Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Universidad y ciencia
versión impresa ISSN 0186-2979
Universidad y ciencia vol.29 no.3 Villahermosa dic. 2013
Artículos
Allometric growth in cuban gar (Atractosteus tristoechus) larvae
Crecimiento alométrico en larvas de manjuarí (Atractosteus tristoechus)
Yamilé Comabella1*, Julia Azanza1, Andrés Hurtado2, Javier Canabal2, Tsai García-Galano1
1 Centro de Investigaciones Marinas. Universidad de la Habana. Calle No. 6 114 e 1ra y 3ra, Miramar, Playa, Cuba, *ycomabella@yahoo.es
2 Knowles Animal Clinics, Florida, United States.
Artículo recibido: 15 de marzo de 2013,
Aceptado: 11 de noviembre de 2013
ABSTRACT
The allometric growth of Cuban gar (Atractosteus tristoechus) was evaluated in larvae reared at a constant temperature (28 ±1 °C), from hatching to 18 days after hatching (DAH). Of the 17 morphometric characters recorded, only six showed isometric growth describing a continuous and gradual change in a very few body characters. From hatching to 14 DAH, the growth in weight presented a slight negative allometry (b = 0.91), but later the growth coefficient increased (b = 2.09) in the exotrophic phase indicating a complete and efficient exogenous feeding thanks to the precocious development of the digestive system. The increase in the length of the head and snout was positively allometric, but their heights and widths were negatively allometric indicating an elongation of the cephalic region that guarantees an efficient food capture. The pectoral and pelvic fins increased in length with a positive allometric growth and biphasic patterns (b = 1.26; b = 2.69, respectively), both with the same inflexion point (8 DAH). The allometries obtained with respect to the head, trunk and tail growth showed a discontinuity and abrupt changes in many of the body sizes and proportions that occur mainly from age 4 (18.6 mm TL) to 8 (23.7 mm TL) DAH. This reflects the priorities of a developing organism, when important organs must first be developed to allow feeding and guarantee a better survival of the juveniles.
Key words: Fish larvae, allometry, development, growth.
RESUMEN
Se evaluó el crecimiento alométrico del manjuarí (Atractosteus tristoechus) en larvas mantenidas a una temperatura constante (28 ±1 °C) desde la eclosión hasta los 18 días después de eclosionadas (DDE). De los 17 caracteres morfométricos medidos, solo seis mostraron crecimiento isométrico describiéndose así un cambio continuo y gradual en muy pocos caracteres corporales. Desde la eclosión y hasta los 14 DDE, el crecimiento en peso exhibió ligera alometría negativa (b = 0.91) pero después el coeficiente de crecimiento se incrementó (b = 2.09) en la fase exotrófica mostrando una completa y eficiente alimentación exógena debido al desarrollo precoz del sistema digestivo. El crecimiento del largo de la cabeza y del hocico fueron positivamente alométricos, pero sus alturas y anchos fueron negativamente alométricos indicando una elongación de la región cefálica que garantiza la eficiente captura del alimento. Las aletas pectorales y pélvicas incrementaron en largo con crecimiento alométrico positivo y patrones bifásicos (b = 1.26; b = 2.69, respectivamente), ambas con el mismo punto de inflexión (8 DDE). Las alometrías obtenidas relacionadas con el crecimiento de la cabeza, el tronco y la cola mostraron una discontinuidad y cambios abruptos en muchos de los tamaños y proporciones corporales que se concentran principalmente entre los 4 (18.6 mm LT) y los 8 (23.7 mm LT) DDE. Esto refleja las prioridades de un organismo en desarrollo, cuando deben ser formados primeramente importantes órganos que permitan la alimentación para así garantizar una mejor supervivencia de los juveniles.
Palabras clave: Larvas de peces, alometría, desarrollo, crecimiento.
INTRODUCTION
The development of fish from fertilisation to sexual maturity is a continuum that is punctuated by developmental events and transitions which may be either gradual and unremarkable or abrupt and quite dramatic (Webb 1999). During growth, fish larvae often go through very complex processes of morphogenesis and differentiation, including changes in morphometric relationships, physiological changes in muscular and internal organ systems, changes in bone remodeling and changes in behaviour. This development, which is regulated by gene expression and influenced by the environment (Gilbert & Bolker 2003), results in different phenotypes with differential relative growths, defined as allometry (Gisbert & Doroshov 2006). This term was coined by Huxley and Teissier (1936) and describes the relationships between organism dimensions and changes in the relative proportions of these dimensions with changes in absolute size (Goldman et al. 1990). Four different concepts of allometry are distinguished: 1-ontogenetic allometry (covariation among characters during growth), 2-phylogenetic allometry (covariation among changes in different traits along the branches of a phylogeny), 3-intraspecific allometry (comparisons between individuals of the same species) and 4-interespecific allometry (comparisons among different species) (Goldman et al. 1990; Klingenberg 1996; Gayon 2000).
The allometric growth model is a widely used method of analysis of relative growth during early larval development (Celik & Cirik 2011). Many fish species exhibit allometric growth during the larval period, from the absorption of the yolk sac to the onset of metamorphosis, and may thus be characterised by their allometric growth patterns (Klingenberg & Froese 1991; Osse & Boogaart 1995; Mello et al. 2006). These patterns of allometric growth reflect morphoanatomical growth priorities in agreement with their importance regarding primary living functions that guarantee an appropriate survival (Sala et al. 2005; Devlin et al. 2012).
