L. Margulis, J.O. Corliss, M.Melkonian, D.J. Chapman (eds.), Handbook of Protoctista. Jones and Bartlett, Boston. Pp. 484–497. [ Links ]
30. Venkataramani, R., I. Kalyanasundaram, R. Kalyanasundaram, 1977. A simple technique for inducing sporulation in cultures of myxomycetes. Transactions of the British Mycological Society 69: 320–322. [ Links ]
31. Wollman, C. 1966. Cultural studies of selected species of Myxomycetes. Ph.D. Dissertation. Univ. of Texas, Austin. 184 p. [ Links ]