Ontogenetic allometry can be used in fishery biology and aquaculture to evaluate the developmental plasticity of species (Koumoundouros et al. 1999; Celik & Cirik 2011). The last decade has seen an increasing interest in allometric growth during early development of fish such as sparids (Kout-touki et al. 2006; Cobán et al. 2009), sturgeons (Gisbert & Doroshov 2006; Huang et al. 2009), catfish (Geerinckx et al. 2008; Huysentruyt et al. 2009), ornamental fish (Roos et al. 2010; Celik & Cirik 2011) and loricariids (Schmidt 2001). However, studies of this type on gar larvae have not been published. The early development of Cuban gar and its growth rate during ontogenesis were first described by Comabella et al. (2010) for specimens reared under laboratory conditions. Atractosteus tristoechus, a vulnerable and endemic species that inhabits the western region of Cuba, could become a promising candidate for aquaculture, considering its high growth rate and good adaptability to culture conditions. Our research efforts have targeted larvae development mainly, since the hatchery phase is considered the most critical for the successful production of a species. A previous morphological study made it evident that a differential relative growth occurs in this species. For this reason, the purpose of the present study was to characterise the allometric growth patterns of Cuban gar larvae.
MATERIALS AND METHODS
The larvae used in the present study were obtained from the induced spawning of domesticated broodstocks of Cuban gar (Atractosteus tristoechus) housed at the Center for Native Ichthy-ofauna Reproduction, Ciénaga de Zapata, Cuba. Induction and spawning conditions occurred as was previously described by Comabella et al. (2010). Fifteen minutes after release from the female, the spawned adhesive eggs were removed from the pond and placed in a 100 L circular fiberglass tank until hatching (68-100 h). The eggs taken from the broodstock pond were transferred to experimental tanks and gradually adapted (4 h) to the control temperature (28 ± 1 °C).
Experimental design, sampling and measurements
After hatching, 300 larvae were distributed in three 15 L circular fiberglass tanks (6.7 larvae L-1), previously conditioned with branches to facilitate larval adherence. The larvae were reared at a constant water temperature of 28 ± 1 °C, under a light regime of 08:00 to 20:00 h, with oxygen levels maintained above 6 ppm. Each morning and after cleaning the bottom, 50% of the water was changed in each tank. The larvae were fed ad libitum with live Moina three times a day (09:00, 14:00, 19:00 h). The experiment included the larval stage of this species (from hatching to 18 days after hatching-DAH), in agreement with Comabella eí al. (2010). Each day, nine larvae were randomly selected, sedated and killed with an overdose of tricaine methanesulphonate (MS 222), and individually weighed on an Ohaus scale (± 0.1 mg). All samples were preserved in 5% phosphate-buffered formaldehyde solution for later examination, and were preserved separately in a horizontal position to avoid deformations of the body until the time of storage.
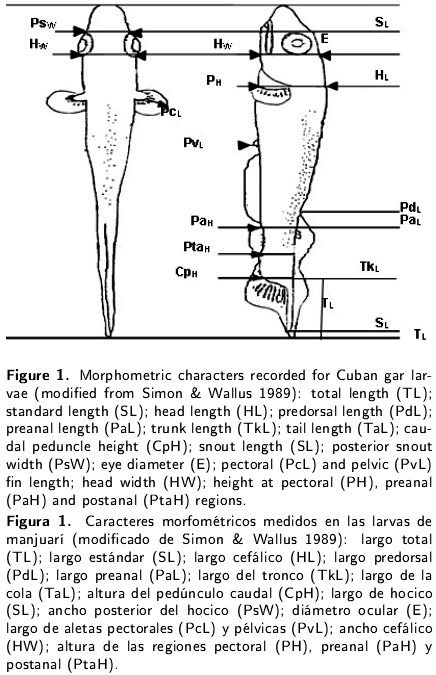
Seventeen morphometric characteristics were recorded using an ocular micrometer and digital calipers (± 0.01 mm): total length (TL), standard length, head length (HL - distance from the tip of the snout to the operculum margin), predorsal length (to the anterior base of the dorsal fin), preanal length (to the anterior base of the anal fin), trunk length (distance between the operculum and the anterior base of the anal fin), tail length (distance between the anus and the tip of the no-tochord), caudal peduncle height (distance between the anterior base of the anal fin and the base of the dorsal fin), snout length (from the tip of the snout to the eye), posterior snout width (before the eyes), eye diameter, pectoral fin and pelvic fin lengths (distance between the anterior base and the fin margin), head width (behind the eyes), height at the pectorals, and preanal and postanal regions (Figure 1). The measurements were taken horizontally or perpendicularly to the axis of the body.
Statistical analyses
Allometric growth was calculated as a power function of X (X = TL or HL for widths) using non-transformed data as: y=a Xb, where y is the recorded character, a is the intercept and b is the growth coefficient (Fuiman 1983). The equations were established from regressions performed on log-transformed data, using TL or HL as the independent variable (Gisbert 1999; Gisbert et al. 2002). When growth was isometric, the growth coefficient was b=l for length, height or width and b = 3 for weight when compared with X (Osse & Boogart 2004). Allometric growth was positive when b was > 1 or 3, and negative when it was < 1 or 3.
Di or triphasic growth can be described by two or three different growth curves, respectively. The X value where the slope changes is called the inflexion point. Inflexion points were determined using iteration procedures according to Snik et al. (1997), Gisbert (1999) and Gisbert et al. (2002). The x-y data set was sorted according to an increasing X. Regression lines were calculated for Xmin to Xintermediate, and for Xintermediate to Xmax, where X intermediate varied iteratively from Xmin + 2 to Xmax -2. Also, t tests were carried out to check whether the growth coefficients for Xmin Xintermediate and Xintermediate Xmax differed significantly. The Xintermediate value that resulted in the largest t was defined as the inflexion point. Growth coefficients were compared statistically using a t-test. The accepted significance level was p < 0.05. STATISTICA ver. 6.0 (StatSoft, Tulsa, Okla.) was used for the analyses.
RESULTS
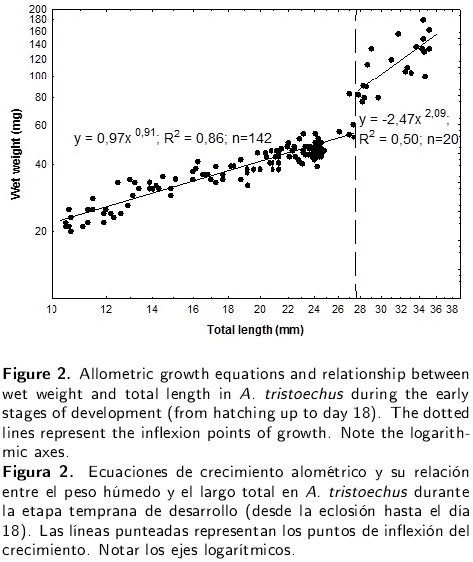
Wet weight growth was negatively allometric (a = -0.09, b = 1.29, R2 = 0.79). Two distinct growth phases were detected: a slow negatively allometric growth from hatching to 14 DAH (27.84 mm TL) and a faster negatively allometric growth from 14 to 18 DAH (Figure 2).
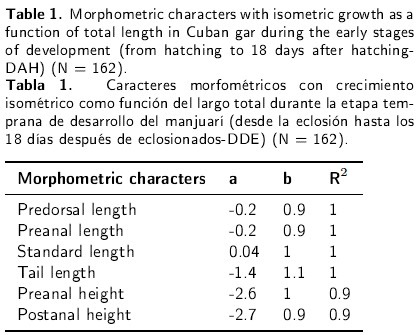
Of all the recorded morphometric characters, only six presented isometric growth as a function of total length during the early stages of development (Table 1). However, other body proportions and growth coefficients changed considerably during this period. The head length and width exhibited biphasic growth patterns with inflexion points at 22.48 mm TL (6 DAH) and 5.8 mm HL (6 DAH) respectively (Figures 3a and 3b).
The head length growth was positively allometric (a = -2.46, b = 1.38, R2 = 0.98) during larval development, though the head width growth was negatively allometric (a = 0.06, b = 0.48, R2 = 0.90). The eye diameter increase was negatively allometric (a = -2.28, b = 0.81, R2 = 0.95) throughout the entire period of development, though its growth could be separated into three different stages (Figure 3c). Growth was negatively allometric (b= 0.8) from hatching to 3-4 DAH (first inflexion point at 17.25 mm TL). A second inflexion point appeared at 24.42 mm TL at 7 DAH, but the eye diameter growth was nearly isometric in the two last stages.
Snout length growth showed a strong positive allometry in relation to total length in the early larvae (a = -6.51, b = 2.36, R2 = 0.96), with an inflexion point at 6 DAH (20.77 mm TL) and similar growth coefficients in both phases (Figure 4a). The posterior snout width also illustrated this biphasic pattern, though its growth was negatively allometric (a = -0.52, b = 0.67, R2 = 0.88) in relation to the head length (Figure 4b).
The growth in length of the trunk was negatively allometric from hatching to 18 DAH (a = 0.08, b = 0.65, R2 = 0.91) and biphasic, with an inflexion point at 14.9 mm TL at 2 DAH (Figure 5a). Pectoral height growth was negatively allometric (b = 0.04) and showed two clearly different phases (Figure 5b). However, the first phase (from hatching to 4 DAH) presented a decrease in pectoral height (b = -0.19). From that inflexion point (18.76 mm TL), pectoral height increased and showed a negative allometric growth (b = 0.68). The other body heights (preanal and postanal), however, recorded an isometric growth (Table 1). The pectoral and pelvic fins increased in length from hatching to 18 DAH, with a positive allometric growth and biphasic patterns (a = -3.65, b = 1.26, R2 = 0.77; a = -8.89, b = 2.69, R2 = 0.85, respectively). The pectoral fins recorded a positive allometric growth from hatching to 8 DAH (24.88 mm) and, from this inflexion point to the end of the study, growth increased isometrically (Figure 5c). A similar pattern occurred in the case of the pelvic fins, although from that point on the growth coefficient (b = 4.04) presented a rapid and positive allometry (Figure 5d).
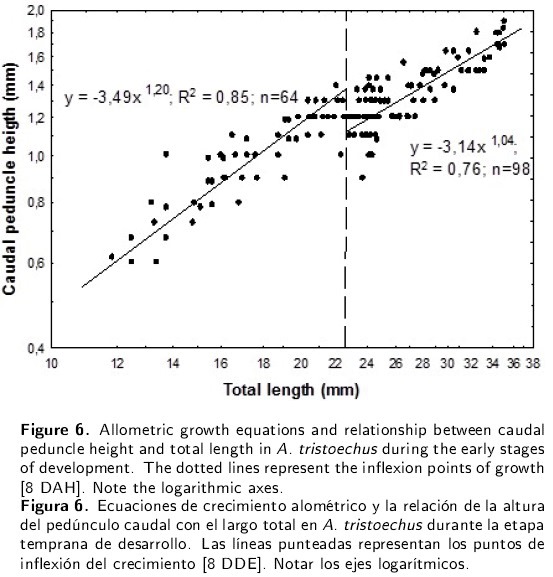
Finally, the growth in the length of the tail showed the same isometric trend in the early larvae as did other length characters presented in Table 1. However, the height of the peduncle showed a negative allometry (a = -2.39, b = 0.82, R2 = 0.79) throughout the 18 days of the experiment (Figure 6). From hatching to 8 DAH (22.87 mm TL), the Cuban gar peduncle height presented a positive allometry, and from that inflexion point to 18 DAH, growth was isometric.
During the early stage of Atractosteus tristoechus development, all inflexion points lay in a remarkably narrow range of body lengths and ages (TL 13.7- 23.7 mm, age 2-8 DAH), particularly from age 4 (18.6 mm TL) to 8 DAH (Figure 7).
DISCUSSION
Most functional systems of fish larvae are incompletely differentiated at the moment of hatching (Eenennaam et al. 2001; Deng et al. 2002). A significant morphogenesis occurs during the early development when the larvae need to adequately and timely form somatic and visceral systems, as well as specialised structures for an optimal interaction with the environment (Dettlaff et al. 1993; Gisbert 1999). The results obtained in the present study reveal that the body proportions of this species change considerably, and many morphological characters present a differential relative growth, indicating times when growth coefficients differ statistically. Punctual and rapid changes (inflexion point) in relation to TL or HL occur in these characters, rather than a continuous and gradual change. Most of the inflexion points recorded lay in a remarkably narrow range of ages (4-8 DAH).
The most drastic morphological changes observed in A. tristoechus occurred during these days. The larvae began to feed exogenously but continued to use the yolk reserves that meet the energetic demands of capturing prey. This transitional period was defined by internal, external and behavioural changes (Comabella et al. 2010; Comabella et al. 2013). The lecithoexotrophic stage is a critical period in larval life due to competition for food and predation (Balon 1985; Coughlin 1991). Propulsion both to capture food and to avoid predators is critical and depends on the development of organs necessary for feeding (Porter & Theilacker 1999; Makrakis et al. 2005) and swimming (Murphy et al. 2007; Huysentruyt et al. 2009). The concurrent development of organs associated with these functions must occur in a mutual balance (Osse et al. 1997; Rodríguez-Mendoza et al. 2011). In the case of the Cuban gar, larval behaviour is characterised by periods of resting, while executing corporal undulation movements. Anguilliform swimming with ample movements over a substantial part of the body is common in fish larvae (Webb & Weihs 1986; Osse & Boogaart 1995), and gradually develops into the characteristic adult swimming pattern (Russo et al. 2007). For these reasons, during the larval stage of many fish, muscle development, rather than fin growth, may be a key factor in the marked increase in swimming performance (Murphy et al. 2007). However, Cuban gar larvae are characterised by an almost immobile behaviour, and these corporal oscillatory movements do not allow them to move in the water column. Therefore, it was not surprising to find that from 2 DAH onwards, the trunk length growth was almost isometric, similar to the preanal and postanal heights, indicating a constant and proportional growth of this part of the body in relation to total length. Significant morphogenesis and growth processes occur in the trunk region: a differentiation and growth of myotomes and the development of digestive organs (Gisbert & Doroshov 2006). The trunk growth pattern observed in A. tristoechus may be explained considering the priority to develop a precocious digestive system, rather than to improve the swimming mechanism with the active participation of the corporal musculature.
Regarding the development of the digestive system, Mendoza et al. (2002) stated that the digestive tract of Atractosteus spatula develops rapidly and is completely formed in 5 DAH larvae. Histological studies carried out on Cuban gar larvae (Comabella et al. 2013) have indicated that the digestive tract in 2 DAH larvae is differentiated into three regions, and the liver and pancreatic tissue are also evident. When the first exogenous feeding takes place, the alimentary canal is well developed and the stomach is morphologically differentiated into three regions, showing the gastric glands in the fundic area, indicating the genesis of chemical digestion. These results are in agreement with data obtained by Comabella et al. (2006), who found significant acid protease activity in Cuban gar larvae (indicative of the beginning of a functional stomach) from 5 DAH onwards. These results reveal the rapid development of this system and ensure that the essential organs for feeding are developed first.
During feeding, when Cuban gar larvae detect a prey item, they twist their entire body into a sinusoidal shape, followed by quick bursts towards the prey, and move their head laterally to place the jaws around the intended prey item (Comabella et al. 2010). This movement may be associated with the standard maneuvering and start movements that are usually observed in fish at this stage (Barros & Higuchi 2007). According to Walker (2004), these actions are commonly associated with predatory strikes that involve both caudal fin movements to generate an impulse and pectoral fins for maneuvering. Positive allometric growth was observed in both the pectoral and pelvic fins during the early development of A. tristoechus. A similar growth has been observed in sturgeons (Gisbert & Doroshov 2006) and red snappers (Williams et al. 2004) during lecithotrophic or exotrophic periods respectively, and was attributed to their crucial function in swimming and maneuvering for feeding. The pectoral fins are the first to appear but the last to obtain a full complement of rays (Betti et al. 2009). They aid in locomotion and prey capture in larval teleosts (Batty 1984; Osse & Boogart 2004). Also, according to Murata et al. (2010), fish with basal pelvic fins (including bichirs, sturgeons, gars and bowfins) often have extremely limited pelvic fin function. In contrast, in more evolved fish, the pelvic fins have a trimming function that reduces pitching and up-ward body displacement during braking. Curiously, the Cuban gar larvae were observed to feed around 5-7 DAH, however from 8 DAH onwards the pectoral fins recorded an isometric growth. For this reason, the relationship between the growth of these fins and the maneuverability function in favour of an effective swimming for feeding as reported for other fish species remains in doubt for Cuban gar. On the other hand, the pelvic fins of A. tristoechus showed a fast positive allometric growth throughout the experiment, with the greatest growth coefficient (b = 4.04) spanning from the depletion of the yolk sac to 8 DAH. What biological explanation could this pattern have? What high-priority functions do the paired fins have in this species? Transformations in the shape, orientation and position of the pectoral and pelvic fins have been well documented (Drucker & Lauder 2002; Lauder & Drucker 2004), yet the
hydrodynamic consequences of this evolutionary variation are poorly understood (Drucker et al. 2005). The other fin involved in the locomotion process is the caudal fin. Atractosteus tristoechus larvae exhibited isometric growth in tail length during the larval stage, in contrast with the positive allometry observed in sturgeon (Gisbert & Doroshov 2006), croaker (Shan & Dou 2009) and catfish (Huysen-truyt et al. 2009), and the negative allometry observed in gilthead sea bream (Russo et al. 2007). The inflexion points recorded for these species have been associated with an improvement in swimming capacity. A possible explanation for these allometric growth patterns is a change in swimming style (Snik et al. 1997; Osse & Boogaart 1999) from anguilliform to subcarangiform, in which the caudal part of the body performs large wide movements and the rest of the body remains relatively rigid (Osse 1990).
Klingenberg and Froese (1991) recorded, for 17 marine species, a strong positive allometry in the body depth behind the anus, indicating that the posterior part of the body became relatively stouter as the larvae grew. These authors related this pattern to a change in swimming style during larval growth, associated with an increasing importance of the tail region for locomotion. However, in our case, apart from the isometric growth in tail length, a negative allometry was recorded for the caudal peduncle depth, with the same inflexion point recorded for the paired fins. Thus, it is now necessary to define these characteristics for our species considering the swimming structures. Detailed studies of gar larvae, in combination with research on larval swimming kinematics, combining laboratory and field studies of locomotion behaviour, could explain the growth pattern obtained in our study.
Also, it is not only the development of structures that guarantee the start of active swimming and an efficient assimilation of external food that is necessary, but improved mechanisms for food capture should also exist, such as the cephalic development that takes place during the first days of the Cuban gar larvae. The growth patterns recorded for the head and the snout in this species were positively allometric for length and negatively allometric for width, indicating a lengthening and narrowing of the cephalic region during early development. Positive allometric growth of the head is a common feature in the early ontogeny of fish like the loricariids (Strauss 1995; Schmidt 2001), sturgeons (Snik et al. 1997; Gisbert 1999; Osse & Boogart 2004) and catfish (Geerinckx et al. 2008; Huysentruyt et al. 2009). Kammerer et al. (2005) examined two gar species (Atractosteus spatula and Lepisosteus osseus) and found a strong positive allometry for jaw length relative to skull size during the transition from larvae to adult, followed by a weak negative allometry as the adult animal continued to grow. Gisbert and Doroshov (2006) and Choo and Liew (2006) considered that a rapid growth in head length is probably linked to the development of nervous (brain), sensory (vision and olfaction), respiratory (gill arches and filaments) and feeding systems. Kolmann and Huber (2009) stated that a positive allometry in feeding performance assists predators in overcoming the functional constraints imposed by their prey, and may confer a competitive advantage over isometric ontogenetic trajectories, facilitating access to exclusive trophic resources earlier in life. In the case of the living gar, predatory behaviour comprises slow overall movements followed by a rapid strike, rather than an active pursuit (Kammerer et al. 2005). Therefore, the elongation of the Cuban gar larvae snout during growth may optimise the capture of rapid swimming prey such as Moina.
The inflexion points for the head and snout lengths-widths of the Cuban gar larvae were recorded at 6 DAH. Our observations revealed that at 4 DAH the yolk sac was externally absent, indicating its depletion, and that the larvae must switch to exogenous feeding. The presence of a functional food intake apparatus is then required as an adaptation to the ichthyophage feeding habits of this family. Given this, it is consistent that nearing the point of yolk sac depletion, external morphogenesis efforts are focused on head elongation in order to complete the most essential apparatus that allows the localisation and uptake of prey of increasing sizes, as a functional priority in fish larvae survival. Of all the morphometric data evaluated, only the pectoral height showed a reduction in absolute size (enantiometry) from hatching to 4 DAH due to the reduction of the yolk sac. Data for this character showed a great dispersion and a poor determination coefficient (R2) that was generated by the difference in the shape and length of the yolk sac among individuals.
The results of the allometric analyses carried out on Cuban gar larvae for the paired and caudal fins, as well as for the growth of the trunk and cephalic region, make it possible to state the following: these fins are the main structures that allow swimming to start at this stage, the morphogenesis efforts in the trunk are focused on the precocious development of the digestive system in order to satisfy the nutritional needs required for rapid growth, and the cephalic development guarantees the efficiency of food capture. These last two aspects allow the beginning of exogenous feeding. Exotrophic larvae, capable of detecting and predating on zooplancton in the water column, were characterised by a pronounced daily increase in weight following an effective assimilation of external nutrients. This was confirmed by an analysis of protein concentration (Comabella et al. 2006) and a study of the effect of starvation (unpublished data). The allometric growth in weight of Cuban gar from hatching to 18 DAH may be divided into two phases: the first 14 days with a slow negative allometric growth and the last 4 days with a faster negative allometric growth. This inflexion point marks the significant differences in the growth coefficient of this variable. The ontogeny of the digestive enzymes (Comabella et al. 2006) also revealed a maximum of enzymatic activity at the same age, indicating that the fast weight growth recorded from 14 to 18 DAH is associated with a complete and efficient exogenous feeding.
Thus, the results obtained in the present study regarding the head, trunk and tail growth in Cuban gar larvae show a continuous and gradual change in few body characters, and a discontinuity and abrupt change in others. This agrees with the theory of saltatory ontogeny formulated by Balon (1985) and applied by other authors like Pavlov (1999), Kovac et al. (1999), Kovac (2002) and Belanger et al. (2010). Fuiman (1983) and Snik et al. (1997) stated that the changes in larvae growth reflect the priorities of the developing organism, optimising growth to increase survival appropriately. Larvae are equipped with numerous temporary organs that are remodeled toward the definitive form present in the adult, using energy in the transformation process. To improve the chance of survival, fish larvae apparently use their available energy considering the most important functions. The concentration of Cuban gar larval inflexion points in a narrow range of ages and total length values may exemplify the priorities of the developing organism. Thus, a rapid development of the head and digestive tract may be necessary for the larvae to be able to satisfy their need to feed on prey at this early stage of development. Also, the inactive behaviour of this species may explain the growth patterns observed for the trunk, tail and paired fins. Allometric growth studies are usually carried out on fish, but they present some limitations. The first is that an allometric equation describes the relationship between two characters but does not explain why the relationship is the way it is (Trombulak 1991). An understanding of the basis for a particular relationship can only come from knowledge of the system itself which may not be obvious, and the interpretation of the changes could be merely speculative. The second limitation is that the relationship may change over time (Trombulak 1991), that it may depend on larval rearing conditions (Koumoundouros et al. 1999; Kouttouki et al. 2006) and that it may vary between wild and reared populations. Also, if samples are preserved for later analysis, the procedures may significantly affect the length and external appearance of the larvae, resulting in body shrinkage and, frequently, in axial curvatures (Koumoundouros et al. 2005). In our case, in order to prevent the effects of stress during preservation as well as the axial curvatures, the specimens were first anesthetised and then preserved in an appropriate position and solution. Although these limitations are real, changes in the growth trajectories of morphological characters during ontogeny are a potentially useful source of information, as they may be caused by marked events in the life history of the species or by rapid ecological changes (Katsanevakis et al. 2007), and should not be overlooked. Information on allometric growth in larval Cuban gar provides insights into the behaviour and phenotype of cultured animals. Besides, it may be used as a reference for their aquacultural monitoring and may become a useful tool in natural environmental studies.
ACKNOWLEDGEMENTS
This study was supported by the Centro de Investigaciones Marinas (CIM) and the Centro de Reproducción de la Ictiofauna Indígena, from Cuba.
LITERATURA CITADA
Balon EK (1985) The theory of saltatory ontogeny and life history models revisited. En: Balon (ed) Early Life History of Fish. Junk Publishers, pp. 13-30. [ Links ] Barros B, Higuchi H (2007) Notes on morphological characters in early developed amazonian leaf fish, Monocirrhus polyacanthus (Polycentridae, Perciformes). Kempffiana 3:18-22. [ Links ]
Batty RS (1984) Development of swimming movements and musculture of larval herring (Clupea harengus). Journal of Experimental Biology 110: 217-229. [ Links ]
Belanger SE, Balon EK, Rawlings JM (2010) Saltatory ontogeny of fishes and sensitive early life stages for ecotoxicology tests. Aquatic Toxicology 97:88-95. [ Links ]
Betti P, Machinandiarena L, Ehrlich MD (2009) Larval development of Argentine hake Merluccius hubbsi. Journal of Fish Biology 74: 235-249. [ Links ]
Celik P, Cirik S (2011) Allometric growth in serpae tetra (Hyphessobrycon serpae) larvae. Journal of Animal and Veterinary Advances 10:2267-2270. [ Links ]
Cobán D, Kamaci HO, Suzer C, Saka A (2009) Allometric growth in hatchery-reared gilthead seabream. North American Journal of Aquaculture 71: 189-196. [ Links ]
Comabella Y, Hernández A, Hurtado A, Canabal J, García-Galano T (2013) Ontogenetic development of the digestive tract in Cuban gar (Atractosteus tristoechus) larvae. Review in Fish Biology and Fisheries 23(2): 245-260. [ Links ]
Comabella Y, Hurtado A, García-Galano T (2010) Morphological and morphometric description of Cuban gar (Atractosteus tristoechus) larvae. Zoological Science 27: 931-938. [ Links ]
Comabella Y, Mendoza R, Aguilera C, Carrillo O, Hurtado A, García-Galano T (2006) Digestive enzyme activity during early larval development of the Cuban gar Atractosteus tristoechus. Fish Physiology and Biochemistry 32: 147-157. [ Links ]
Coughlin DJ (1991) Ontogeny of feeding behaviour of first-feeding Atlantic salmon (Salmo salar). Canadian Journal of Fisheries and Aquatic Science 48: 1896-1904. [ Links ]
Choo CK, Liew HC (2006) Morphological development and allometric growth patterns in the juvenile seahorse Hippocampus kuda Bleeker. Journal of Fish Biology 69: 426-445. [ Links ]
Deng X, Eenennaam JPV, Doroshov SI (2002) Comparison of early life stages and growth of green and white sturgeon. En: Winkle WV, Anders PJ, Secor DH, Dixon DA (eds) Biology, Management and Protection of North American Sturgeon. American Fisheries Society Symposium 28. pp. 237-248. [ Links ]
Dettlaff TA, Ginsburg AS, Schmalhausen 0I (1993) Sturgeon fishes: developmental biology and aquaculture. Springer-Verlag, Berlin. [ Links ]
Devlin RH, Vandersteen WE, Uh M, Stevens ED (2012) Genetically modified growth affects allometry of eye and brain in salmonids. Canadian Journal of Zoology 90: 193-202. [ Links ]
Drucker EG, Lauder GV (2002) Wake dynamics and locomotor function in fishes: Interpreting evolutionary patterns in pectoral fin design. Integrative Comparative Biology 42: 997-1008. [ Links ]
Drucker EG, Walker JA, Westneat MW (2005) Mechanics of Pectoral Fin Swimming in Fishes. En: Fish Physiology, pp. 369-423. [ Links ]
Eenennaam JPV, Web MAH, Deng X, Doroshov SI, Mayfield RB, Cech JJ, Hillemeier DC, Willson TE (2001) Artificial spawning and larval rearing of Klamath River green sturgeon. Transactions of the American Fisheries Society 130: 159-165. [ Links ]
Fuiman LA (1983) Growth gradients in fish larvae. Journal of Fish Biology 23: 117-123. [ Links ]
Gayon J (2000) History of the concept of allometry. American Zoologist 40: 748-758. [ Links ]
Geerinckx T, Verhaegen Y, Adriaens D (2008) Ontogenetic allometries and shape changes in the suckermouth armoured catfish Ancistrus cf. triradiatus Eigenmann (Loricariidae, Siluriformes), related to suckermouth attachment and yolksac size. Journal of Fish Biology 72: 803-814. [ Links ]
Gilbert SF, Bolker JA (2003) Ecological developmental biology: preface to the symposium. Evolution & Development 5: 3-8. [ Links ]
Gisbert E (1999) Early development and allometric growth patterns in Siberian sturgeon and their ecological significance. Journal of Fish Biology 54: 852-862. [ Links ]
Gisbert E, Doroshov SI (2006) Allometric growth in green sturgeon larvae. Journal of Applied Ichthyolgy 22 (Suppl. 1):202-207. [ Links ]
Gisbert E, Merino G, Muguet JB, Bush D, Piedrahita RH, Conklin DE (2002) Morphological development and allometric growth patterns in hatchery-reared California halibut larvae. Journal of Fish Biology 61: 1217-1229. [ Links ]
Gisbert E, Sarasquete MC, Williot P, Castelló-Orvay F (1999) Histochemistry of the development of the digestive system of Siberian sturgeon during early ontogeny. Journal of Fish Biology 55: 596-616. [ Links ]
Goldman CA, Snell RR, Thomason JJ, Brown DB (1990) Principles of allometry. En: Goldman CA (ed) Tested Studies for Laboratory Teaching, pp. 43-72. [ Links ]
Huang P, Song C, Zhang L-Z, Zhang T, Huang X-R, Wang B (2009) Allometric growth of artificial bred Siberian sturgeon Acipenser baeri larvae and juveniles. Chinese Journal of Ecology 04. 681-687 [ Links ]
Huxley JS, Teissier G (1936) Terminology of relative growth. Nature 137:780-781. [ Links ]
Huysentruyt F, Moerkerke B, Devaere S, Adriaens D (2009) Early development and allometric growth in the armoured catfish Corydoras aeneus (Gill, 1858). Hydrobiologia 627: 45-54. [ Links ]
Kammerer CH, Grande L, Westneat MW (2005) Comparative and developmental functional morphology of the jaws of living and fossil gars (Actinopterygii: Lepisosteidae). Journal of Morphology 263: 1-15. [ Links ]
Katsanevakis S, Thessalou-Legaki M, Karlou-Riga C, Lefkaditou E, Dimitriou E, Verriopoulos G (2007) Information-theory approach to allometric growth of marine organisms. Marine Biology 151: 949-959. [ Links ]
Klingenberg CP (1996) Multivariate allometry. En: Marcus LF (ed) Advances in Morphometries. Plenum Press, New York pp. 23-49. [ Links ]
Klingenberg CP, Froese R (1991) A multivariate comparison of allometric growth patterns. Systematic Zoology 40: 410-419. [ Links ]
Kolmann MA, Huber D (2009) Scaling of feeding biomechanics in the horn shark Heterodontus francisci: ontogenetic constraints on durophagy. Zoology 112: 351-361. [ Links ]
Koumoundouros G, Divanach P, Kentouri M (1999) Ontogeny and allometric plasticity of Dentex dentex (Osteichthyes: Sparidae) in rearing conditions. Marine Biology 135: 561-572. [ Links ]
Koumoundouros G, Kouttouki S, Georgakopoulou E, Papadakis I, Maingot E, Kaspiris P, Kiriakou Y, Georgiou G, Divanach P, Kentouri M, Mylonas C (2005) Ontogeny of the shi drum Umbrina cirrosa (Linnaeus 1758), a candidate new species for aquaculture. Aquaculture Research 36: 1265-1272. [ Links ]
Kouttouki S, Georgakopoulou E, Kaspiris P, Divanach P, Koumoundouros G (2006) Shape ontogeny and variation in the sharpsnout seabream Diplodus puntazzo (Cetti, 1777). Aquaculture Research 37:655-663. [ Links ]
Kovac V (2002) Synchrony and heterochrony in ontogeny (offish). Journal of Theoretical Biology 217: 499-507. [ Links ]
Kovac V, Copp GH, Francis MP (1999) Morphometry of the stone loach Barbatula barbatula: do mensural characters reflect the species' life history thresholds? Environmental Biology of Fishes 56: 105-115. [ Links ]
Lauder GV, Drucker EG (2004) Morphology and experimental hydrodynamics offish fin control surfaces. IEEE Journal of Oceanic Engineering 29:556-571. [ Links ]
Makrakis MC, Nakatani K, Bialetzki A, Sanches PV, Baumgartnera G, Gomes LC (2005) Ontogenetic shifts in digestive tract morphology and diet of fish larvae of the Itaipu Reservoir, Brazil. Environmental Biology of Fishes 72: 99-107. [ Links ]
Mello FT, Iglesias C, Borthagaray Al, Mazzeo N, Vilches J, Larrea D, Ballabio R (2006) Ontogenetic allometric coefficient changes: implications of diet shift and morphometric traits in Hoplias malabaricus (Bloch) (Characiforme, Erythrinidae). Journal of Fish Biology 69: 1770-1778. [ Links ]
Mendoza R, Aguilera C, Rodríguez G, González M, Castro R (2002) Morphophysiological studies on alligator gar (Atractosteus spatula) larval development as a basis for their culture and repopulation of their natural habitats. Fish Biology and Fisheries 12: 133-142. [ Links ]
Murata Y, Tamura M, Aita Y, Fujimura K, Murakami Y, Okabe M, Okada N, Tanaka M (2010) Allometric growth of the trunk leads to the rostral shift of the pelvic fin in teleost fishes. Developmental Biology 347: 236-245. [ Links ]
Murphy BF, Leis JM, Kavanagh KD (2007) Larval development of the Ambon damselfish Pomacentrus amboinensis, with a summary of pomacentrid development. Journal of Fish Biology 71: 569-584. [ Links ]
Osse JW, Boogaart JGM (1995) Fish larvae, allometric growth and the aquatic environment. ICES Marine Sciences Symposium 201: 21-34. [ Links ]
Osse JW (1990) Form changes in fish larvae in relation to changing demands of function. Netherland Journal of Zoology 40:362-385. [ Links ]
Osse JW, Boogaart JGM (1999) Dynamic morphology of fish larvae, structural implications of friction forces in swimming, feeding and ventilation. Journal of Fish Biology 55: 156-174. [ Links ]
Osse JW, Boogaart JGM, Snik GMJ, Sluys LVD (1997) Priorities during early growth offish larvae. Aquaculture 155: 249-258. [ Links ]
Osse JW, Boogart JGM (2004) Allometric growth in fish larvae: timing and function. American Fisheries Society Symposium 40: 167-194. [ Links ]
Pavlov DA (1999) Features of transition from larva to juvenile in fishes with different types of early ontogeny. Environmental Biology Fisheries 56:41-52. [ Links ]
Porter S, Theilacker G (1999) The development of the digestive tract and eye in larval walleye pollock Theragra chalcogramma. Fisheries Bulletin 97: 722-729. [ Links ]
Rodríguez-Mendoza R, Muñoz M, Saborido-Rey F (2011) Ontogenetic allometry of the bluemouth Helicolenus dactylopterus dactylopterus (Teleostei: Scorpaenidae), in the Northeast Atlantic and Mediterranean based on geometric morphometries. Hydrobiologia 670: 5-22. [ Links ]
Roos G, Wassenbergh SV, Herrel A, Adriaens D, Aerts P (2010) Snout allometry in seahorses: insights on optimisation of pivot feeding performance during ontogeny. Journal of Experimental Biology 213: 2184-2193. [ Links ]
Russo T, Costa C, Cataudella S (2007) Correspondence between shape and feeding habit changes throughout ontogeny of gilthead sea bream Sparus aurata L. 1758. Journal of Fish Biology 71: 629-656. [ Links ]
Sala R, Santamaría CA, Crespo S (2005) Growth of organ systems of Dentex dentex (L) and Psetta maxima (L) during larval development. Journal of Fish Biology 66: 315-326. [ Links ]
Schmidt RE (2001) Loricaria cataphracta: parental care and description of early larvae. Ichthyological Explorations of Freshwaters 12: 235-240. [ Links ]
Shan X-J, Dou S-Z (2009) Allometric growth of croaker Miichthys miiuy larvae and juveniles and its ecological implication. Oceanología et Limnología Sinica 06. [ Links ]
Simon T, Wallus R (1989) Contributions to the early life histories of gar (Actinopterygii: Lepisosteidae) in the Ohio and Tennesse river basins with emphasis on larval development. Transactions of the Kentucky Academy of Science 50: 59-74. [ Links ]
Snik GMJ, Boogaart JGM, Osse JW (1997) Larval growth patterns in Cyprinus carpió and Clarias gariepinus with attention to the finfold. Journal of Fish Biology 50: 1339-1352. [ Links ]
Strauss RE (1995) Metamorphic growth-gradient changes in South American loricariid catfishes Lorcariichthys maculatus and Pseudohemiodon laticeps. Studies on Neotropical Fauna and Environment 30: 177-191. [ Links ]
Trombulak SC (1991) Allometry in biological systems. En: Goldman CA (ed) Tested Studies for Laboratory Teaching, pp. 49-68. [ Links ]
Walker J A (2004) Kinematics and performance of maneuvering control surfaces in teleost fishes. IEEE Journal of Oceanic Engineering 3: 572-584. [ Links ]
Webb JF (1999) Larvae in Fish Development and Evolution. En: The Origin and Evolution of Larval Forms. Academic Press, pp. 109-158. [ Links ]
Webb PW, Weihs D (1986) Functional morphology of early life history stages of fishes. Transactions of the American Fisheries Society 115: 115-127. [ Links ]
Williams K, Papanikos N, Phelps RP, Shardo JD (2004) Development, growth and yolk utilization of hatchery-reared red snapper Lutjanus campechanus larvae. Marine Ecology Progress Series 275: 231-239. [ Links ]